Published online Jun 16, 2024. doi: 10.12998/wjcc.v12.i17.3019
Revised: April 17, 2024
Accepted: April 19, 2024
Published online: June 16, 2024
Processing time: 96 Days and 7.3 Hours
Chronic eczema significantly impacts daily life, social interactions, and quality of life; however, no curative treatment has been identified.
To determine the clinical efficacy of acupoint injection for chronic eczema and its influence on peripheral blood T cells.
Eighty patients with chronic eczema treated at our hospital between June 2022 and March 2023 were randomly assigned to a control group (n = 40), which rec
No difference in overall response rates were found between the observation and control groups (100% vs 90%, respectively; P > 0.05); however, the observation group had a higher marked response rate than the control group (87.5% vs 52.5%; P < 0.05). Both groups had decreased Eczema Area and Severity Index scores and increased pruritus after treatment (P < 0.05), particularly in the observation group (P < 0.05). The observation group had an adverse reaction rate of 2.5% (1/40), which did not differ significantly from that of the control group (P > 0.05). The observation group exhibited higher post-treatment INF-γ and IL-2 but lower IL-4 levels than the control group (P < 0.05); however, no significant inter-group difference was observed in post-treatment IL-10 levels (P > 0.05).
Acupoint injection of triamcinolone acetonide is safe and effective in treating chronic eczema. Its therapeutic mechanism is related to the regulation of peripheral blood T cell levels, inhibition of inflammatory reactions, and mitigation of immune imbalance.
Core Tip: No cure has been found for chronic eczema. In this study, patients with eczema were treated with either routine Western medicine treatment or routine Western medicine treatment plus acupoint injection of triamcinolone acetonide. The clinical efficacy of the two treatment regimens was validated based on therapeutic efficacy, incidence of adverse reactions, and changes in serum cytokine INF-γ and IL-2/4/10 levels before and after treatment. Acupoint injection of triamcinolone acetonide was found safe and effective in treating chronic eczema, and its therapeutic mechanism is related to regulating peripheral blood T cell levels, inhibiting inflammatory reactions, and regulating immune imbalance.
- Citation: Gan HH, Yang G, Shen TT. Efficacy of acupoint injection in the treatment of chronic eczema and its influence on peripheral blood T cells. World J Clin Cases 2024; 12(17): 3019-3026
- URL: https://www.wjgnet.com/2307-8960/full/v12/i17/3019.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i17.3019
Eczema is a type of inflammatory dermatosis triggered by multiple internal and external factors. It is characterized by polymorphic skin lesions, exudation tendency, and intense pruritus[1] and has a high and continuously increasing incidence. Among different eczema types, chronic eczema has the highest incidence. It causes severe, lingering, long-lasting skin itching that is prone to recurrence, seriously affecting patients’ daily living activities, social interactions, and quality of life[2]. No completely curative treatment has yet been found for chronic eczema[3]. Topical glucocorticoid preparations are still the mainstay of eczema treatment, and antihistamines can be appropriately selected according to the patient’s condition to relieve itching and inflammation[4]. Traditional Chinese medicine (TCM) characteristic therapy offers unique properties and advantages that would help treat and prevent eczema[5]. Li et al[6] found that the Gu-Ben-Hua-Shi formula can significantly improve atopic dermatitis symptoms in mice. Its therapeutic mechanism is based on the regulation of the NLRP3 signaling pathway. Zhu et al[7] reported that Wenguanmu ointment can inhibit symptoms of 2,4-dinitrochlorobenzene-induced eczema in a mouse model by inhibiting the CKLF-1/NF-κB pathway. According to Thanik et al[8], TCM variously promotes the recovery of skin lesions, improving the quality of life of patients with mode
This study investigated the clinical efficacy of acupoint injection in the treatment of chronic eczema and elucidated its mechanism of action.
We selected 80 patients with chronic eczema who visited the dermatology clinic of Jinshan Hospital of Fudan University between June 2022 and March 2023. The patients were randomly assigned to the control or observation groups (n = 40 each). The observation group included 25 men and 15 women aged 58.68 ± 14.85 years on average, with a median course of 6.5 months. In the control group, the male-to-female ratio, mean age, and median disease course were 23:17, 62.50 ± 17.11 years, and 6 months, respectively. No significant inter-group differences were observed in general demographic data (P > 0.05), suggesting comparability (Table 1).
| Factors | Observation group (n = 40) | Control group (n = 40) | χ2/t/Z | P value |
| Sex | 0.208 | 0.648 | ||
| Male | 25 (62.5) | 23 (57.5) | ||
| Female | 15 (37.5) | 17 (42.5) | ||
| Age | 58.68 ± 14.85 | 62.50 ± 17.11 | -1.068 | 0.289 |
| Course of disease | 6.50 (5.00, 16.50) | 6.00 (4.00, 12.00) | -0.560 | 0.575 |
The inclusion criteria were as follows: (1) Meeting the clinical diagnostic criteria for chronic eczema; (2) Age > 18 years, regardless of sex or ethnicity; and (3) Voluntary participation with informed consent provided.
Patients were excluded based on the following criteria: (1) Known allergies to triamcinolone acetonide and/or lidocaine hydrochloride injection; (2) Dainting during previous acupuncture treatment; (3) Pregnant or lactating; (4) Topical use of hormonal drugs within the last 15 d; (5) Oral treatment with hormones, immunosuppressants, or retinoids in the last 30 d; (6) Serious heart, liver, kidney, or other visceral disease; malignant tumors; mental illness; or other skin diseases; and (7) Infectious diseases.
The control group were treated with 10 mg ebastine tablets, taken orally once a day (Approval No.: Imported Drug Reg
Primary outcome measures: The skin lesion area and severity index were assessed before and after treatment by referring to the Eczema Area And Severity Index (EASI)[9].
Skin lesion area: The proportion of the skin lesion area to the rest of the body (upper limbs, lower limbs, trunk, head, and neck) was calculated, with 0 points for no rash; 1 point for < 10% rash; 2 points for 10%–19%; 3 points for 20%–49%; 4 for 50%–69%; 5 for 70%–89%; and 6 for 90%–100%.
Severity of skin lesions: Manifestations of skin lesions, such as erythema (E), edema/papulation (I), exudation/crusting (C), exfoliation (Ex), and lichenization (L), were assessed. Each clinical manifestation was assigned a score of 0–3 points (0: none; 1: mild; 2: moderate; and 3: severe) based on severity, with a half score (0.5 point) allowed. The severity of the skin lesion was scored based on the total skin lesion (E + I + C + Ex + L) score.
EASI score = head area score × head severity (E + I + C + Ex + L) score × 0.1 + upper limb area score × upper limb severity (E + I + C + Ex + L) score × 0.2 + trunk area score × trunk severity (E + I + C + Ex + L) score × 0.3 + lower limb area score × lower limb severity (E + I + C + Ex + L) score × 0.4. Therapeutic efficacy was assessed before and after treatment using the Symptom Score Reduction Index (SSRI): SSRI = [(pretreatment symptom score − posttreatment symptom score) / pretreatment symptom score] × 100%.
SSRI > 90% indicated a cure; 60% ≤ SSRI ≤ 90% indicated a marked response; 20% ≤ SSRI ≤ 59% indicated an improvement; and SSRI < 20% indicated a non-response. The overall and marked response rates were calculated as follows: Overall response rate (ORR) = (cure cases + marked response cases + improvement cases) / total cases × 100%; Marked response rate = (cured cases + marked response cases) / total cases × 100%.
Secondary outcome measures: Patients were evaluated for pruritus before and after treatment using the Visual Analogue Scale (VAS) according to the symptom management theory. A 10-cm-long linear ruler was used for the assessment, with one end indicating 0 points (no itching) and the other 10 points (most severe itching. The middle section indicated varying degrees of itching. The patient was asked to mark the point on the ruler that best represented the intensity of itching. The results were calculated using a 10-point scale, with a higher score indicating a greater degree of itching.
Serum inflammatory cytokines: Before and after treatment, venous blood (4 mL) was collected on an empty stomach in the early morning and centrifuged (3000 rpm for 10 min). The upper layer containing liquid serum was collected and frozen at −80°C for further testing. Operated in strict accordance with the specifications, IFN-γ and IL-2/4/10 contents were analyzed using an enzyme-linked immunosorbent assays kits strictly according to the manufacturer’s instructions (Shanghai Keshun Biotechnology Co., Ltd.).
Safety observation and evaluation: Adverse reactions and severity and skin damage severity after treatment were recorded. In addition, local symptoms, such as skin irritation, erythema, desquamation, dryness, pruritus, and skin atrophy, at the acupoint injection site were noted. Laboratory safety tests that were performed before and after treatment included routine tests for blood and urine, liver and renal functions, and blood sugar. Detailed records were kept of the symptoms, occurrence time, duration, correlation with the test drugs (irrelevant, possibly irrelevant, possibly relevant, and relevant), measures taken, and outcomes of adverse reactions. The adverse reaction rate was then calculated as follows: Adverse reaction rate = number of cases of adverse reactions / total cases × 100%.
The data were analyzed using SPSS 25.0, and statistical significance was set at P < 0.05. Normally distributed continuous data were expressed as mean ± SD and analyzed using the independent samples t-test and paired-samples t-test to identify inter- and intra-group differences, respectively. Data with non-normal distribution were expressed as median (quartiles) and compared for inter- and intra-group differences using the Mann–Whitney rank sum test and Wilcoxon rank sum test, respectively. Categorical variables were expressed as number (percentage) and analyzed using the chi-square test or Fisher’s exact probability test.
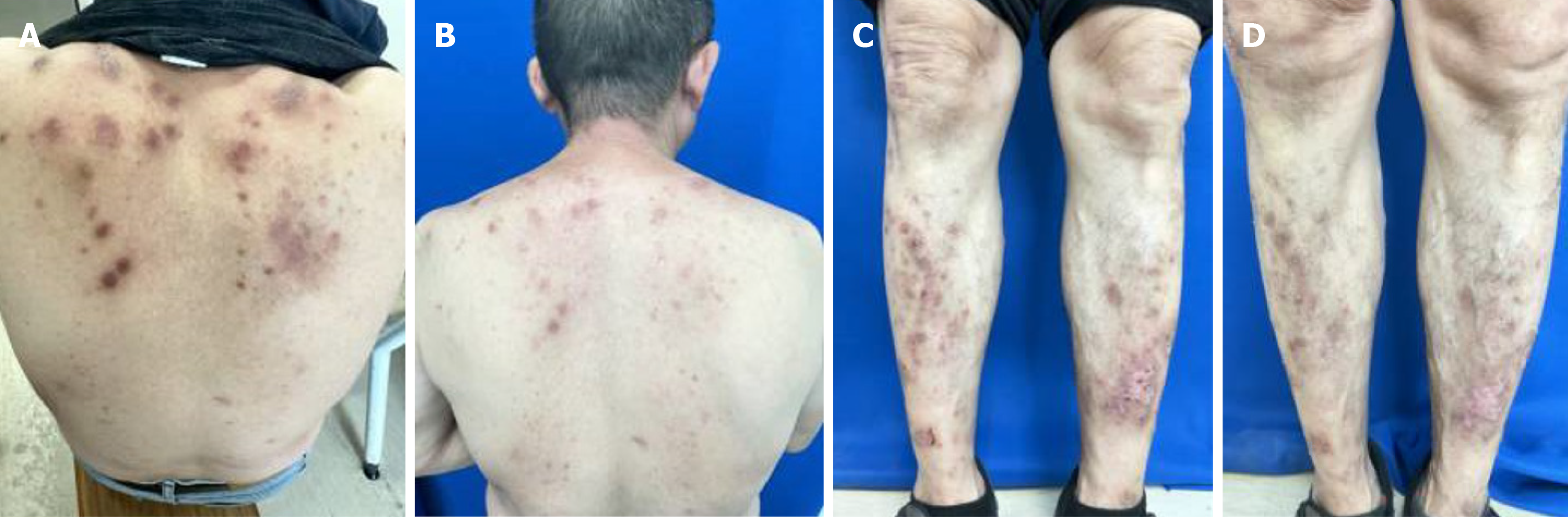
All 80 patients completed the treatment as required, with no withdrawals (see Figure 1 for post-treatment cases). No significant difference in ORR was observed between the observation and control groups (100% vs 90%, respectively; P > 0.05). However, the observation group showed a significantly higher marked response rate than that of the control group (87.5% vs 52.5%, respectively; P < 0.05; Table 2).
| Groups | n | Curative effect | Response rate | Marked response rate | |||
| Non-response | Response | Marked response | Cure | ||||
| Observation group | 40 | 0 (0.0) | 5 (12.5) | 31 (77.5) | 4 (10.0) | 40 (100.0) | 35 (87.5) |
| Control group | 40 | 4 (10.0) | 15 (37.5) | 19 (47.5) | 2 (5.0) | 36 (90.0) | 21 (52.5) |
| χ2 | 11.667 | ||||||
| P value | 0.1161 | 0.001 | |||||
The two groups did not differ significantly in pretreatment EASI and VAS scores (P > 0.05). However, the post-treatment EASI score in the observation group was lower than that in the control group (P < 0.05). In both groups, the EASI scores decreased significantly from the baseline after treatment (P < 0.05). Both groups also showed lower VAS scores after treatment (P < 0.05), particularly in the observation group (P < 0.05; Table 3).
| Groups | n | EASI score | VAS score | ||||||
| Before treatment | After treatment | t value | P value | Before treatment | After treatment | t value | P value | ||
| Observation group | 40 | 7.52 ± 3.25 | 2.22 ± 1.30 | 12.002 | 0 | 6.05 ± 1.54 | 1.98 ± 1.07 | 21.747 | 0 |
| Control group | 40 | 7.30 ± 3.96 | 3.19 ± 2.52 | 9.058 | 0 | 6.08 ± 1.72 | 2.93 ± 1.27 | 12.498 | 0 |
| t value | 0.278 | −2.17 | −0.069 | −3.615 | |||||
| P value | 0.782 | 0.034 | 0.945 | 0.001 | |||||
Forty healthy people who concurrently underwent physical examination at our hospital were selected for comparison. INF-γ and IL-2 Levels were lower, whereas IL-4 and IL-10 Levels were higher, in the observation group than in the control group (P < 0.05; Table 4). The two patient groups did not differ significantly in pretreatment levels of INF-γ, IL-2, IL-4, and IL-10 (P > 0.05). However, the observation group exhibited higher INF-γ and IL-2 and lower IL-4 levels than the control group after treatment (P < 0.05), No significant inter-group difference was found in post-treatment IL-10 levels (P > 0.05). The INF-γ and IL-2 levels in the observation group were significantly higher than the baseline after treatment, whereas IL-4 and IL-10 were significantly lower (P < 0.05). In the control group, no significant change was observed in INF-γ levels before and after treatment (P > 0.05). However, IL-2 levels were higher and IL-4 and IL-10 were lower after treatment (P < 0.05; Table 5).
| Parameters | Case group | Healthy control group | t value | P value |
| INF-γ | 311.75 ± 136.86 | 399.17 ± 129.35 | -2.582 | 0.011 |
| IL-2 | 35.54 ± 19.11 | 57.25 ± 20.51 | -4.479 | 0.000 |
| IL-4 | 79.40 ± 9.62 | 60.72 ± 9.99 | 7.712 | 0.000 |
| IL-10 | 66.00 ± 8.78 | 56.16 ± 6.90 | 4.659 | 0.000 |
| Parameters | Time point | Observation group | Control group | t value | P value |
| INF-γ | Before treatment | 321.29 ± 135.22 | 302.21 ± 139.53 | 0.621 | 0.536 |
| After treatment | 394.88 ± 115.70a | 334.38 ± 137.47 | 2.13 | 0.036 | |
| IL-2 | Before treatment | 38.15 ± 19.98 | 32.93 ± 18.07 | 1.224 | 0.225 |
| After treatment | 56.51 ± 18.00a | 47.13 ± 20.04a | 2.203 | 0.031 | |
| IL-4 | Before treatment | 81.01 ± 8.78 | 77.79 ± 10.25 | 1.508 | 0.136 |
| After treatment | 61.31 ± 7.60a | 67.20 ± 9.00a | −3.165 | 0.002 | |
| IL-10 | Before treatment | 65.94 ± 8.85 | 66.06 ± 8.83 | −0.063 | 0.95 |
| After treatment | 60.55 ± 11.86a | 62.00 ± 8.84a | −0.623 | 0.535 |
No serious adverse reactions occurred in either group. No symptoms of local skin irritation, hirsutism, atrophy, or telangiectasia were observed after the topical use of hormones. No abnormalities were noted in routine tests for blood and urine, liver and kidney functions, and blood sugar before or after treatment. In the observation group, soreness and discomfort at the local injection site were relieved within 24 h after acupoint injection, and no weakness of limbs was noted. Furthermore, no skin atrophy or depigmentation of concave pigment was observed at the acupoint injection site. The adverse reaction rate in the observation group was 2.5%, attributed to one patient who developed trunk folliculitis on the third week of treatment, which resolved after topical medication. The adverse reaction rate in the control group was 0. Three patients in the observation group experienced recurrence 1 month after the end of treatment, which was a recurrence rate of 7.5%. Comparatively, 7 cases in the control group recurred, with a recurrence rate of 17.5%; however, the EASI and VAS scores were lower than the baseline. The two groups showed no marked differences in adverse reaction and recurrence rates (P > 0.05; Table 6).
| Groups | Adverse reactions | Recurrence |
| Observation group | 1 (2.5) | 3 (7.5) |
| Control group | 0 (0.0) | 7 (17.5) |
| χ2 | 1.829 | |
| P value | 1.000 | 0.176 |
Eczema is a common and frequently occurring disease in dermatology, with an incidence of 7.5% in China and 10.7% in the United States[10]. Eczema is caused by immune dysfunction, which is often associated with delayed allergic reactions[11,12]. The expression of inflammatory cytokines in the skin of patients with eczema is significantly increased compared with that in healthy individuals[13]. Research indicates that the pathogenesis of eczema may be related to imbalanced T cell subsets (Th1/Th2)[14]. Th1/Th2 cell subpopulations can regulate each other through the different cytokines they secrete[15]. Th1 cells primarily secrete IFN-α, IL-2, IFN-γ, and TNF-β, which are called Th1-type cytokines[16]. IFN-γ, as the representative Th1-type cytokine, has pro-inflammatory effects, and its main activity centers in immune regulation[17]. It is an important immunomodulatory factor in the body that promotes Th1 cell maturation and inhibits Th2 cell proliferation. It not only suppresses Th2 cell production of IL-4 but also inhibits B cell secretion of IgE, thereby inhibiting hypersensitivity reactions caused by high IgE levels[18]. Th2 cells primarily secrete IL-4, IL-5, IL-10, and IL-13, among others, which are called Th2-type cytokines[19]. IL-4, as the representative Th2-type, exerts anti-inflammatory effects[20]. IL-4 can inhibit Th1 cells and their functions, induce B cells to produce IgE, promote B cell proliferation, induce low-affinity receptor for IgE expression, and play a vital role in modulating IgE production, thereby participating in the development of allergic diseases[21,22]. Abnormal Th2-type cytokine secretions are strongly related to the occurrence, development, and prognosis of eczema[23].
Chronic eczema falls into the category of “wet sores” in TCM, which is mainly caused by wind-dampness-heat soaking the skin due to insufficiency of natural endowment; improper diet-induced movement; transformation disorders of the spleen and stomach; endogenous dampness and heat; exogenous wind-heat; and interactions between internal and external pathogens[15]. Therefore, treatment should involve cooling blood, dispelling wind, and relieving itching. Quchi is the confluence of the large intestine channel of hand-Yangming, which is good for clearing heat and relieving exterior syndrome; dispelling wind and eliminating rash; and regulating meridians, Qi, and blood combined with the characteristics of Yangming meridian[24]. Xuehai is the acupoint of the spleen meridian of foot-Taiyin, which can regulate spleen meridian Qi, modulate spleen transport function, promote blood circulation, and nourish blood to dispel wind[25]. The combination of the two acupoints based on syndrome differentiation functions in dispelling wind-evil, relieving itching, clearing heat, and removing dampness[26]. Acupoint injection is a modified TCM treatment that combines acupuncture stimulation with the properties of Western drugs and their penetrating effects on acupoints, exerting a comprehensive therapeutic effect[27]. Triamcinolone acetonide is a long-acting glucocorticoid with potent and lasting anti-inflammatory and anti-allergic effects[28]. Local injection can inhibit the transformation of B cells into plasma cells and interfere with humoral immunity[29] Furthermore, it stabilizes the lysosomal membrane, inhibits leukocytes and macrophages from migrating outside the blood vessels, and reduces inflammation[30]. It can also increase mast cell stability and reduce histamine release[31]. In the current study, the post-treatment EASI and VAS (pruritus assessment) scores of both groups decreased from baseline, with even lower scores in the observation group. Moreover, a significantly higher marked response rate was noted in the observation group. Hence, the combination of Western medicine and acupoint injection is effective in alleviating clinical symptoms and reducing severity of chronic eczema, with remarkable clinical effects. In terms of safety, no significant inter-group difference was found in the total adverse reaction rate, demonstrating the favorable safety profile of the combination therapy. Furthermore, the higher post-treatment serum INF-γ and IL-2 levels and lower IL-4 levels in the observation group indicates that acupoint injection of triamcinolone acetonide can promote IFN-γ and IL-2 (Th1-type cytokine) secretion, and inhibit the IL-4 (Th2-type cytokine) secretion, thereby correcting the Th1/Th2 imbalance in patients with chronic eczema at the cytokine level.
Acupoint injection of triamcinolone acetonide can effectively treat chronic eczema by regulating immune balance to improve the T lymphocyte level and inhibit inflammatory reactions. In addition, the treatment is effective in alleviating clinical symptoms with obvious curative effects and confers convenience and safety, which indicates is potential clinical application.
| 1. | Plötz SG, Wiesender M, Todorova A, Ring J. What is new in atopic dermatitis/eczema? Expert Opin Emerg Drugs. 2014;19:441-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Liu Y, Dong Y, Sun DH, Zhang XL. Effect of Shenling Baizhu Powder Combined with Polysulfonic Acid Mucopolysaccharide Encapsulation on Inflammatory Cytokines and Peripheral Blood T Lymphocyte Subsets in Elderly Patients with Chronic Eczema and Blood Deficiency. Zhonghua Zhongyiyao Xuekan. 2020;38:166-169. [DOI] [Full Text] |
| 3. | Teasdale E, Muller I, Sivyer K, Ghio D, Greenwell K, Wilczynska S, Roberts A, Ridd MJ, Francis N, Yardley L, Thomas KS, Santer M. Views and experiences of managing eczema: systematic review and thematic synthesis of qualitative studies. Br J Dermatol. 2021;184:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Lax SJ, Harvey J, Axon E, Howells L, Santer M, Ridd MJ, Lawton S, Langan S, Roberts A, Ahmed A, Muller I, Ming LC, Panda S, Chernyshov P, Carter B, Williams HC, Thomas KS, Chalmers JR. Strategies for using topical corticosteroids in children and adults with eczema. Cochrane Database Syst Rev. 2022;3:CD013356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Wang MC, Chou YT, Kao MC, Lin QY, Chang SY, Chen HY. Topical Chinese herbal medicine in treating atopic dermatitis (eczema): A systematic review and meta-analysis with core herbs exploration. J Ethnopharmacol. 2023;317:116790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 6. | Li X, Feng L, Zhong T, Mo X, Wang D, Gu J, Chen D, Zeng X, Yan F. Gu-Ben-Hua-Shi (AESS) formula ameliorates atopic dermatitis via regulating NLRP3 signaling pathways. Saudi Pharm J. 2023;31:101792. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Zhu L, Li XJ, Gu C, Gao Y, Zhang CS, Wang LY, Chen NH, Li G. Mongolian medicine Wenguanmu ointment treats eczema by inhibiting the CKLF-1/NF-κB pathway. J Ethnopharmacol. 2023;313:116549. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 8. | Thanik E, Wisniewski JA, Nowak-Wegrzyn A, Sampson H, Li XM. Effect of traditional Chinese medicine on skin lesions and quality of life in patients with moderate to severe eczema. Ann Allergy Asthma Immunol. 2018;121:135-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Zhao B. Eczema area and severity index scoring method. Zhonghua Pifuke Zazhi. 2004;37:3-4. |
| 10. | Zhang JZ. Guidelines for the diagnosis and treatment of eczema (2011). Zhonghua Pifuke Zazhi. 2011;44:5-6. |
| 11. | Vallion R, Kerdine-Römer S. Regulation of the immune response to contact sensitizers by Nrf2. Contact Dermatitis. 2022;87:13-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Stalder JF, Tennstedt D, Deleuran M, Fabbrocini G, de Lucas R, Haftek M, Taieb C, Coustou D, Mandeau A, Fabre B, Hernandez-Pigeon H, Aries MF, Galliano MF, Duplan H, Castex-Rizzi N, Bessou-Touya S, Mengeaud V, Rouvrais C, Schmitt AM, Bottino R, Cottin K, Saint Aroman M. Fragility of epidermis and its consequence in dermatology. J Eur Acad Dermatol Venereol. 2014;28 Suppl 4:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Kim JE, Kim JS, Cho DH, Park HJ. Molecular Mechanisms of Cutaneous Inflammatory Disorder: Atopic Dermatitis. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Eyerich K, Novak N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy. 2013;68:974-982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Dong C. Cytokine Regulation and Function in T Cells. Annu Rev Immunol. 2021;39:51-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 349] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 16. | Zhou C, Li C, Gong GZ, Wang S, Zhang JM, Xu DZ, Guo LM, Ren H, Xu M, Xie Q, Pan C, Xu J, Hu Z, Geng S, Zhou X, Wang X, Mi H, Zhao G, Yu W, Wen YM, Huang L, Wang XY, Wang B. Analysis of immunological mechanisms exerted by HBsAg-HBIG therapeutic vaccine combined with Adefovir in chronic hepatitis B patients. Hum Vaccin Immunother. 2017;13:1989-1996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Kak G, Raza M, Tiwari BK. Interferon-gamma (IFN-γ): Exploring its implications in infectious diseases. Biomol Concepts. 2018;9:64-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 398] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 18. | Annunziato F, Romagnani C, Romagnani S. The 3 major types of innate and adaptive cell-mediated effector immunity. J Allergy Clin Immunol. 2015;135:626-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 559] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 19. | Waśkiel-Burnat A, Osińska M, Salińska A, Blicharz L, Goldust M, Olszewska M, Rudnicka L. The Role of Serum Th1, Th2, and Th17 Cytokines in Patients with Alopecia Areata: Clinical Implications. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 20. | Wojdasiewicz P, Poniatowski ŁA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 1135] [Article Influence: 103.2] [Reference Citation Analysis (0)] |
| 21. | Zhu CC, Pan HJ, Zhu QG. Mechanism of Th2 related inflammatory factors in the pathogenesis of eczema. Yaoxue Shijian Zazhi. 2019;37:9-13. |
| 22. | Diao QC, Liu Y. Expert Consensus on Chinese Medicine Diagnosis and Treatment of Eczema (Eczema). Zhongguo Zhongxiyijiehe Pifuxingbingxue Zazhi. 2021;20:517-521. |
| 23. | Ma CS. T-helper-2 cells and atopic disease: lessons learnt from inborn errors of immunity. Curr Opin Immunol. 2023;81:102298. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 24. | Qiao R, Kasimu A, Chen D, Gao C, Li B. Abdominal Massage to Improve Motor Dysfunction in Rats with Cerebral Palsy. J Vis Exp. 2023;. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Zhang R, Ai BW. [Professor AI Bing-wei's treatment experience of acupuncture for anovulatory infertility]. Zhongguo Zhen Jiu. 2019;39:293-295. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Lin HL, Lin LC. Discussion on the law of acupuncture point selection for chronic eczema based on data mining technology. Shanghai Zhengjiu Zazhi. 2022;41:102-106. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Wang H, Yuan DW, Tang HF, Lv BT. Clinical study on Zusanli injection combined with abdominal acupoint application in the treatment of gastrointestinal dysfunction after laparoscopic cholecystectomy. Xiandai Xiaohua Ji Jieruzhenliao. 2023;28:472-475. [DOI] [Full Text] |
| 28. | Karaulov AV, Ilina NI, Shartanova N, Maslakov A, Lucio L. Triamcinolone Acetonide in the Treatment of Perennial Allergic Rhinitis: A post hoc Efficacy Analysis of a Phase III Study Performed in Russia. Int Arch Allergy Immunol. 2022;183:168-175. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Koletsa T, Patsatsi A, Kostopoulos I, Kartsios C, Korantzis I, Sotiriadis D. A case of a primary cutaneous plasmacytoma presenting in adolescence. Am J Dermatopathol. 2012;34:537-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Xie Y, Ju X, Beaudin S, Wiltshire L, Oliveria JP, MacLean J, Sommer DD, Cusack R, Li O, Banerjee P, Keith PK, O'Byrne PM, Bauer RN, Staton T, Gauvreau GM, Sehmi R. Effect of intranasal corticosteroid treatment on allergen-induced changes in group 2 innate lymphoid cells in allergic rhinitis with mild asthma. Allergy. 2021;76:2797-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Giordano F, Corvino A, Scognamiglio A, Citi V, Gorica E, Fattorusso C, Persico M, Caliendo G, Fiorino F, Magli E, Perissutti E, Santagada V, Severino B, Pavese RC, Petti F, Martelli A, Calderone V, Frecentese F. Hybrids between H(2)S-donors and betamethasone 17-valerate or triamcinolone acetonide inhibit mast cell degranulation and promote hyperpolarization of bronchial smooth muscle cells. Eur J Med Chem. 2021;221:113517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |