Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2837
Revised: March 7, 2024
Accepted: April 10, 2024
Published online: June 6, 2024
Processing time: 167 Days and 21.6 Hours
Adult-onset Still’s disease (AOSD) is a rare systemic inflammatory disorder characterized by fever, arthritis, skin rash, and systemic symptoms. The etiology of AOSD is unknown; however, it is thought to be related to immune dysregulation. Although a rare disease, AOSD can significantly impact reproductive health, particularly during pregnancy. This case study assesses the implications of pregnancy in a patient with AOSD, as well as the potential for heredity of the disease. Neonatal hemophagocytic lympho-histiocytosis (HLH) is a rare and life-threatening disorder characterized by hyperinflammation and uncontrolled activation of immune cells, leading to multiple organ dysfunction. This case report aimed to introduce neonatal HLH from a mother with AOSD.
This case study presents a 29-year-old female with AOSD who became pregnant and gave birth to a premature infant who was diagnosed with neonatal HLH. AOSD can significantly impact pregnancy and childbirth, as it may become more severe during pregnancy, with an increased risk of fetal loss and preterm birth. The management of AOSD during pregnancy involves the use of nonsteroidal anti-inflammatory drugs and glucocorticoids, as well as immunosuppressive agents in severe cases. However, the use of immunosuppressive agents during pregnancy may be associated with potential risks to the fetus. The hereditary implications of AOSD are unclear; however, available evidence suggests that genetic factors may play a role in the disease development.
AOSD can have significant implications for pregnancy and childbirth, including an increased risk of fetal loss and preterm birth. Neonatal HLH, a complication of AOSD in pregnancy, requires prompt diagnosis and management. Women with AOSD who are considering pregnancy should discuss their options with their healthcare provider and develop a management plan that addresses the potential risks to both mother and fetus.
Core Tip: This case study explores the intricate challenges faced by a 29-year-old woman with adult-onset Still’s disease (AOSD) during pregnancy, resulting in the premature birth of an infant diagnosed with neonatal hemophagocytic lympho-histiocytosis. Pregnancy exacerbates the risks associated with AOSD, including heightened chances of fetal loss and preterm birth. The management of AOSD during pregnancy involves utilizing nonsteroidal anti-inflammatory drugs, glucocorticoids, and, in severe cases, immunosuppressive agents despite potential fetal risks such as low birth weight and preterm birth, highlighting the delicate balance required in addressing the complexities of AOSD during gestation.
- Citation: Kang JH. Managing adult-onset Still's disease in pregnancy: A case report. World J Clin Cases 2024; 12(16): 2837-2841
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2837.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2837
Adult-onset Still’s disease (AOSD) is a rare inflammatory condition with uncertain origins, likely stemming from immune dysregulation[1], posing significant considerations during pregnancy. Given its potential impact on reproductive health, understanding the implications of pregnancy in patients with AOSD and the potential hereditary aspects of the disease are crucial. Additionally, neonatal hemophagocytic lympho-histiocytosis (HLH), a severe disorder characterized by immune hyperactivation, presents a rare but life-threatening complication in newborns born to mothers with AOSD, warranting further investigation and clinical attention.
Neonatal HLH is a rare and life-threatening disorder characterized by hyperinflammation and uncontrolled activation of immune cells, leading to multiple organ dysfunction. This case report aimed to introduce neonatal HLH from a mother with AOSD.
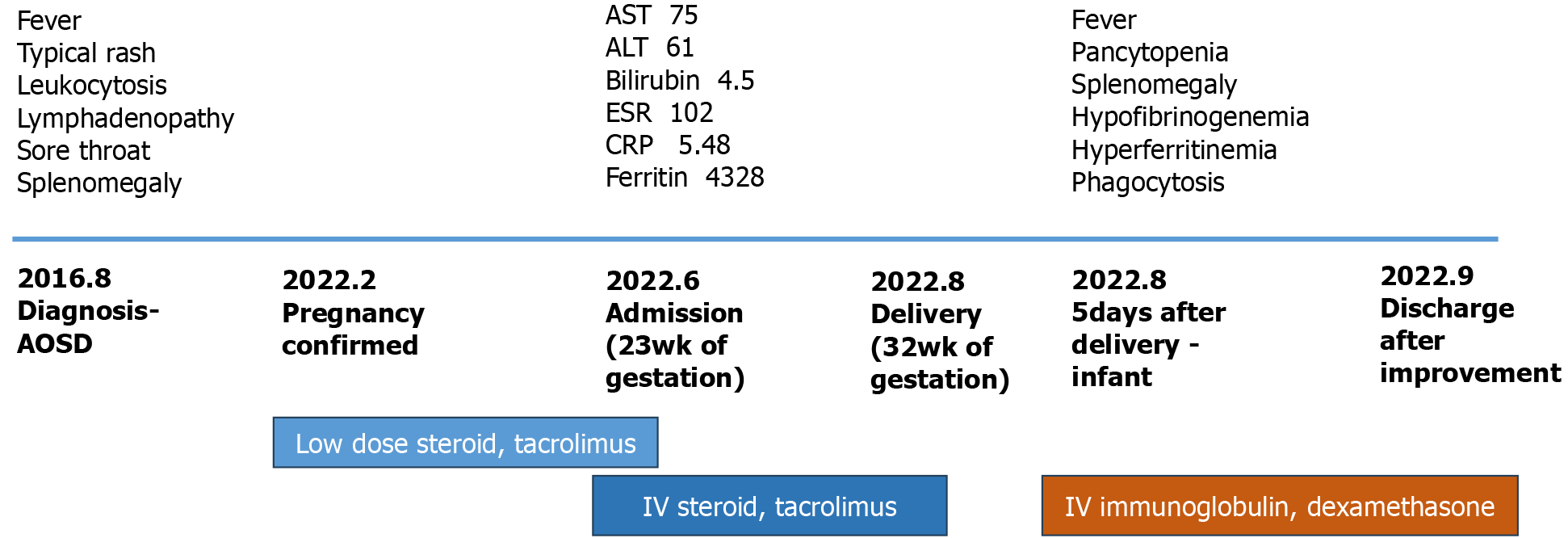
In the first trimester of pregnancy, she was maintained in a stable state with a low-dose steroid and tacrolimus. From the second trimester, she was hospitalized due to sudden elevation of liver function test markers, including aspartate aminotransferase, alanine transaminase, and bilirubin, fever, and rash. Due to frequent recurrent flares, she was admitted repeatedly and was treated with intravenous steroid injections. At 32 wk of gestation, she suddenly went into labor and gave birth through emergent delivery by cesarean section. The male infant was born prematurely and was cared for in the neonatal intensive care unit. At 5 d of age, the male infant developed fever, pancytopenia, hepatosplenomegaly, and hypofibrinogenemia (Figure 1).
The male infant was born prematurely and was cared for in the neonatal intensive care unit. At 5 d of age, he developed fever, pancytopenia, hepatosplenomegaly, and hypofibrinogenemia.
A 29-year-old female reported to the emergency room with complaints of a prolonged high fever for more than 1 month, a non-pruritic, salmon pink-colored rash in the trunk, sore throat, multiple lymphadenopathies including both axillary and left para-aortic areas, and splenomegaly. Her white blood cell count was 20100/μL, including 92.5% of neutrophils. Her ferritin level was 14602 ng/mL, and her C-reactive protein level was 15.66 mg/dL. A biopsy finding of the left axillary lymph node presented reactive lymphadenopathy, and the blood culture results were negative. Her laboratory test results for rheumatoid factor, anti-neutrophil cytoplasmic antibody, and anti-nuclear antibody, were negative. According to the criteria by Yamaguchi et al[2], this patient was diagnosed with AOSD (Figure 1). The patient was prescribed intravenous methylprednisolone 1 mg/kg for 3 wk. Moreover, her symptoms improved, and her dosage of steroids was tapered while undergoing additional treatment with oral methotrexate. She was maintained in the outpatient department for more than 3 years without any flares. Then, she was suddenly hospitalized with fever, generalized skin rash, myalgia, and arthralgia. She was treated with 2-wk high-dose intravenous methylprednisolone therapy, with no effect. Her symptoms, such as fever, rash, and arthralgia, were not controlled, and cyclosporin (100 mg once daily) was added to her therapy during hospitalization. Despite receiving high doses of intravenous methylprednisolone, methotrexate, and cyclosporin for 6 months post-diagnosis, her symptoms, including fever, skin rash, and arthritis, persisted intermittently and remained uncontrolled. Consequently, the patient underwent monthly intravenous administration of tocilizumab, an interleukin (IL)-6 inhibitor. Following tocilizumab therapy, her symptoms improved, and this positive response was sustained. After 1 year, the tocilizumab therapy was discontinued, and she became pregnant after marriage.
The patient has no specific family or personal history.
The newborn presented with symptoms of high fever and distress, accompanied by labored breathing, prompting immediate medical attention.
The hemoglobin level of the male infant was 7.9 g/dL, and the platelet count was 34 × 109/L. His ferritin level was 12825 ng/mL, and he had hypofibrinogenemia.
The abdominal ultrasound of the male infant showed hepatosplenomegaly.
According to the modified 2009 diagnostic criteria of familial HLH[3], the male infant satisfied seven parameters, including fever, splenomegaly, anemia and thrombocytopenia, hypofibrinogenemia, hyperferritinemia, elevated soluble IL-2 receptor, and evidence of phagocytosis on bone marrow biopsy (Figure 1).
The infant was treated with dexamethasone, immunosuppressive therapy, and intravenous immunoglobulin (IVIG).
After 3 wk of intensive treatment, the baby improved and was discharged, while follow-up at the outpatient clinic continued. Moreover, his symptoms were well-controlled 6 months after therapy discontinuation.
AOSD can significantly affect pregnancy and childbirth, as the disease may become more severe during pregnancy due to hormonal changes and immune dysregulation. Similarly, patients with AOSD have an increased risk of fetal loss and preterm birth. In a study of 40 pregnant patients with AOSD, 10 (25%) had fetal loss, and 14 (35%) had preterm delivery. Additionally, patients with AOSD may have an increased risk of preeclampsia and gestational diabetes[3]. Therefore, close monitoring and management of AOSD during pregnancy is essential to ensure the best possible outcome for mother and child.
Management of AOSD during pregnancy may involve the use of nonsteroidal anti-inflammatory drugs and glucocorticoids, as well as disease-modifying antirheumatic drugs (DMARDs) in severe cases. However, the use of DMARDs during pregnancy may be associated with potential risks to the fetus, including fetal malformations and growth retardation[4]. Therefore, the use of DMARDs should be carefully considered and balanced against the potential benefits of controlling disease activities.
The hereditary implications of AOSD are not well understood; however, evidence suggests that genetic factors may play a role in disease development. In a study of 11 patients with AOSD, 5 had a family history of rheumatologic diseases, suggesting a potential genetic predisposition to the disease. Additionally, several genetic variants have been associated with AOSD, including human leukocyte antigen-DRB1 and IL1 polymorphism[5].
Neonatal HLH is a rare and life-threatening disorder characterized by hyperinflammation and uncontrolled activation of immune cells, leading to multiple organ dysfunction. Early diagnosis and prompt treatment are essential for improving outcomes in affected infants. Genetic testing should be performed to identify underlying mutations and guide appropriate management strategies. The management of neonatal HLH typically involves a combination of che
The risk of heredity in AOSD is not well-defined; however, the disease is likely multifactorial, with genetic and environmental factors contributing to its development. Therefore, the risk of heredity may be influenced by the genetic background of the patient, as well as environmental factors such as infections and stress.
This case serves as evidence that the intricate interplay of genetic predisposition, the unique circumstances of pregnancy in a mother with AOSD, and various environmental factors can collectively contribute to the development of neonatal HLH[7]. The underlying genetic background of AOSD in the mother, compounded by pregnancy-induced physiological changes, may influence the fetal environment, potentially predisposing the newborn to HLH. Moreover, the management of AOSD during pregnancy, including the use of medications like glucocorticoids and immunosuppressive agents, could further influence the fetal immune response. Additionally, environmental factors such as infections and stressors during pregnancy may exacerbate the risk of HLH development in the newborn. Overall, this case underscores the complex nature of disease pathogenesis, highlighting the need for a comprehensive understanding and management of genetic and environmental factors in similar clinical scenarios.
Pregnancy in patients with AOSD can be associated with significant risks, including fetal loss, preterm birth, and maternal complications. Therefore, close monitoring and management of disease activity during pregnancy are essential to ensure the best possible outcome for both mother and child. Additionally, the hereditary implications of AOSD are not well understood; however, evidence suggests genetic factors may play a role in disease development. Further research is necessary to understand the underlying mechanisms of AOSD and develop appropriate management strategies for pregnant patients with AOSD.
Learning points: (1) AOSD can have significant implications for pregnancy and childbirth, including an increased risk of fetal loss and preterm birth; (2) Neonatal HLH can be a complication of AOSD in pregnancy and requires prompt diagnosis and management; and (3) Women with AOSD who are considering pregnancy should discuss their options with their healthcare provider and develop a management plan that takes into account the potential risks to both the mother and the fetus.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s classification
Scientific Quality: Grade A, Grade D
Novelty: Grade B, Grade C
Creativity or Innovation: Grade A, Grade C
Scientific Significance: Grade A, Grade C
P-Reviewer: Hrgovic Z, Germany; Wu ZQ, China S-Editor: Che XX L-Editor: A P-Editor: Zhao S
| 1. | Feist E, Mitrovic S, Fautrel B. Mechanisms, biomarkers and targets for adult-onset Still's disease. Nat Rev Rheumatol. 2018;14:603-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 2. | Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, Kashiwazaki S, Tanimoto K, Matsumoto Y, Ota T. Preliminary criteria for classification of adult Still's disease. J Rheumatol. 1992;19:424-430. [PubMed] |
| 3. | Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am Soc Hematol Educ Program. 2009;127-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 241] [Article Influence: 15.1] [Reference Citation Analysis (1)] |
| 4. | Wang Z, Chi H, Feng T, Du Q, Zeng T, Teng J, Liu H, Cheng X, Ye J, Shi H, Sun Y, Hu Q, Jia J, Liu T, Wan L, Wu X, Zhou Z, Yang C, Su Y. Pregnancy Outcomes in Patients With Adult-Onset Still's Disease: A Cohort Study From China. Front Med (Lausanne). 2020;7:566738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Peterson EA, Lynton J, Bernard A, Santillan MK, Bettendorf B. Rheumatologic Medication Use During Pregnancy. Obstet Gynecol. 2020;135:1161-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Youm JY, Woo JH, Kim TH, Bae SC, Yoo DH. Interleukin-1beta and interleukin-1 receptor antagonist gene polymorphisms in Korean patients with adult-onset Still's disease. Scand J Rheumatol. 2007;36:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | McLean J, Katebian R, Suh E, Mirza K, Amin S. Neonatal Hemophagocytic Lymphohistiocytosis. Neoreviews. 2019;20:e316-e325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |