Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3061
Revised: April 23, 2024
Accepted: April 29, 2024
Published online: June 16, 2024
Processing time: 87 Days and 4.5 Hours
Many guidelines have recommended renin-angiotensin system inhibitors (RASI) as the first-line treatment for patients with chronic kidney disease (CKD). We studied RASI prescription trends from 2010 to 2019, and analyzed the characteristics associated with RASI prescription in Chinese hospitalized CKD patients.
To study the prescription of renin angiotensin system inhibitors in hospitalized patients with CKD in China.
It was retrospectively, cross-sectional reviewed RASI prescriptions in hospitalized CKD patients in China from 2010 to 2019. RASI prescribing trends were analyzed from 2010 to 2019, and bivariate and multivariate logistic regression analyses were conducted to identify characteristics associated with RASI prescription.
A total of 35090 CKD patients were included, with 10043 (28.6%) RASI prescrip
The frequency of RASI prescriptions showed an initial increase but a slight decreasing trend in more recent years. CKD patients with certain characteristics such as elderly age, advanced disease stage, surgery department admission, or acute kidney injury were less likely to receive RASI prescriptions. In the application of RASI in hospitalized CKD patients is insufficient. The actual clinical practice needs to be improved. The development of related research is helpful to guide the correct choice of clinical treatment strategy.
Core Tip: This study examines the prescribing trends of renin-angiotensin system inhibitors (RASI) in hospitalized Chinese chronic kidney disease (CKD) patients from 2010 to 2019. Despite guidelines recommending RASI as first-line therapy, findings reveal a peak in prescriptions around 2015 and 2016, followed by a slight decline. Factors such as younger age, male gender, and non-acute conditions increased RASI prescription likelihood. The research highlights a need for improved adherence to clinical guidelines in RASI application to enhance CKD patient care outcomes.
- Citation: Zhang C, Duan ZY, Nie SS, Zhang Z, Guo XR, Zhang CY, Dong J, Cai GY. Renin-angiotensin system inhibitors prescriptions in Chinese hospitalized chronic kidney disease patients. World J Clin Cases 2024; 12(17): 3061-3075
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3061.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3061
Chronic kidney disease (CKD) is a worldwide public health problem. Currently, the estimated CKD prevalence is more than 10% worldwide, an increase of 29.3% from 1990[1,2]. Increased CKD prevalence has contributed significantly to global morbidity and mortalit[3]. Appropriate management of CKD can not only slow the deterioration of the kidney function, but also reduce the risk of cardiovascular disorders, having a great impact on both the individual patient and society[4].
In the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines for the Evaluation and Management of CKD, close blood pressure monitoring and control was noted as an essential step to effectively reducing the progression of CKD and cardiovascular diseases[5]. The renin-angiotensin-aldosterone system (RAAS) is activated in patients with CKD[6]. Renin-angiotensin system inhibitors (RASI) have been shown to protect against renal impairment and reduce cardiovascular events independent of their blood pressure-lowering effects[7]. RASI has been recommended as first-line treatment for hypertensive patients with CKD, especially those with albuminuria[8,9]. It is also recommended that adult CKD patients with or without diabetes but experiencing albuminuria receive RASI[5]. However, there is significant variation in RASI prescribing practices among different countries, and adherence to the KDIGO guidelines is poor[10]. With a prevalence of approximately 11%, China has the highest number of patients with CKD[2,11]. A recently published study reported that only 71.2% of hypertensive patients with CKD received RASI[12]. However, the characteristics associated with RASI prescription in these CKD patients were not studied.
In the present study, we retrospectively reviewed RASI prescription in many hospitalized CKD patients over a 10-year period. Here, we report RASI prescribing trends and the characteristics associated with RASI prescription, with the purpose of improving CKD management and patient outcomes.
We performed a retrospective cross-sectional study and reviewed the hospital medical records of patients who were admitted to and discharged from the First Medical Center of the Chinese People’s Liberation Army General Hospital from January 2010 to December 2019. The study protocol was approved by the hospital ethics committee.
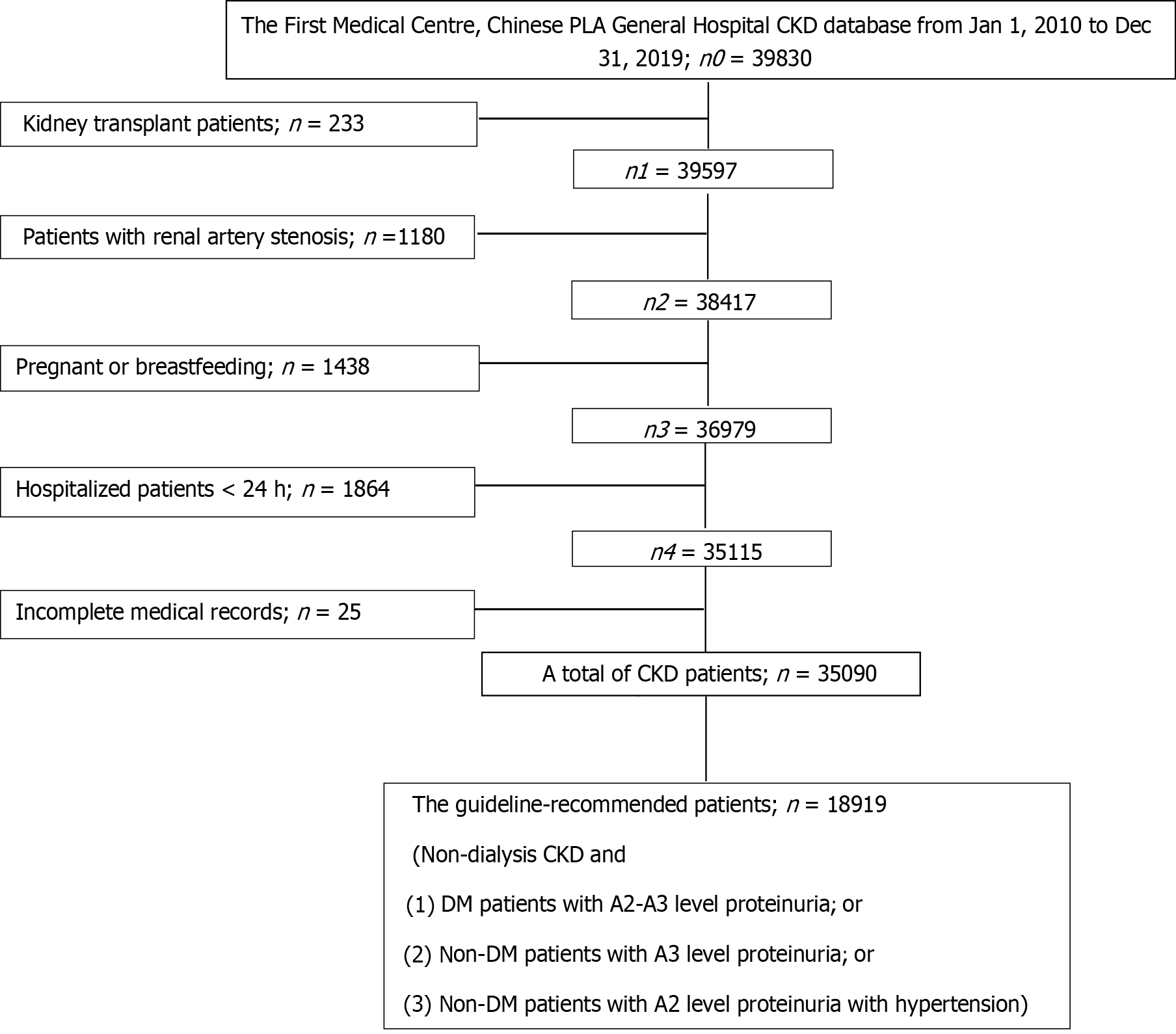
The inclusion criteria included adult patients (age ≥ 18-year-old) with a hospital discharge diagnosis of CKD based on the International Classification of Diseases, Tenth Revision (ICD-10). The exclusion criteria were patients with: (1) History of renal transplantation; (2) Bilateral renal artery stenosis; (3) Pregnant or breastfeeding; (4) Length of hospital stay < 24 h; or (5) Incomplete medical records.
Data were collected by reviewing the hospital medical records. The baseline characteristics included age, gender, height, weight, race, and medical history of other diseases such as hypertension, diabetes, cerebrovascular disease (heart failure, coronary artery disease, myocardial infarction, atrial fibrillation, or stroke), hyperlipidemia, and peripheral vascular disease. Blood pressure was the initial measurement at hospital admission. Laboratory test results were also collected based on the initial examination results at hospital admission and included fasting blood glucose, creatinine, potassium, total cholesterol, hemoglobin, bicarbonate, urinalysis, 24-h total urine protein excretion rate (PER), and urine albumin to creatinine ratio (ACR). Echocardiography results were reviewed to record the left ventricular ejection fraction. Treatments including hemodialysis, peritoneal dialysis, and combined hemodialysis and peritoneal dialysis were documented. Information on medications at hospital discharge including angiotensin II receptor blockers, angiotensin converting enzyme inhibitors, calcium channel blockers, beta-blockers, diuretics, were reviewed. In addition, hospital admission information including admitting department, year of admission were also collected. The cause of CKD including glomerulonephritis, IgA nephropathy, hypertension, and diabetes were documented. In patients with multiple hospital admissions during the study period, only information collected during the first admission was reviewed.
CKD stage was determined based on the initial calculated estimated glomerular filtration rate (eGFR). The eGFR was based on the initial serum creatinine (Scr) level at hospital admission and calculated with the CKD-EPI-Asian formula[13]. Female Scr ≤ 0.7 mg/dL, eGFR = 151 × (0.993) age × (Scr/0.7) - 0.328. Female Scr > 0.7 mg/dL, eGFR = 151 × (0.993) age × (Scr/0.7) - 1.210. Male Scr ≤ 0.9 mg/dL, eGFR = 149 × (0.993) age × (Scr/0.7) - 0.415. Male Scr > 0.9 mg/dL, eGFR = 149 × (0.993) age × (Scr/0.7) - 1.210; CKD stages were defined as G1 with eGFR ≥ 90 mL/min/1.73 m2, G2 with eGFR 60-89 mL/min/1.73 m2, G3 with eGFR 30-59 mL/min/1.73 m2, G4 with eGFR 15-29 mL/min/1.73 m2, and G5 with eGFR < 15 mL/ min/1.73 m2[4].
Albuminuria was staged as A1 (PER < 150 mg/d or ACR < 30 mg/g), A2 (PER 150-500 mg/d or ACR 30-300 mg/g), and A3 (PER > 500 mg/d or ACR > 300 mg/g) based on the 2012 KDIGO guidelines for CKD management[14].
Acute kidney injury was defined as a Scr level that increased ≥ 26.5 μmol/L (≥ 0.3 mg/dL) within 48 h, or a Scr level that increased ≥ 1.5 times higher than the baseline level within 7 d[5].
The patients were assigned into a RASI group and a non-RASI group based on hospital discharge medication in CKD patients and KDIGO guideline-recommended CKD patients. Since elderly CKD patients (age ≥ 60-year-old) and CKD patients with a history of cardiovascular or cerebrovascular diseases have increased risk of morbidity and mortality, we analyzed these subgroups separately.
Continuous data are presented as mean ± SD and were compared by student’s t test, or median with interquartile range and compared by the Mann-Whitney U test, depending on normality test results. Categorical variables are represented as numbers with percentages (%) and compared using Chi square test. Bivariate comparisons were performed first between the CKD patients with or without the RASI prescriptions. Then, stepwise multivariate logistic regression analyses were used to analyze the characteristics associated with RASI prescription in hospitalized CKD patients and guideline-recommended CKD patients. All statistical analyses were performed with SPSS 22.0 software (SPSS, IBM, New York, United States). All tests were two-sided, with a P < 0.05 considered statistically significant.
A total of 35090 CKD patients were included in the analysis (Figure 1). The mean age was 50.3 ± 16.9 years old, with 21803 (62.1%) male patients and 11581 (33.0%) patients ≥ 60-year-old. The majority of the patients were of Han race (31743, 90.5%). The most common cause of CKD was glomerulonephritis (GN) (14127, 40.3%). The most common medical history was hypertension (20514, 58.5%). There were 13607 (38.8%), 9420 (26.8%), 4568 (13.0%), and 7495 (21.4%) patients with stage G1-2, G3, G4, and G5 CKD, respectively. RASI prescriptions were found in 10043 (28.6%) patients. Among all the patients included in this analysis, 18919 (53.9%) patients met the criteria for RASI treatment based on the 2012 KDIGO guidelines for CKD management. Of these, 7246 (72.2%) patients received RASI prescriptions.
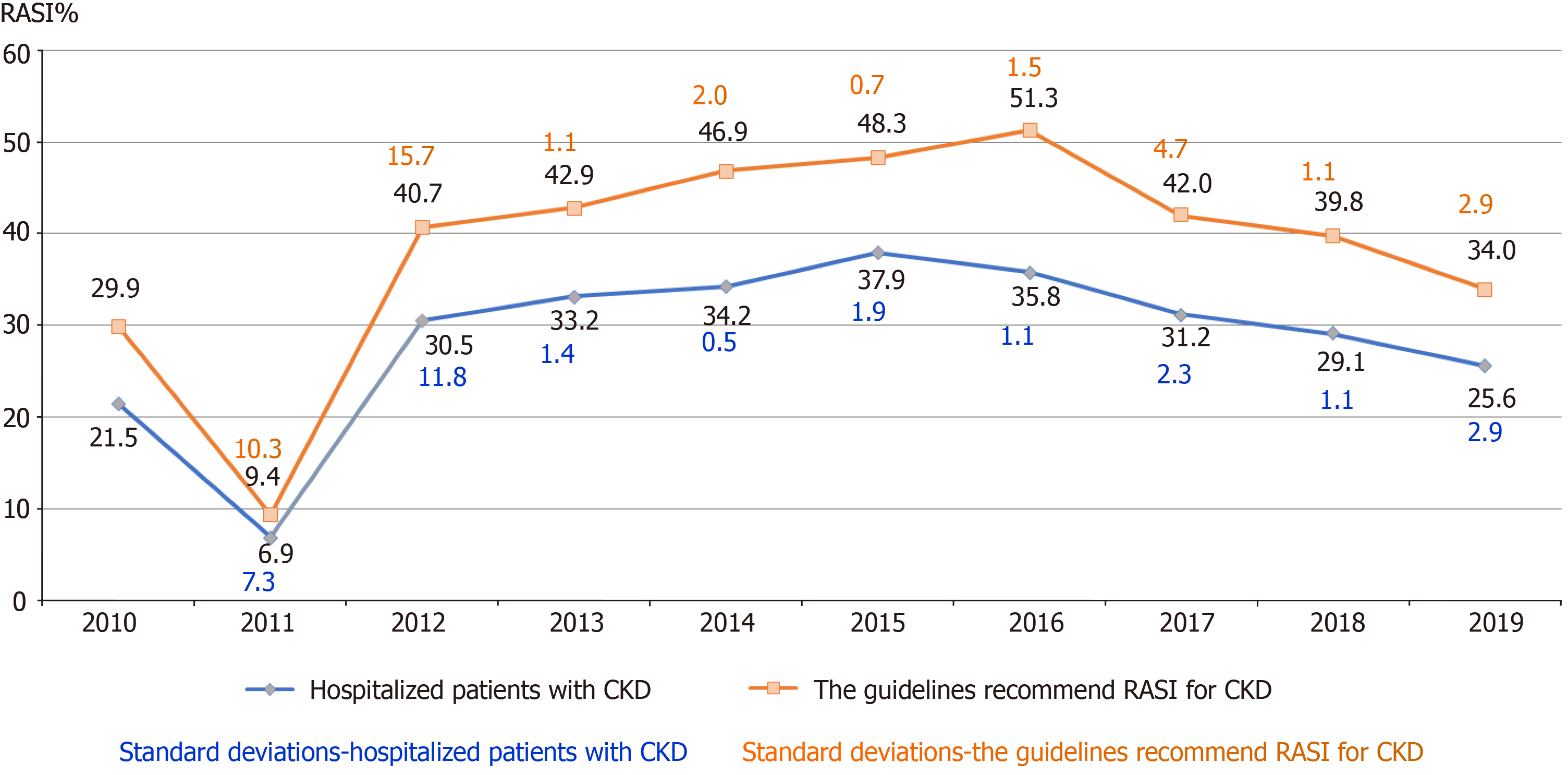
In general, there was an increasing trend in the frequency of RASI prescriptions in all CKD patients and guideline-recommended CKD patients (Figure 2). The most significant increase was observed from 2011 to 2016 (an increase of 13.4%). The peak percentages of RASI prescriptions were observed in 2015 and 2016. However, the frequency of RASI prescriptions showed a slight decreasing trend after 2016. Subgroup analyses in the elderly patients showed similar trends in RASI prescriptions from 2011 to 2016.
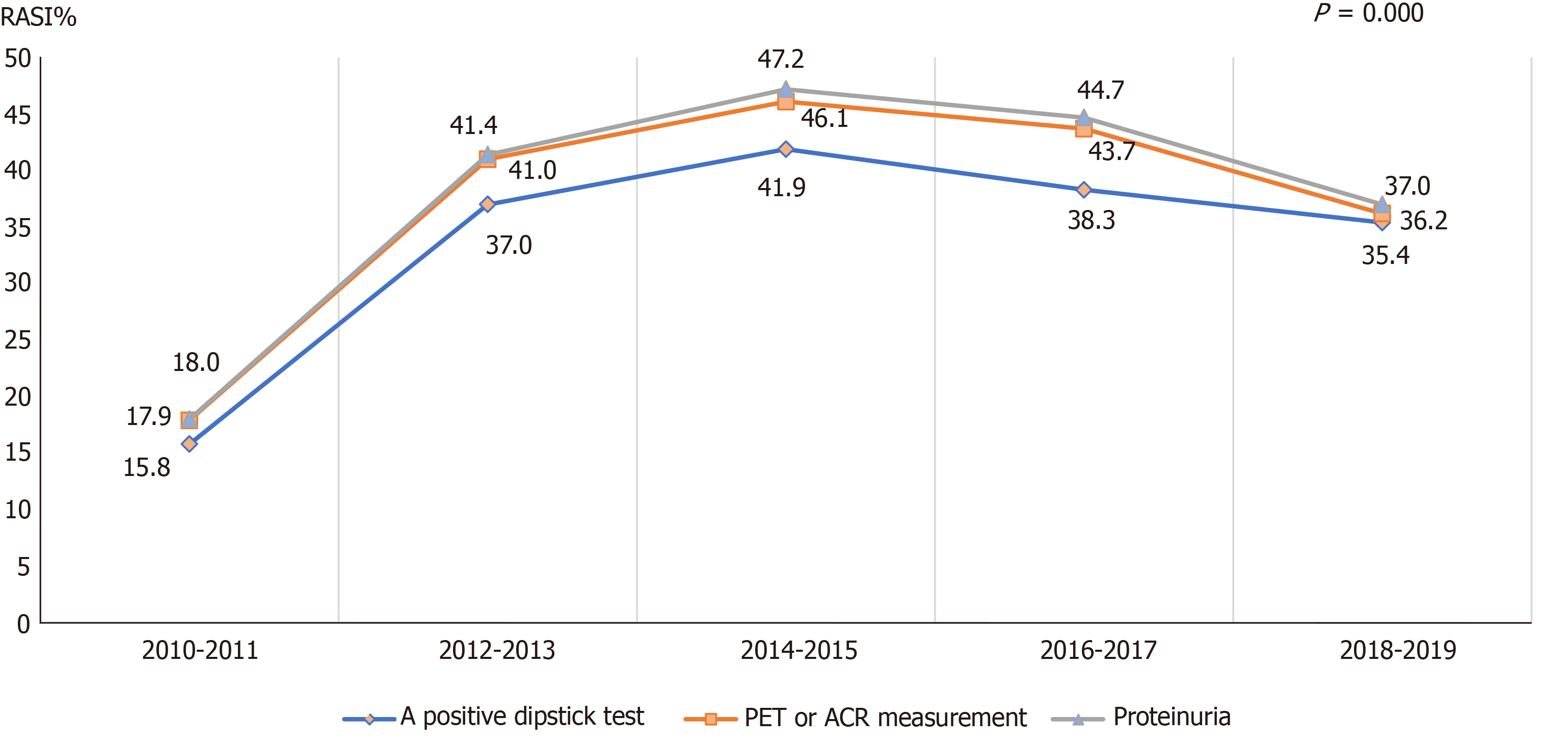
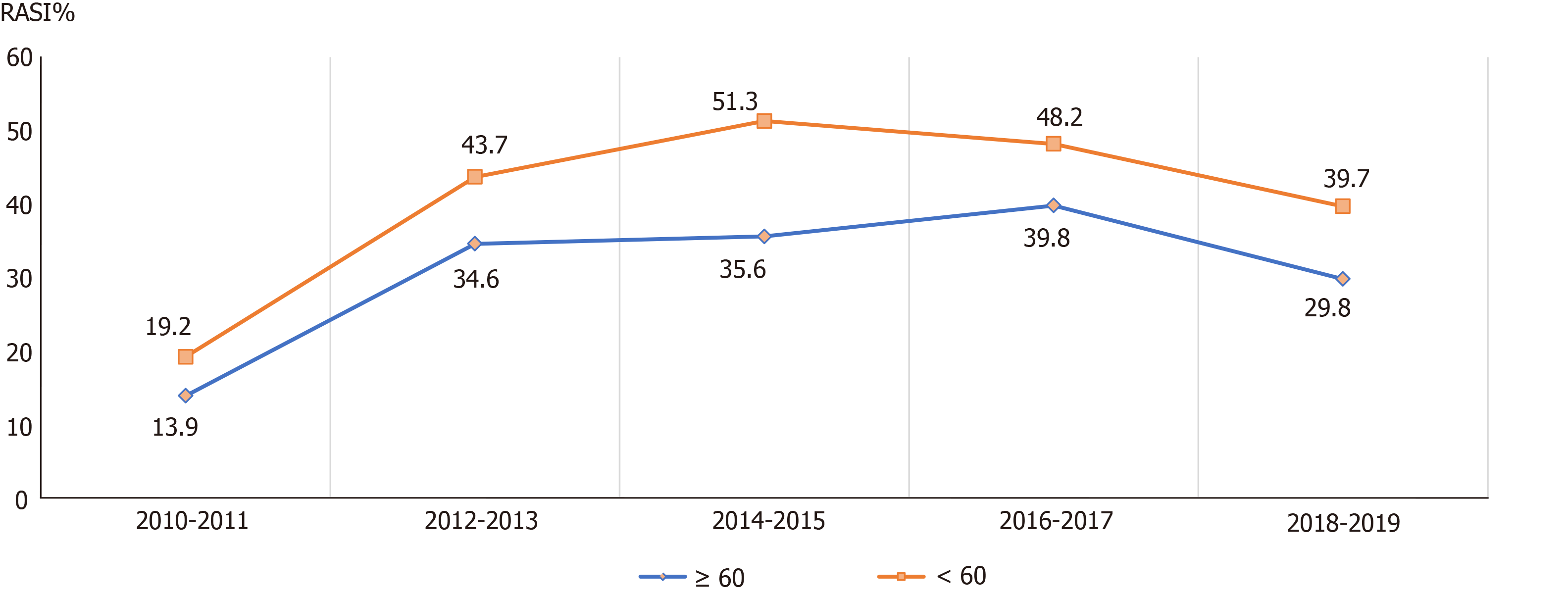
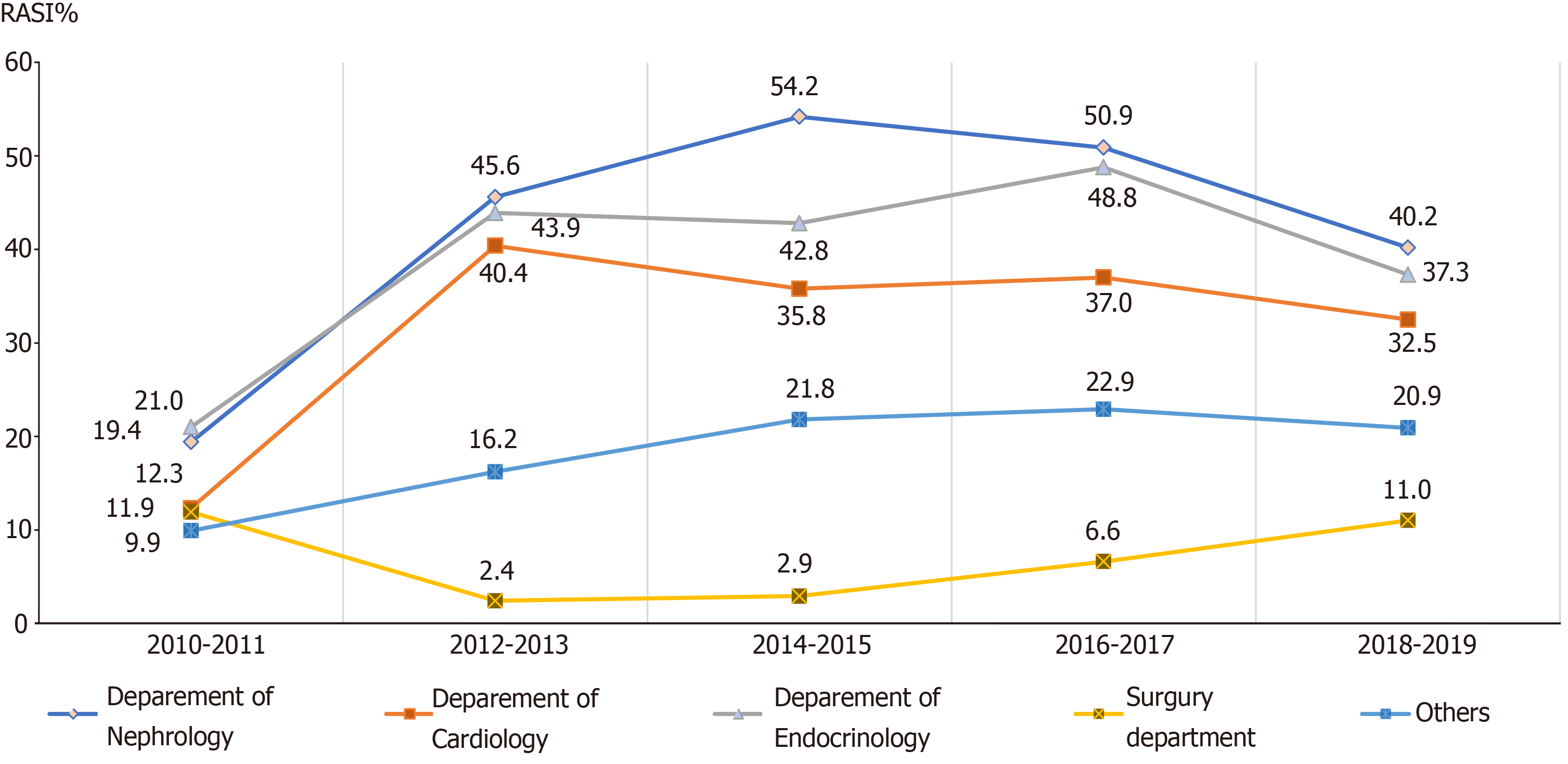
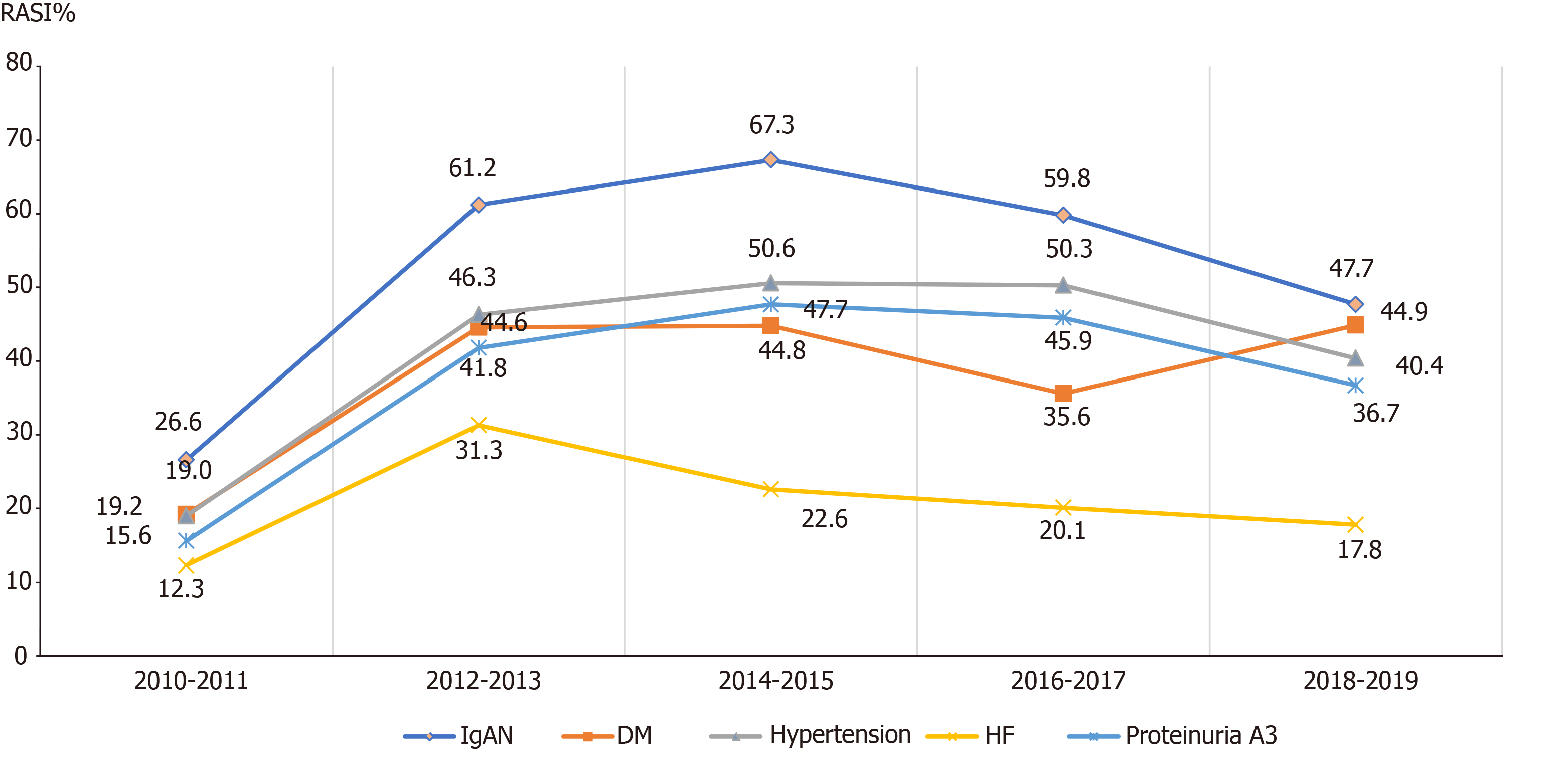
In CKD patients with proteinuria, as well as the guideline-recommended patients in different age groups, different admission departments, and different etiologies, similar increasing trends of the RASI prescriptions were also observed from 2011 to 2019 (Figures 3-6). Of note, the surgery department had a persistent low frequency of RASI prescriptions, only about 10% throughout the 10-year study period.
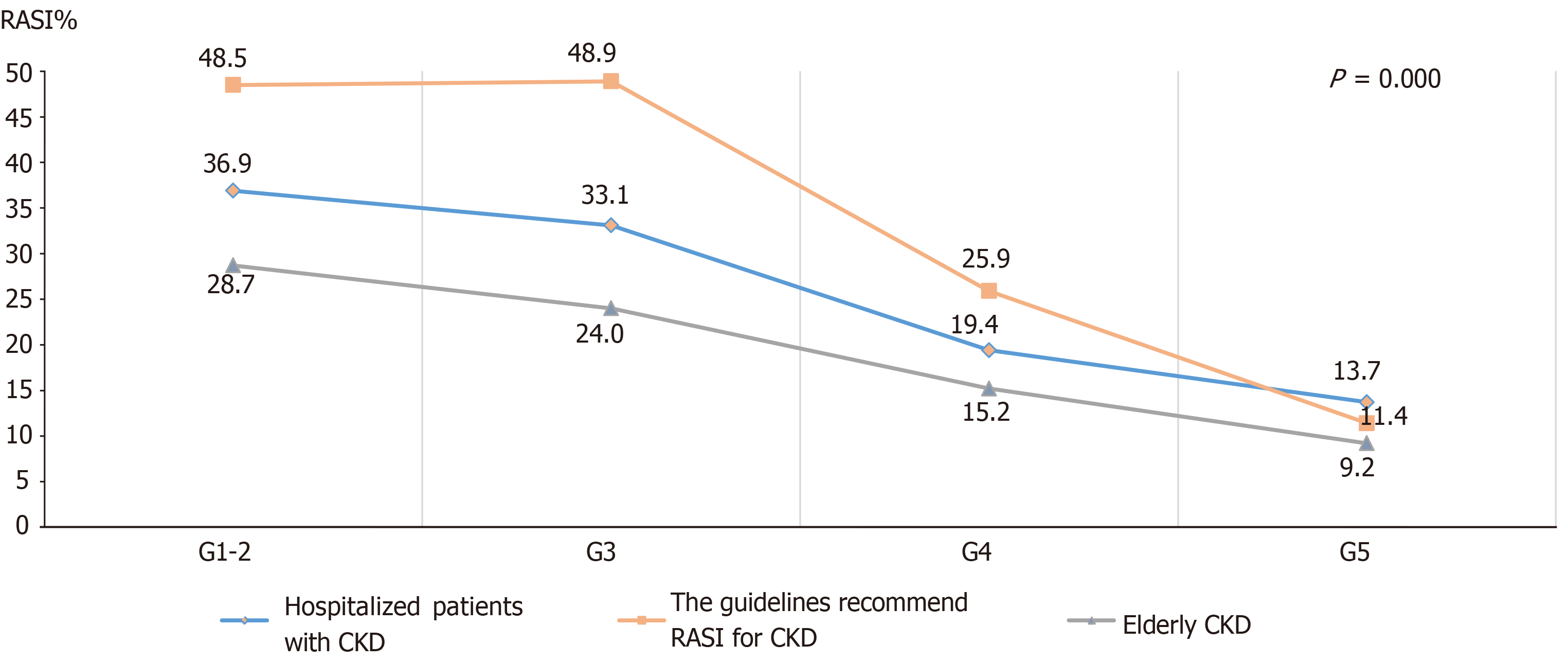
When comparing the characteristics of CKD patients with or without the RASI prescriptions, RASI prescriptions were more frequently found in patients < 60-year-old, discharged from the department of nephrology, GN as the CKD cause, and with a medical history of hypertension, diabetes, or hyperlipidemia. Patients with higher blood pressure (≥ 140 or 90 mmHg for systolic and diastolic blood pressure, respectively), stage G1-2 CKD, hemoglobin ≥ 120 g/L, low bicarbonate levels (< 22 mmol/L), low serum potassium levels (< 5.5 mmol/L), stage A3 albuminuria, or on kidney replacement therapy were more likely to receive RASI prescriptions. Detailed comparisons between CKD patients with or without RASI prescriptions are shown in Table 1. The RASI prescription analysis in patients with different CKD stages in different patient populations (all patients, guideline-recommended patients, and elderly patients) showed that patients with advanced CKD were less likely to receive RASI (Figure 7).
| Characteristic | All | No RASI | RASI | P value |
| Patients | 35090 | 25047 | 10043 | |
| Beijing patients | 6756 (19.3) | 5045 (20.1) | 1711(17.0) | |
| Guideline-recommend patients | 18919 (53.9) | 11673 (61.7) | 7246 (72.2) | < 0.001 |
| Calendar year | < 0.001 | |||
| 2010–2011 | 6679 (19.0) | 5785 (23.1) | 894 (8.9) | < 0.001 |
| 2012–2013 | 7487 (21.3) | 5104 (20.4) | 2383 (23.7) | |
| 2014–2015 | 7205 (20.5) | 4606 (18.4) | 2599 (25.9) | |
| 2016–2017 | 6770 (19.3) | 4503 (18.0) | 2267 (22.6) | |
| 2018–2019 | 6949 (19.8) | 5049 (20.2) | 1900 (18.9) | |
| Age ≥ 60 yr | 11581 (33.0) | 7688 (76.6) | 2355 (23.4) | < 0.001 |
| Men | 21803 (62.1) | 15493 (61.9) | 6310 (62.8) | |
| Ethnic group | ||||
| Han Chinese | 31743 (90.5) | 22686 (90.6) | 9057 (90.2) | |
| BMI (kg/m2) | 24.74 (22.18, 27.4406) | 24.44 (21.91, 27.12) | 25.48 (28.26, 22.99) | |
| Age, yr | 50.33 ± 16.924 | 51.15 ± 17.097 | 46.97 ± 15.760 | < 0.001 |
| Systolic blood pressure (mmHg) | 138 ± 26 | 140 ± 18 | 135 ± 42 | |
| Diastolic blood pressure (mmHg) | 79 ± 12 | 77 ± 14 | 82 ± 9 | |
| Blood glucose (mmol/L) | 8.1 ± 4.3 | 7.9 ± 4.1 | 8.5 ± 5.1 | |
| Hemoglobin (g/dL) | 110 ± 25.6 | 111.4 ± 27 | 107.3 ± 26.2 | |
| Serum phosphorus (mmol/L) | 1.7 ± 0.3 | 1.1 ± 0.3 | 1.4 ± 0.4 | |
| CO2 (mmol/L) | 22.7 ± 3.6 | 23.8 ± 2.5 | 20.4 ± 4.6 | |
| Serum potassium (mmol/L) | 4.3 ± 0.7 | 4.1 ± 0.6 | 4.8 ± 0.9 | |
| Serum creatinine (mg/dL) | 2.4 (1.1, 3.2) | 2.2 (1.1, 3.0) | 2.8 (1.4, 6.2) | |
| Cholesterol (mmol/L) | 4.50 (3.7000, 5.5500) | 4.7000 (3.9300, 5.8200) | 4.3900 (3.6000, 5.41000) | |
| Admitting department | < 0.001 | |||
| Department of Nephrology | 18072 (51.5) | 10669 (42.6) | 7403 (73.7) | |
| Department of Cardiology | 4460 (12.7) | 3233 (12.9) | 1227 (12.2) | |
| Department of Endocrinology | 2306 (6.6) | 1504 (6) | 802 (8.0) | |
| Department of Neurology | 343 (1.0) | 279 (1.1) | 64 (0.6) | |
| Department of Respiratory | 497 (1.4) | 462 (1.8) | 35 (0.3) | |
| Surgery department | 4192 (11.9) | 4135 (16.5) | 57 (0.6) | |
| Others | 5220 (14.9) | 5765 (19) | 455 (4.5) | |
| Cause of CKD | ||||
| Glomerulonephritis | 14127 (40.3) | 2954 (31.8) | 6173 (61.5) | < 0.001 |
| IgA nephropathy | 4153 (11.8) | 2050 (8.2) | 2103 (20.9) | < 0.001 |
| Diabetic kidney disease | 4150 (11.8) | 2696 (10.8) | 1454 (14.5) | < 0.001 |
| Hypertensive nephropathy | 1800 (5.1) | 1004 (4.0) | 796 (7.9) | < 0.001 |
| Others | 16769 (47.7) | 12409 (49.5) | 4340 (43.3) | < 0.001 |
| Medical history | ||||
| Hypertension | 20514 (58.5) | 13533 (54) | 6981 (69.5) | < 0.001 |
| Diabetes mellitus | 9949 (28.4) | 6892 (27.5) | 3057 (30.4) | < 0.001 |
| Coronary heart disease | 5728 (16.3) | 4244 (16.9) | 1484 (14.8) | < 0.001 |
| Atrial fibrillation | 1050 (3) | 836 (3.3) | 214 (2.1) | < 0.001 |
| Heart failure | 2879 (8.2) | 2306 (9.2) | 573 (5.7) | < 0.001 |
| Myocardial infarction | 687 (2) | 527 (2.1) | 160 (1.6) | 0.002 |
| Stroke | 1440 (4.1) | 1116 (4.5) | 324 (3.2) | < 0.001 |
| Peripheral arterial disease | 1074 (3.1) | 678 (2.7) | 396 (3.9) | < 0.001 |
| Hyperlipidemia | 3423 (9.8) | 2166 (8.6) | 1257 (12.5) | < 0.001 |
| Acute kidney injury | 6804 (19.4) | 5740 (22.9) | 1064 (10.6) | < 0.001 |
| Systolic blood pressure (mmHg) | < 0.001 | |||
| ≥ 140 | 15467 (44.1) | 10701 (42.7) | 4766 (47.5) | |
| 120 ≤ SBP < 140 | 6403 (18.3) | 4921 (19.6) | 1482 (14.8) | |
| 90 ≤ SBP < 120 | 13002 (37.1) | 3781 (37.6) | 9221 (36.8) | |
| < 90 | 218 (0.6) | 14 (0.1) | 204 (0.8) | |
| Diastolic blood pressure (mmHg) | < 0.001 | |||
| ≥ 90 | 10519 (30.0) | 6797 (27.1) | 3722 (37.1) | |
| 60 ≤ DBP < 90 | 23365 (66.6) | 17231 (68.8) | 6134 (61.1) | |
| < 60 | 1206 (3.4) | 1019 (4.1) | 187 (3.4) | |
| Staging of CKD | < 0.001 | |||
| G1–2 | 13607 (38.8) | 8592 (34.3) | 5015 (49.9) | |
| G3 | 9420 (26.8) | 6305 (25.2) | 3115 (31) | |
| G4 | 4568 (13) | 3684 (14.7) | 884 (8.8) | |
| G5 | 7495 (21.4) | 6466 (25.8) | 1029 (10.2) | |
| Hemoglobin (g/L) | < 0.001 | |||
| ≥ 120 | 18113.0 | 11645 (46.5) | 6468 (64.5) | |
| 100 ≤ HB < 120 | 8402.0 | 6324 (25.3) | 2078 (20.7) | |
| 70 ≤ HB< 100 | 7201.0 | 5932 (23.7) | 1269 (12.6) | |
| < 70 | 1374.0 | 1146 (4.6) | 228 (2.3) | |
| CO2 < 22 (mmol/L) | 7314 (20.8) | 5380 (21.5) | 1934 (19.3) | < 0.001 |
| Serum potassium (mmol/L) | < 0.001 | |||
| < 5.5 | 33555 (97.5) | 23749 (97.1) | 9806 (98.6) | |
| 5.5-6.0 | 592 (1.7) | 494 (2.0) | 98 (1.0) | |
| > 6.0 | 257 (0.7) | 216 (0.9) | 41 (0.4) | |
| Dipstick test positive | 25806 (73.5) | 17158 (68.5) | 8648 (86.1) | < 0.001 |
| Albuminuria | 21905 (62.43) | 13744 (54.87) | 8161 (37.3) | < 0.001 |
| A1 | 1228 (5.6) | 919 (3.7) | 309 (3.1) | |
| A2 | 3416 (15.6) | 2167 (8.7) | 1249 (12.4) | |
| A3 | 17262 (78.8) | 10658 (42.6) | 6603 (65.7) | |
| Unmeasured LVEF | 32981 (94.0) | 9531 (94.9) | 23450 (93.6) | < 0.001 |
| Medication use | ||||
| Calcium channel blocker | 9636 (27.5) | 5540 (22.1) | 4096 (40.8) | < 0.001 |
| Beta-blocker | 6193 (17.6) | 3679 (14.7) | 2514 (25) | < 0.001 |
| Diuretics | 3469 (9.9) | 1975 (7.9) | 1494 (14.9) | < 0.001 |
| Kidney replacement therapy | 1177 (3.4) | 728 (2.9) | 449 (4.5) | < 0.001 |
| Hemodialysis | 726 (2.1) | 464 (1.9) | 262 (2.6) | < 0.001 |
| Peritoneal dialysis | 494 (1.4) | 285 (1.1) | 209 (2.1) | < 0.001 |
Of the 18919 patients who met the criteria for RASI treatment based on the 2012 KDIGO guidelines for CKD management, male patients and young patients (< 60-year-old), as well as patients with CKD stages 1 to 3, admitted to the nephrology department, with GN as the etiology, or with a history of hypertension were more likely to receive a RASI prescription (Table 2).
| Characteristic | 2010-2011 | 2012-2013 | 2014-2015 | 2016-2017 | 2018-2019 | P value | ||||||||||
| All | No RASI | RASI | All | No RASI | RASI | All | No RASI | RASI | All | No RASI | RASI | All | No RASI | RASI | ||
| Patients | 3518 (18.6) | 636 (8.8) | 2882 (24.7) | 4306 (22.8) | 1798 (24.8) | 2508 (21.5) | 3876 (20.5) | 1848 (25.5) | 2028 (17.4) | 3291 (17.4) | 1512 (20.9) | 1779 (15.2) | 3928 (20.8) | 1452 (20.0) | 2476 (21.2) | < 0.001 |
| Age ≥ 60 yr | 722 (20.5) | 100 (15.7) | 622 (21.6) | 934 (21.7) | 323 (18.0) | 611 (24.4) | 886 (22.9) | 315 (17.0) | 571 (28.2) | 872 (26.5) | 347 (22.9) | 525 (29.5) | 1082 (27.5) | 322 (22.2) | 760 (30.7) | < 0.001 |
| Male | 2101 (59.7) | 393 (61.8) | 1708 (59.3) | 2552 (59.3) | 1079 (60.0) | 1473 (58.7) | 2335 (60.2) | 1131 (61.2) | 1204 (59.4) | 2048 (62.2) | 954 (63.1) | 1094 (61.5) | 2513 (64.0) | 911 (62.7) | 1602 (64.75) | 0.172 |
| Admitting department | < 0.001 | |||||||||||||||
| Department of Nephrology | 2540 (72.2) | 493 (77.5) | 2047 (71.0) | 3186 (74.0) | 1452 (80.8) | 1734 (69.1) | 2779 (71.7) | 1506 (81.5) | 1273 (62.8) | 2350 (71.4) | 1196 (79.1) | 1154 (64.9) | 2869 (73.0) | 1153 (79.4) | 1716 (69.3) | |
| Department of Cardiology | 171 (4.9) | 21 (3.3) | 150 (5.2) | 245 (5.7) | 99 (5.5) | 146 (5.8) | 232 (6.0) | 83 (4.5) | 149 (7.3) | 211 (6.4) | 78 (5.2) | 133 (7.5) | 271 (6.9) | 88 (6.1) | 183 (7.4) | |
| Department of endocrinology | 372 (10.6) | 78 (12.3) | 294 (10.2) | 401 (9.3) | 176 (9.8) | 225 (9.0) | 397 (10.2) | 170 (9.2) | 227 (11.2) | 322 (9.8) | 157 (10.4) | 165 (9.3) | 332 (8.5) | 124 (8.5) | 208 (8.4) | |
| Surgery department | 42 (1.2) | 5 (0.8) | 37 (1.3) | 42 (1.0) | 1 (0.1) | 41 (1.6) | 68 (1.8) | 2 (0.1) | 66 (3.3) | 76 (2.3) | 5 (0.3) | 71 (4.0) | 9 (0.6) | 9 (0.6) | 73 (2.9) | |
| Others | 393 (11.17) | 39 (6.13) | 354 (12.28) | 432 (10.03) | 70 (3.9) | 362 (14.4) | 400 (10.3) | 87 (4.7) | 313 (15.4) | 332 (10.1) | 76 (5.0) | 256 (14.4) | 24 (1.7) | 78 (5.4) | 296 (12.0) | |
| Glomerulonephritis | 1987 (56.5) | 403 (63.4) | 1584 (55.0) | 2559 (59.4) | 1216 (67.6) | 1343 (53.5) | 2333 (60.2) | 1375 (74.4) | 958 (47.2) | 1873 (56.9) | 1056 (69.8) | 817 (45.9) | 2281 (58.1) | 1041 (71.7) | 1240 (50.1) | < 0.001 |
| IgA nephropathy | 668 (19.0) | 178 (28.0) | 490 (17.0) | 763 (17.7) | 467 (26.0) | 296 (11.8) | 651 (16.8) | 438 (23.7) | 213 (10.5) | 527 (16.0) | 315 (20.8) | 212 (11.9) | 620 (15.8) | 296 (20.4) | 324 (13.1) | < 0.001 |
| Diabetic kidney disease | 641 (18.2) | 136 (21.4) | 505 (17.5) | 680 (15.8) | 320 (17.8) | 360 (14.4) | 581 (15.0) | 261 (14.1) | 320 (15.8) | 550 (16.7) | 241 (15.9) | 309 (17.4) | 738 (18.8) | 275 (18.9) | 463 (18.7) | 0.654 |
| Hypertensive nephropathy | 256 (7.3) | 43 (6.8) | 213 (7.4) | 384 (8.9) | 186 (10.3) | 198 (7.9) | 197 (5.1) | 137 (7.4) | 60 (3.0) | 140 (4.3) | 78 (5.2) | 62 (3.5) | 495 (12.6) | 247 (17.0) | 248 (10.0) | < 0.001 |
| Medical history | ||||||||||||||||
| Diabetes mellitus | 1051 (29.9) | 202 (31.8) | 849 (29.5) | 1252 (29.1) | 559 (31.1) | 693 (27.6) | 1090 (33.1) | 488 (32.3) | 602 (33.8) | 1360 (34.6) | 484 (33.3) | 876 (35.4) | 1195 (30.4) | 536 (36.9) | 659 (26.6) | 0.770 |
| Hypertension | 2261 (64.3) | 430 (67.6) | 1831 (63.5) | 2723 (63.2) | 1261 (70.1) | 1462 (58.3) | 2405 (62.0) | 1218 (65.9) | 1187 (58.5) | 2031 (61.7) | 1021 (67.5) | 1010 (56.8) | 2719 (69.2) | 1098 (75.6) | 1621 (65.5) | < 0.001 |
| Heart failure | 146 (4.2) | 18 (2.8) | 128 (4.4) | 227 (5.3) | 71 (3.9) | 156 (6.2) | 186 (4.8) | 42 (2.3) | 144 (7.1) | 179 (5.4) | 36 (2.4) | 143 (8.0) | 247 (6.3) | 44 (3.0) | 203 (8.2) | < 0.001 |
| Acute kidney injury | 587 (16.7) | 71 (11.2) | 516 (17.9) | 636 (14.8) | 171 (9.5) | 465 (18.5) | 504 (13.0) | 115 (6.2) | 389 (19.2) | 495 (15.0) | 103 (6.8) | 392 (22.0) | 556 (14.2) | 80 (5.5) | 476 (19.2) | < 0.001 |
| Staging of CKD | < 0.001 | |||||||||||||||
| G1–2 | 1537 (43.7) | 350 (55.0) | 1187 (41.2) | 1895 (44.0) | 958 (53.3) | 937 (37.4) | 1756 (45.3) | 1050 (56.8) | 706 (34.8) | 1400 (42.5) | 824 (54.5) | 576 (32.4) | 1541 (39.2) | 762 (52.5) | 779 (31.5) | |
| G3 | 743 (21.1) | 157 (24.7) | 586 (20.3) | 976 (22.7) | 502 (27.9) | 474 (18.9) | 948 (24.5) | 540 (29.2) | 408 (20.1) | 835 (25.4) | 498 (32.9) | 337 (18.9) | 1010 (25.7) | 509 (35.1) | 501 (20.2) | |
| G4 | 444 (12.6) | 59 (9.3) | 385 (13.4) | 593 (13.8) | 179 (10.0) | 414 (16.5) | 529 (13.6) | 173 (9.4) | 356 (17.6) | 472 (14.2) | 140 (9.3) | 332 (18.7) | 603 (15.4) | 132 (9.1) | 471 (19.0) | |
| G5 | 794 (22.6) | 70 (11.0) | 724 (25.1) | 842 (19.6) | 159 (8.8) | 683 (27.2) | 643 (16.6) | 85 (4.6) | 558 (27.5) | 584 (17.7) | 50 (3.3) | 534 (30.0) | 774 (19.7) | 49 (3.4) | 725 (29.3) | |
| Serum potassium (mmol/L) | < 0.001 | |||||||||||||||
| < 5.5 | 3405 (96.8) | 616 (96.9) | 2789 (96.8) | 4206 (97.7) | 1753 (97.5) | 2453 (97.8) | 3808 (98.2) | 1821 (98.5) | 1987 (98.0) | 3217 (97.8) | 14719 (97.3) | 1746 (98.1) | 3830 (97.5) | 1436 (98.9) | 2394 (96.7) | |
| 5.5-6.0 | 77 (2.2) | 13 (2.0) | 64 (2.2) | 71 (1.6) | 33 (1.8) | 38 (1.5) | 50 (1.3) | 20 (1.1) | 30 (1.5) | 50 (1.5) | 28 (1.9) | 22 (1.2) | 74 (1.9) | 12 (0.8) | 62 (2.5) | |
| > 6.0 | 36 (1.0) | 7 (1.1) | 29 (1.0) | 29 (0.7) | 12 (0.7) | 17 (0.7) | 18 (0.5) | 7 (0.4) | 11 (0.5) | 24 (0.7) | 13 (0.9) | 11 (0.6) | 24 (0.6) | 4 (0.3) | 20 (0.8) | |
| Albuminuria | 0.096 | |||||||||||||||
| A2 | 451 (12.8) | 95 (14.9) | 356 (12.4) | 556 (12.9) | 254 (14.1) | 302 (12.0) | 492 (12.7) | 221 (12.0) | 271 (13.4) | 416 (12.6) | 201 (13.3) | 215 (12.1) | 478 (12.2) | 183 (12.6) | 295 (11.9) | |
| A3 | 3067 (87.2) | 541 (85.1) | 2526 (87.6) | 3750 (87.1) | 1544 (85.9) | 2206 (88.0) | 3384 (87.3) | 1627 (88.0) | 1757 (86.6) | 2875 (87.4) | 1311 (86.7) | 1564 (87.9) | 3475 (87.8) | 1269 (87.4) | 2181 (88.1) | |
We further performed multivariate logistic regression analysis and analyzed the characteristics that were associated with RASI prescriptions in CKD patients. Table 3 and Table 4 show the analysis results in all CKD patients and the guideline-recommended CKD patients, respectively. Both analyses indicated that male patients, diagnosed more recently, of younger age, discharged from the nephrology department, lower CKD stage, history of hypertension or diabetes, proteinuria, GN as the CKD etiology, and non-AKI were associated with RASI prescriptions. In addition, in the guideline-recommended patients, patients with a diastolic blood pressure ≥ 90 mmHg, with a history of IgA nephropathy, or without heart failure were more likely to receive RASI prescriptions.
| Characteristic | P value | Odds ratio (95%CI) |
| Gender, male versus female | 0.012 | 1.077 (1.016, 1.141) |
| Year of admission | < 0.001 | |
| 2012-2013 | 3.532 (3.214, 3.881) | |
| 2014-2015 | 4.529 (4.118, 4.982) | |
| 2016-2017 | 4.070 (3.694, 4.485) | |
| 2018-2019 | 2.818 (2.555, 3.107) | |
| Age, yr | < 0.001 | |
| 60 ≤ age < 70 | < 0.001 | 0.764 (0.707, 0.826) |
| 70 ≤ age < 80 | < 0.001 | 0.703 (0.633, 0.781) |
| 80 ≤ age < 90 | < 0.001 | 0.558 (0.462, 0.675) |
| ≥ 90 | 0.185 | 0.544 (0.221, 1.338) |
| Departments | < 0.001 | |
| Cardiology | < 0.001 | 0.599 (0.542, 0.663) |
| Endocrinology | < 0.001 | 0.486 (0.430, 0.548) |
| Neurology | < 0.001 | 0.271 (0.202, 0.364) |
| Pulmonary | < 0.001 | 0.112 (0.078, 0.161) |
| Surgery | < 0.001 | 0.024 (0.018, 0.031) |
| Others | < 0.001 | 0.150 (0.133, 0.169) |
| Stage of CKD | < 0.001 | |
| CKD2 | 0.259 | 1.050 (0.965, 1.142) |
| CKD3 | < 0.001 | 0.807 (0.743, 0.875) |
| CKD4 | < 0.001 | 0.260 (0.234, 0.289) |
| CKD5 | < 0.001 | 0.149 (0.133, 0.166) |
| Acute kidney injury | < 0.001 | 0.857 (0.788, 0.932) |
| Hypertension | < 0.001 | 2.798 (2.624, 2.984) |
| Diabetes | < 0.001 | 1.245 (1.164, 1.332) |
| Glomerulonephritis | < 0.001 | 1.179 (1.091, 1.275) |
| Proteinuria | < 0.001 | 1.553 (1.434, 1.680) |
| Characteristics | P value | Odds ratio (95%CI) |
| Gender, male versus female | 0.020 | 1.089 (1.014, 1.170) |
| Year of admission | < 0.001 | |
| 2012-2013 | 3.795 (3.384, 4.255) | |
| 2014-2015 | 4.799 (4.269, 5.394) | |
| 2016-2017 | 4.648 (4.117, 5.247) | |
| 2018-2019 | 2.913 (2.589, 3.276) | |
| Age, yr | < 0.001 | |
| 60 ≤ age < 70 | 0.008 | 0.873 (0.790, 0.964) |
| 70 ≤ age < 80 | 0.001 | 0.767 (0.660, 0.891) |
| 80 ≤ age < 90 | 0.008 | 0.639 (0.459, 0.889) |
| ≥ 90 | 0.290 | 0.320 (0.039, 2.647) |
| Diastolic blood pressure | < 0.001 | |
| ≥ 90 mmHg | < 0.001 | 1.195 (1.110, 1.287) |
| < 60 mmHg | 0.004 | 0.666 (0.506, 0.877) |
| Department | < 0.001 | |
| Cardiology | < 0.001 | 0.731 (0.624, 0.857) |
| Endocrinology | < 0.001 | 0.523 (0.459, 0.595) |
| Neurology | < 0.001 | 0.361 (0.221, 0.591) |
| Pulmonary | < 0.001 | 0.113 (0.061, 0.208) |
| Surgery | < 0.001 | 0.107 (0.068, 0.169) |
| Others | < 0.001 | 0.299 (0.260, 0.344) |
| Stage of CKD | < 0.001 | |
| CKD2 | 0.548 | 1.031 (0.933, 1.139) |
| CKD3 | < 0.001 | 0.808 (0.732, 0.890) |
| CKD4 | < 0.001 | 0.245 (0.217, 0.277) |
| CKD5 | < 0.001 | 0.880 (0.077, 0.101) |
| Acute kidney injury | 0.003 | 0.835 (0.742, 0.939) |
| Hypertension | < 0.001 | 2.513 (2.318, 2.724) |
| Diabetes | < 0.001 | 1.218 (1.118, 1.327) |
| IgA nephropathy | < 0.001 | 1.498 (1.368, 1.640) |
| Heart failure | 0.045 | 0.825 (0.685, 0.995) |
| Proteinuria | < 0.001 | 1.400 (1.227, 1.597) |
CKD is a long-term progressive deterioration of renal function and is associated with RAAS activation[6]. RAAS activation can elevate blood pressure, which, together with many other factors, can cause further renal injury[15]. Many guidelines have recommended RASI as the first-line treatment for patients with CKD[5,8,16-18]. China has the largest patient population with CKD[2]. CKD is associated with many other illnesses such as cardiovascular diseases, anemia, and electrolyte imbalance, which significantly increases morbidity and mortality in affected patients[3]. This suggests that there is an urgent need to manage CKD in China. However, there is limited research investigating RASI usage and its associated prescribing patterns in China, especially in patients who are admitted to the hospital. In the present study, we reviewed real-world data on RASI prescriptions in a large population of hospitalized CKD patients in China over a 10-year period. We analyzed trends of the RASI prescription and the characteristics influencing its prescribing.
Over a 10-year-period from 2010 to 2019, there was an increasing trend of RASI prescriptions. The most significant increase occurred from 2011 to 2012, when the KDIGO published its guidelines for CKD management[5]. The peak frequencies of RASI prescriptions occurred in 2015 and 2016, after publication of the guidelines for blood pressure management and glycemia treatment in the United States[17,18]. Both guidelines suggested RASI as the first-line treatment for CKD patients with hypertension, diabetes, or proteinuria. However, the frequency of RASI prescription showed a slight decreasing trend after 2016. This suggests that persistent education on RASI treatment should be given to healthcare providers who manage CKD patients.
In the present study, 28.6% CKD patients received RASI prescriptions. In CKD patients who met the criteria for RASI treatment based on the 2012 KDIGO guidelines, 72.2% received RASI prescriptions. In a previous multi-country study in CKD patients in Brazil, Germany, France, and the United States, Germany and the United States had the highest (80%) and lowest (52%) RASI prescription percentages, respectively[19]. Another study reported a significant variation among different countries in RASI prescriptions (from 54% to 91%) for blood pressure management in CKD patients[20]. A recent study in China reported that 71.2% of CKD patients received RASI prescriptions[12]. Our current study results are consistent with these previous findings, suggesting a significant number of CKD patients did not receive RASI prescriptions even though it is recommended by many guidelines as the first-line treatment for CKD patients.
Next, we analyzed RASI prescribing patterns in CKD patients with different characteristics. Most of the patients had stage 1 to 3 CKD. Results showed that there was a decreasing trend in RASI prescriptions with increasing CKD stage. Approximately 40% of stage 1 to 2 CKD patients received RASI where only 13% of stage 5 CKD patients received it. This RASI prescribing pattern was similar in CKD patients who met the criteria for RASI treatment based on the 2012 KDIGO guidelines. However, the KDIGO guidelines do not support stopping RASI treatment in patients with advanced CKD[5,9]. Another previous study reported similar results showing that RASI treatments were less likely to be prescribed to patients with advanced CKD, even though treatments with other anti-hypertensive agents could cause poor outcomes[21]. The authors suggested that more clinical trials should be performed to compare different anti-hypertensive agents in patients with advanced CKD.
RASI is the recommended treatment for CKD patients with hypertension, diabetes, and proteinuria[5,9]. In our study, GN, IgA, hypertension, and diabetes were the main causes of CKD. However, only CKD patients with GN and IgA nephropathy were more likely to receive RASI. In addition, fewer than 50% of CKD patients with hypertension and diabetes received RASI. CKD with proteinuria is another indication for RASI treatment[9,22,23]. Our study showed that fewer than 50% of CKD patients with proteinuria received RASI, however, CKD patients with more severe proteinuria were more likely to receive RASI. These results suggested that healthcare providers were more likely to be familiar with treatments for GN- or IgA-induced CKD, but require more education on RASI treatment in CKD patients with hypertensin, diabetes, and proteinuria.
Elderly CKD patients carry a high risk of morbidity and mortality[24]. In our study, compared with young CKD patients, CKD patients > 60-year were less likely to receive RASI. Similar findings have been reported by previous studies[25-27]. Fewer elderly patients received RASI prescriptions, which might be due to concerns of decreased renal reserve, potential electrolyte imbalance, concurrent multiple medical problems, or interactions with other medications[28].
In the clinic, hyperkalemia and AKI are the main reasons for interruption of RASI treatment, as RASI can increase serum potassium levels and decrease eGFR during the initial stage of treatment[29,30]. This was consistent with our present study results. Patients with hyperkalemia or AKI were less likely to receive RASI prescriptions, suggesting that healthcare providers were aware of the potential side effects of RASI treatment.
We did not identify any previous studies comparing RASI prescriptions in CKD patients admitted to different medical specialties. In the present study, we found that the nephrology department prescribed the most RASI treatments for CKD patients. This result was not surprising, as most patients with CKD are admitted to the nephrology department, and nephrologists are the physicians who are most familiar with the KDIGO guidelines. The surgery department was the least likely service to prescribe RASI treatment. This might be because CKD patients were admitted into the surgery department for surgical issues and not the management of renal function or blood pressure control. Surgeons would treat the acute surgical issue and pay little attention to the management of renal function or hypertension.
We further performed a multivariate logistic regression analysis and evaluated the characteristics that were associated with RASI prescriptions in CKD patients. The results were consistent with the bivariate analysis. Of the CKD patients, male patients, diagnosed more recently, younger age, admission to the nephrology department, lower CKD stage, history of hypertension or diabetes, proteinuria, GN as the CKD etiology, and non-AKI were associated with RASI prescriptions. In addition, in the guideline-recommended CKD patients, those patients with a diastolic blood pressure ≥ 90 mmHg, with a history of IgA nephropathy, or without heart failure were more likely to receive RASI prescriptions. The different analysis results between all CKD patients and the guideline-recommended patients might be due to different proportions of patients with IgA nephropathy, heart failure, and abnormal blood pressure measurements between the two groups of patients.
The strengths of our study include its large sample size and the long observational period of over 10 years. The limitations of our study include its retrospective cross-sectional design with potential biases during the data collection and analyses. The diagnosis of CKD, as well as other medical issues including hypertension, diabetes, and IgA nephropathy were based on the hospital discharge ICD-10 diagnostic codes, which might not be accurate. Our research study was performed in hospitalized patients at a single hospital in a city in China. Although our hospital is a tertiary care hospital with more than 80% of patients from different areas of China, the single-center research could still limit the generalizability of the study results. We also did not collect data on the RASI dosage and patient treatment outcomes. Further research should address these limitations.
In conclusion, our study results showed that the frequency of RASI prescriptions increased initially after the publication of relevant CKD management guidelines but showed a slight decreasing trend in more recent years. Certain CKD patient populations including elderly patients, patients with advanced CKD, patients admitted to the surgery department, and patients with AKI were less likely to receive RASI prescriptions. The application of RASI in hospitalized CKD patients is not enough, relevant research will help to guide the correct choice of clinical treatment strategy.
| 1. | Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12:7-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 1325] [Article Influence: 441.7] [Reference Citation Analysis (0)] |
| 2. | GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4349] [Cited by in RCA: 3809] [Article Influence: 761.8] [Reference Citation Analysis (0)] |
| 3. | Carney EF. The impact of chronic kidney disease on global health. Nat Rev Nephrol. 2020;16:251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 231] [Article Influence: 46.2] [Reference Citation Analysis (1)] |
| 4. | Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322:1294-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 1016] [Article Influence: 169.3] [Reference Citation Analysis (0)] |
| 5. | Taler SJ, Agarwal R, Bakris GL, Flynn JT, Nilsson PM, Rahman M, Sanders PW, Textor SC, Weir MR, Townsend RR. KDOQI US commentary on the 2012 KDIGO clinical practice guideline for management of blood pressure in CKD. Am J Kidney Dis. 2013;62:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 6. | Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33:363-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 276] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 7. | Zhang Y, He D, Zhang W, Xing Y, Guo Y, Wang F, Jia J, Yan T, Liu Y, Lin S. ACE Inhibitor Benefit to Kidney and Cardiovascular Outcomes for Patients with Non-Dialysis Chronic Kidney Disease Stages 3-5: A Network Meta-Analysis of Randomised Clinical Trials. Drugs. 2020;80:797-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr, Williamson JD, Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2649] [Cited by in RCA: 2550] [Article Influence: 364.3] [Reference Citation Analysis (0)] |
| 9. | Cheung AK, Chang TI, Cushman WC, Furth SL, Hou FF, Ix JH, Knoll GA, Muntner P, Pecoits-Filho R, Sarnak MJ, Tobe SW, Tomson CRV, Lytvyn L, Craig JC, Tunnicliffe DJ, Howell M, Tonelli M, Cheung M, Earley A, Mann JFE. Executive summary of the KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021;99:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 193] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 10. | Stengel B, Muenz D, Tu C, Speyer E, Alencar de Pinho N, Combe C, Yamagata K, Reichel H, Fliser D, Massy ZA, Lopes AA, Jadoul M, Winkelmayer WC, Pisoni RL, Robinson BM, Pecoits-Filho R; CKDopps investigators. Adherence to the Kidney Disease: Improving Global Outcomes CKD Guideline in Nephrology Practice Across Countries. Kidney Int Rep. 2021;6:437-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 11. | Zhang L, Wang F, Wang L, Wang W, Liu B, Liu J, Chen M, He Q, Liao Y, Yu X, Chen N, Zhang JE, Hu Z, Liu F, Hong D, Ma L, Liu H, Zhou X, Chen J, Pan L, Chen W, Li X, Wang H. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1333] [Cited by in RCA: 1543] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 12. | Liu B, Wang Q, Wang Y, Wang J, Zhang L, Zhao M; C-STRIDE study group. Utilization of antihypertensive drugs among chronic kidney disease patients: Results from the Chinese cohort study of chronic kidney disease (C-STRIDE). J Clin Hypertens (Greenwich). 2020;22:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Das SK, Roy DK, Chowdhury AA, Roy AS, Ahammed SU, Asadujjaman M, Rabbani MG, Islam MS, Barman GC, Chanda K, Hossain MB, Salahuddin AZ, Saha S, Das SK, Ara J. Correlation of eGFR By MDRD and CKD-EPI Formula with Creatinine Clearance Estimation in CKD Patients and Healthy Subjects. Mymensingh Med J. 2021;30:35-42. [PubMed] |
| 14. | Vaidya SR, Aeddula NR, Doerr C. Chronic Renal Failure (Nursing). StatPearls. Treasure Island. 2022. Available from: https://www.scirp.org/reference/referencespapers?referenceid=3394327. |
| 15. | Muñoz-Durango N, Fuentes CA, Castillo AE, González-Gómez LM, Vecchiola A, Fardella CE, Kalergis AM. Role of the Renin-Angiotensin-Aldosterone System beyond Blood Pressure Regulation: Molecular and Cellular Mechanisms Involved in End-Organ Damage during Arterial Hypertension. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 168] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 16. | American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44:S151-S167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (1)] |
| 17. | James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5429] [Cited by in RCA: 5504] [Article Influence: 500.4] [Reference Citation Analysis (0)] |
| 18. | Pinsker JE, Shank T, Dassau E, Kerr D. Comment on American Diabetes Association. Approaches to glycemic treatment. Sec. 7. In Standards of Medical Care in Diabetes-2015. Diabetes Care 2015;38(Suppl. 1):S41-S48. Diabetes Care. 2015;38:e174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Pecoits-Filho R, Fliser D, Tu C, Zee J, Bieber B, Wong MMY, Port F, Combe C, Lopes AA, Reichel H, Narita I, Stengel B, Robinson BM, Massy Z; CKDopps Investigators. Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens (Greenwich). 2019;21:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 20. | Alencar de Pinho N, Levin A, Fukagawa M, Hoy WE, Pecoits-Filho R, Reichel H, Robinson B, Kitiyakara C, Wang J, Eckardt KU, Jha V, Oh KH, Sola L, Eder S, de Borst M, Taal M, Feldman HI, Stengel B; International Network of Chronic Kidney Disease cohort studies (iNET-CKD). Considerable international variation exists in blood pressure control and antihypertensive prescription patterns in chronic kidney disease. Kidney Int. 2019;96:983-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 21. | Ku E, McCulloch CE, Vittinghoff E, Lin F, Johansen KL. Use of Antihypertensive Agents and Association With Risk of Adverse Outcomes in Chronic Kidney Disease: Focus on Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers. J Am Heart Assoc. 2018;7:e009992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 22. | Qiao Y, Shin JI, Chen TK, Sang Y, Coresh J, Vassalotti JA, Chang AR, Grams ME. Association of Albuminuria Levels With the Prescription of Renin-Angiotensin System Blockade. Hypertension. 2020;76:1762-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Chu CD, Powe NR, McCulloch CE, Banerjee T, Crews DC, Saran R, Bragg-Gresham J, Morgenstern H, Pavkov ME, Saydah SH, Tuot DS; Centers for Disease Control and Prevention Chronic Kidney Disease Surveillance Team. Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use Among Hypertensive US Adults With Albuminuria. Hypertension. 2021;77:94-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 24. | Mallappallil M, Friedman EA, Delano BG, McFarlane SI, Salifu MO. Chronic kidney disease in the elderly: evaluation and management. Clin Pract (Lond). 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 25. | Savarese G, Dahlström U, Vasko P, Pitt B, Lund LH. Association between renin-angiotensin system inhibitor use and mortality/morbidity in elderly patients with heart failure with reduced ejection fraction: a prospective propensity score-matched cohort study. Eur Heart J. 2018;39:4257-4265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Winkelmayer WC, Fischer MA, Schneeweiss S, Wang PS, Levin R, Avorn J. Underuse of ACE inhibitors and angiotensin II receptor blockers in elderly patients with diabetes. Am J Kidney Dis. 2005;46:1080-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Pappoe LS, Winkelmayer WC. ACE inhibitor and angiotensin II type 1 receptor antagonist therapies in elderly patients with diabetes mellitus: are they underutilized? Drugs Aging. 2010;27:87-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Roux-Marson C, Baranski JB, Fafin C, Exterman G, Vigneau C, Couchoud C, Moranne O; Investigators PSPA. Medication burden and inappropriate prescription risk among elderly with advanced chronic kidney disease. BMC Geriatr. 2020;20:87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Mansfield KE, Nitsch D, Smeeth L, Bhaskaran K, Tomlinson LA. Prescription of renin-angiotensin system blockers and risk of acute kidney injury: a population-based cohort study. BMJ Open. 2016;6:e012690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Weir MR, Lakkis JI, Jaar B, Rocco MV, Choi MJ, Kramer HJ, Ku E. Use of Renin-Angiotensin System Blockade in Advanced CKD: An NKF-KDOQI Controversies Report. Am J Kidney Dis. 2018;72:873-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |