Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2722
Revised: February 21, 2024
Accepted: April 18, 2024
Published online: June 6, 2024
Processing time: 159 Days and 1.2 Hours
Giant cell tumor of bone is a locally aggressive and rarely metastasizing tumor, and also a potential malignant tumor that may develop into a primary malignant giant cell tumor.
To evaluate the role of multimodal imaging in the diagnosis of giant cell tumors of bone.
The data of 32 patients with giant cell tumor of bone confirmed by core-needle biopsy or surgical pathology at our hospital between March 2018 and March 2023 were retrospectively selected. All the patients with giant cell tumors of the bone were examined by X-ray, computed tomography (CT) and magnetic resonance imaging (MRI), and 7 of them were examined by positron emission tomography (PET)-CT.
X-ray imaging can provide overall information on giant cell tumor lesions. CT and MRI can reveal the characteristics of the internal structure of the tumor as well as the adjacent relationships of the tumor, and these methods have unique advantages for diagnosing tumors and determining the scope of surgery. PET-CT can detect small lesions and is highly valuable for identifying benign and malignant tumors to aid in the early diagnosis of metastasis.
Multimodal imaging plays an important role in the diagnosis of giant cell tumor of bone and can provide a reference for the treatment of giant cell tumors.
Core Tip: Multimodal imaging plays an important role in the diagnosis of giant cell tumors. X-ray, computed tomography, magnetic resonance imaging, and positron emission tomography-computed tomography imaging have their own advantages and can provide a reference for the treatment of giant cell tumors.
- Citation: Kou MQ, Xu BQ, Liu HT. Multimodal imaging in the diagnosis of bone giant cell tumors: A retrospective study. World J Clin Cases 2024; 12(16): 2722-2728
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2722.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2722
Giant cell tumor of bone (GCTB), also known as an osteoclast tumor, is a locally aggressive and rarely metastasizing tumor that accounts for approximately 5% of primary bone tumors[1]. Research about the incidence and demographics of GCTB showed the incidence was 1.7 per million inhabitants per year with a male to female ratio of 1:1.38 and a median age of 35 years[2]. GCTB is a potential malignant tumor that may develop into a primary malignant giant cell tumor (PMGCT)[3]. GCTB generally occurs at the metaphyseal end of the long bone. Multiple cases are usually solitary and rare. The most common sites for GCTB are the proximal tibia, distal femur, distal radius, and proximal humerus, with approximately 60% of lesions occurring around the knee joint as well as around the vertebral column and pelvis. This study retrospectively analyzed 32 patients with giant cell tumor confirmed by core-needle biopsy or surgical pathology at our hospital and compared the results of X-ray, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)-CT examinations with the objective of improving the diagnosis of GCTB and guiding clinical treatment.
The data of 32 patients with giant cell tumor of bone confirmed by core-needle biopsy or surgical pathology at Shaanxi Provincial People’s Hospital between March 2018 and March 2023 were retrospectively selected. There were 11 males and 21 females aged 38.5 ± 7.6 years. All the patients with giant cell tumors of the bone were examined by X-ray, CT and MRI, and 7 of them were examined by PET-CT. The distribution of cases by location were as follows: 13 cases, upper tibia; 9 cases, lower femur; 3 cases, neck of the femur; 1 case, proximal humerus; 1 case, distal ulna; 2 cases, pelvis; and 3 cases, vertebral column. The study protocol was approved by the Medical Ethical Committee of Shaanxi Provincial People’s Hospital.
The examination methods used included X-ray, CT, MRI, and PET-CT. Digital X-ray photographs of the anteroposterior and lateral sides of the lesion and adjacent joints were taken with Digital Radiography (Siemens Fluorospot Compact FD, Germany). CT scan was performed using a Toshiba Medical Systems 2nd-generation 640 slice volumetric CT scanner (Aquilion ONE) with a scanning range of lesions and adjacent joints and a scanning layer thickness and spacing of 5 mm. The MRI devices used were a Philips 1.5T dual gradient magnetic resonance imaging system and a Toshiba 1.5T magnetic resonance imaging system, with a specified scanning layer thickness and an interlayer spacing of 5 mm. The main SE sequences used for cross-sectional, coronal, and sagittal scans were T1WI (TR500 ms, TE 20 ms) and T2WI (TR3000 ms, E90 ms). 18F-Fluorodeoxyglucose (FDG) PET-CT was performed with an Ingenuity TF PET-CT scanner produced by Philips in the Netherlands for diagnosis. Prior to the examination, patients were instructed to fast for 8 h, and their blood sugar levels were controlled below 11.0 mmol/L to improve the quality of the examination. Then, the patient was given an intravenous injection of 18F-FDG at a dose of 0.10-0.15 MBq/kg, after which the patient was allowed to rest for 60 min. PET-CT was subsequently performed, with the layer thickness controlled at approximately 2.0 mm. After the PET-CT scanning was completed, the fused images were obtained.
The examination results were jointly diagnosed by two radiologists with more than 10 years of work experience, who observed and recorded the number, location, and size of lesions observed using various imaging methods as well as the imaging features of skeletal lesions (bone destruction, cortical bone, boundaries, periosteal reactions, and whether soft tissue tumors were merged). PET-CT was used to delineate the region of interest in the area of increased uptake, and volume segmentation was performed on the lesion to obtain the metabolic parameters and maximum standardized uptake value (SUVmax) of the primary tumor lesion.
Descriptive analysis of the data was conducted by using GraphPad Prism 10 statistical software. The consistency of the evaluation results of GCTB by two observers was tested using the kappa test: a kappa value ≤ 0.20 indicated no consistency, 0.20 < a kappa value ≤ 0.40 indicated poor consistency, 0.40<a kappa value ≤ 0.6 indicated moderate consistency, 0.60 < a kappa value ≤ 0.80 indicated good consistency, and 0.80 < a kappa value ≤ 1.00 indicated excellent consistency.
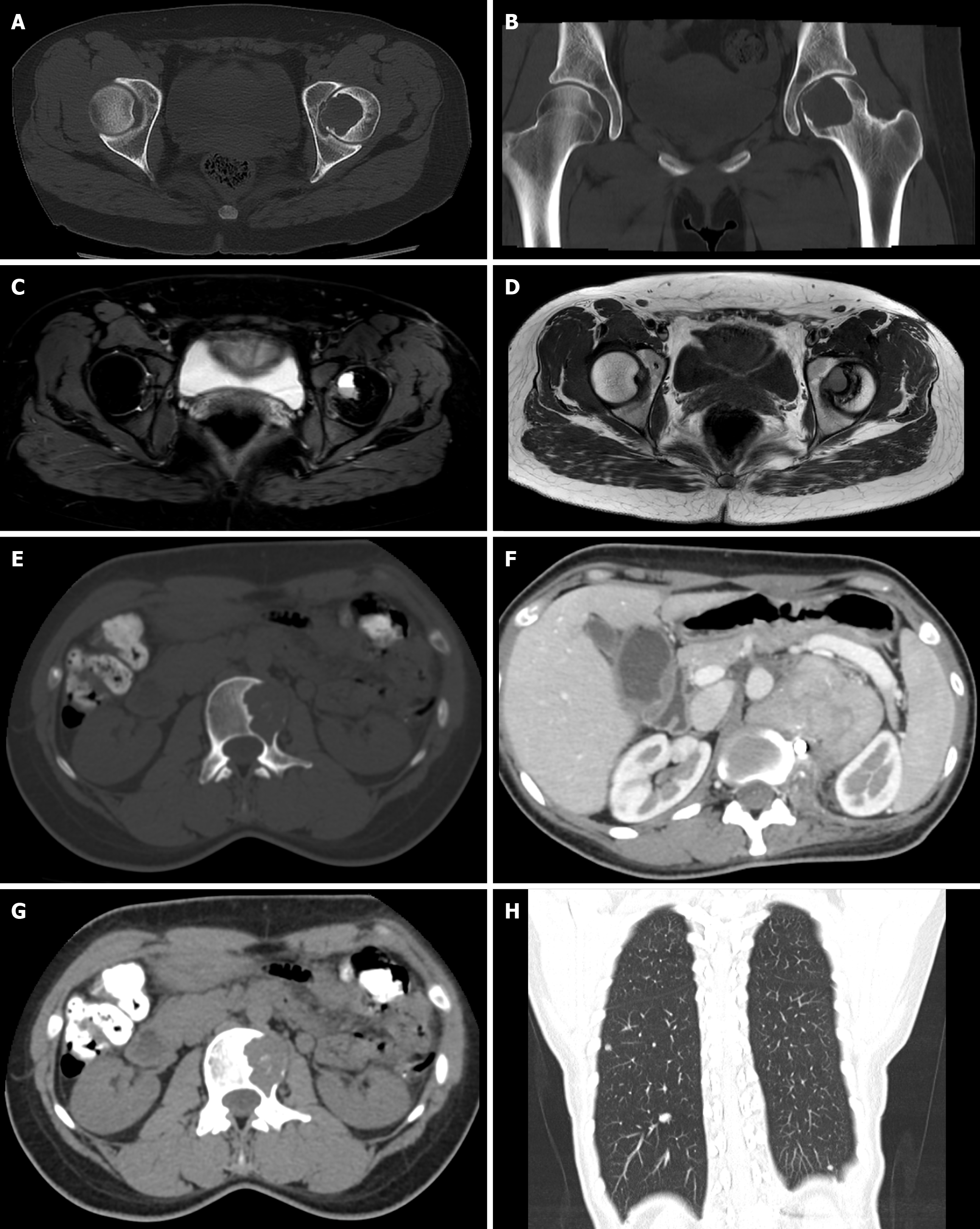
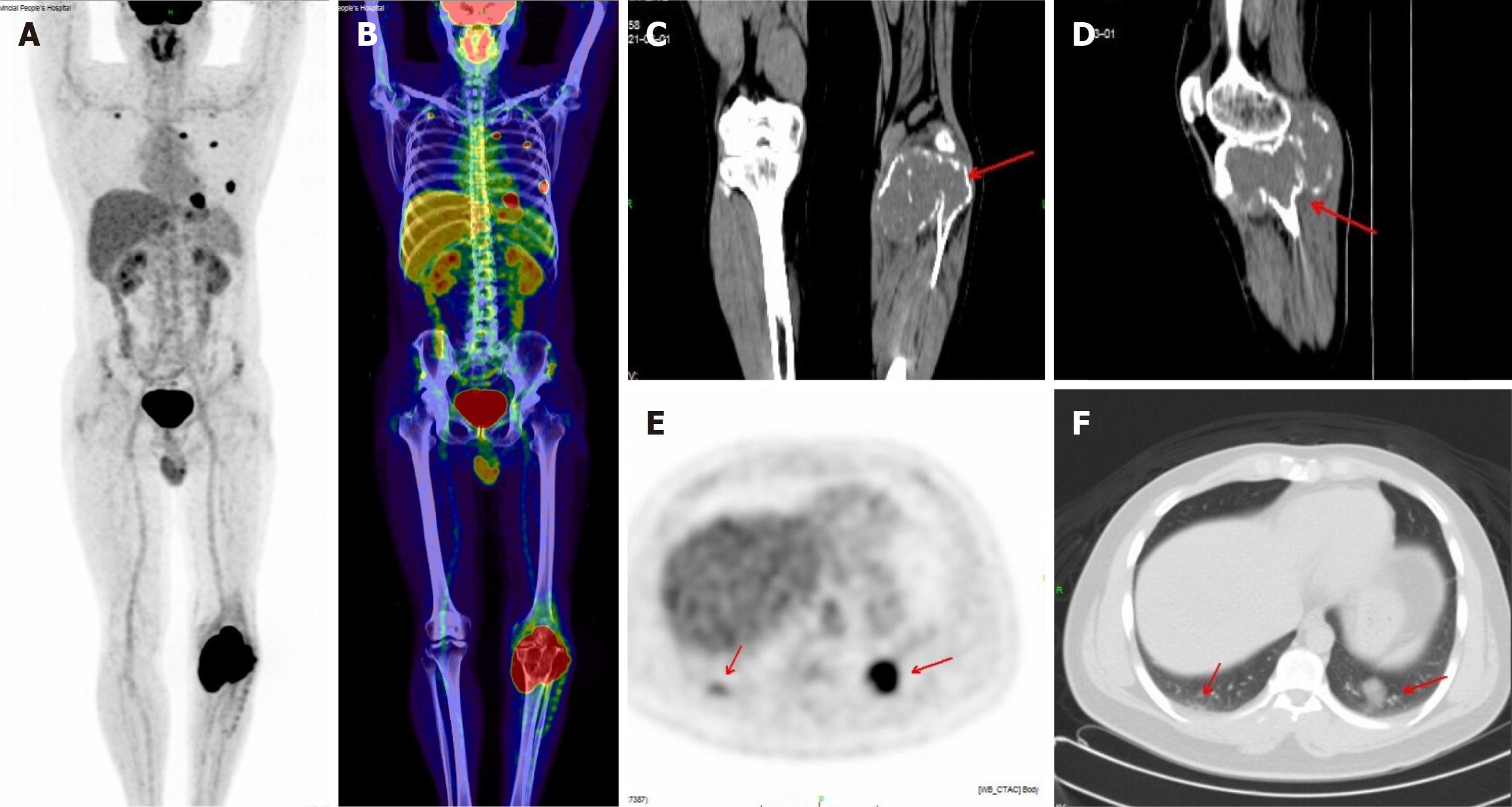
A total of 32 patients with giant cell tumors of bone were retrospectively selected. All the patients with GCTB were examined by X-ray, CT and MRI, and 7 of those patients were examined by PET-CT. There were 13 cases in the upper tibia, 9 cases in the lower femur, 3 cases in the neck of the femur, 1 case in the proximal humerus, 1 case in the distal ulna, 2 cases in the pelvis, and 3 cases in the vertebral column (Figures 1 and 2). The consistency test between the two reviewers yielded a kappa value of 0.875, which indicated excellent consistency (Table 1).
| Locations of GCTB | Cases | Proportions (%) |
| Upper tibia | 13 | 40.625 |
| Lower femur | 9 | 28.125 |
| The neck of the femur | 3 | 9.375 |
| Proximal humerus | 1 | 3.125 |
| Distal ulna | 1 | 3.125 |
| Pelvis | 2 | 6.250 |
| Vertebral column | 3 | 9.375 |
| Kappa value | 0.875 | |
22 patients had circular or elliptical low-density areas in the lesion area, 19 had cortical expansion and thinning, 11 had trabecular septa forming soap bubbles in the destruction area, 8 had unilateral osteolytic destruction, 5 had sclerotic margins, 11 had cortical interruption, 2 had fibrous ossification in the destruction area, 1 had multiple layers of bone shells in soft tissue, 1 had a vertebral compression fracture, and 7 did not have obvious abnormalities on X-ray imaging.
Expansive growth was found in 15 patients, bone shell formation was found in 14 patients, 10 patients had eccentric growth, 5 patients had central growth, 5 patients had mild to moderate sclerosis at the edges of the lesions, 9 patients had varying sizes and numbers of bone ridges inside the lesions, 11 patients had cortical perforation destruction, lesions reached the articular surface in 3 patients, and 10 patients had extraosseous soft tissue masses.
Compared to that on the tumor, the surrounding muscle tissue on T1WI has a low signal intensity and equal signal intensity, while on T2WI, it has an uneven high signal intensity. The cortical bone has a low signal intensity. A total of 12 patients had a circular low-signal zone at the edge of the tumor, while 8 patients had an incomplete circular low-signal shadow. Three patients had small patchy or circular high-signal areas of the tumor on T1WI and T2WI, indicating subacute bleeding. Eight patients had a low signal on T1WI and a high signal on T2WI, indicating cystic degeneration or necrosis of the tumor. Multiple fibrous septa could be seen penetrating the tumor body in 8 patients, resulting in a compartmentalized lesion. Three patients had a liquid–liquid plane, with a low signal on T1WI compared to the lower layer, a high signal on T2WI, and a higher signal on the upper layer.
Seven patients were examined via PET-CT. Among them, 2 patients had lung metastasis, both invading surrounding soft tissues, and 2 patients had GCTB involving the articular surface. GCTB showed a high metabolic zone at the tumor site, with an SUVmax of 25.6 ± 4.3 (range 22.5-28.6). The SUVmax of the metastatic pulmonary nodules was 17.3 and 13.8, respectively (Figure 2).
Giant cell tumors of bone mostly occur in mature bones. The age at predilection is 20-45 years[1]. They are generally believed to originate from bone mesenchymal cells, accounting for approximately 22.7% of benign bone tumors, and they represent the sixth most common primary bone tumor[3]. GCTB is more common in the long bones of the limbs, most commonly in the distal femur, proximal tibia, proximal humerus, and distal radius, and may occur in some cases in the ilium, foot, skull, mandible, and vertebrae column[4]. Patients often seek medical attention due to pain and fatigue in the affected area. GCTB is the most common lesion associated with aneurysmal bone cysts, accounting for approximately 39% of cases. GCTB is a borderline tumor with strong local invasiveness and easy recurrence after curettage surgery, which can lead to distant metastasis. Approximately 1%-2% of GCTBs can develop into primary malignant cell tumors (PMRGs)[5]. PMGCTs are a type of tumor in which both malignant and benign GCTB components are observed in the tumor tissue and account for approximately 1-5% of the total number of GCTBs[6]. Imaging findings mainly include osteolytic destruction of the long bone end, an incomplete bone shell, and the formation of large soft tissue masses without ossification, calcification, or periosteal reactions[7]. The severity of GCTB is assessed mainly by imaging findings, which are mainly classified as Campanacci grade[8]: Grade I—dilated bone destruction is limited to the mature bone shell, with clear boundaries, an intact or thinning bone cortex, moderate nuclear concentration, limited to the lesion, and mild or absent clinical symptoms; Grade II—most commonly manifested as osteolytic bone destruction with unclear boundaries, but the tumor is limited to a thin layer of cortical bone, and there is a concentration of nuclides in the surrounding tissues of tumor grade; and Grade III—commonly seen in recurrent GCTB, can be treated with pathological fractures, manifested as indistinct osteolytic bone destruction or even bone disappearance; the tumor breaks through the bone cortex to form a soft tissue mass, and nuclides accumulate around the tumor.
GCTB can cause single or multichambered cystic expansive bone destruction, with intact or incomplete bone shells visible at the edges. In areas with a weaker bone cortex, tumors are prone to break through and infiltrate into surrounding soft tissues, forming masses. GCTB often occurs at the bone end of long bones in the limbs. Like GCTB in long tubular bones, spinal GCTB still shows typical signs of expansive growth. Markedly swollen lesions often break through the bone cortex and grow outward to form soft tissue masses. X-ray findings reveal eccentric, osteolytic, and expansive bone destruction at the bone end, with soap bubble-like changes[9]. The lesions are mostly solitary, with clear edges and no obvious sclerosis. The destruction area can reach under the cartilage, and the bone cortex around the lesion becomes thinner. When the tumor is large and invasive, continuous new bone cortex and surrounding soft tissue masses may appear, but there is little periosteal reaction[10]. When pathological fractures occur, a periosteal reaction can be observed. When tumors occur in long bones, the extent of invasion by the elongated bone diameter is often greater than the extent of invasion by the elongated bone diameter[11]. CT and MRI are considerably better than plain films at displaying soft tissue masses, bone septations, and lesion involvement, but MRI is more effective at displaying these masses. Most lesions have clear boundaries and uniform density on CT, with no calcification, periosteal reaction, or new bone formation within the lesion. After enhancement, they exhibit uniform enhancement[12]. On MRI, the lesion T1WT shows a mixed signal dominated by a medium signal or uniform medium signal or a low signal dominated by a mixed signal, while T2WT shows a mixed signal dominated by a medium signal or medium high signal, with clear edges and visible circular low signal bands. The parenchymal part of the lesion is uniformly enhanced after enhancement. Liquefaction necrosis within the lesion, visible bleeding, formation of surrounding soft tissue masses, and surrounding edema are observed[12]. The lesion is located beneath the articular cartilage, resulting in bone destruction and joint deformation. The imaging manifestations of GCTB, to a certain extent, reflect its pathological and histological characteristics. In the early and middle stages, tumors often have a fibrous capsule with clear boundaries with the surrounding area, and sometimes, low signal loops can be observed on MRI. The linear low-signal interval of the tumor tissue is defined as fibrous tissue, consistent with the soap bubble sign observed on plain film. On CT, it shows cystic density, while on MRI, it shows high signal density on T1 and T2, indicating cystic transformation and necrotic areas; on CT, it shows high density, while on MRI, it shows short T1 and long T2 signals, indicating bleeding. Two different types of fluids form a liquid-liquid plane.
Due to its high cost, PET-CT is relatively less commonly used in clinical applications for GCTB. The purpose of PET-CT for patients with primary bone tumors is to determine the location of the primary tumor lesion and to perform efficacy testing[13]. PET-CT mainly reveals an abnormal increase in FDG uptake at the lesion site, while co-image CT reveals osteolytic bone destruction. FDG uptake is abnormally increased in both benign and malignant GCTB[13,14]. Whole-body PET-CT imaging can not only reveal FDG uptake in lesions but also reveal bone changes, the degree of destruction, and the relationship between the lesion and surrounding tissues. PET-CT is superior to CT for detecting small lesions and evaluating tumor recurrence. In this study, the SUVmax of GCTB significantly increased, with an average of 25.6 ± 4.3. An increase in the SUV is correlated with the pathological grade of GCTB.
Typically, GCTB is not difficult to diagnose based on its location at onset, age of onset, and imaging features. A partial lack of specific manifestations, especially in GCTB with only solid components, requires differentiation from osteosarcoma and metastatic tumors[10]. Osteosarcoma is highly invasive and often accompanied by periosteal reactions. There are more obvious soft tissue masses outside the bone, but the swelling is not obvious[15]. GCTB reveals expansive features, such as bone destruction, and soft tissue masses outside the bone are relatively small. There is often a history of multiple primary diseases.
Spinal osteosarcoma often needs to be differentiated from primary aneurysmal bone cysts, hemangiomas, osteo
Multimodal imaging plays an important role in the diagnosis of giant cell tumors. X-ray, CT, MRI, and PET-CT images have their own advantages and can provide a reference for the treatment of giant cell tumors.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade C
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Wang Y, United States S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Agawa R, Kato I, Kawabata Y, Takeyama M, Fujii S. Histological and immunohistochemical analyses of osteoclast maturation in giant cell tumor of bone. Pathol Res Pract. 2024;254:155128. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Verschoor AJ, Bovée JVMG, Mastboom MJL, Sander Dijkstra PD, Van De Sande MAJ, Gelderblom H. Incidence and demographics of giant cell tumor of bone in The Netherlands: First nationwide Pathology Registry Study. Acta Orthop. 2018;89:570-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 3. | Hayashida K, Kawabata Y, Kato I, Kamiishi T, Matsuo K, Takeyama M, Inaba Y. Clinical and pathological analysis of giant cell tumor of bone with denosumab treatment and local recurrence. J Orthop Sci. 2022;27:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 4. | Li SL, Kong YG, Zou Y, Yu X, Ouyang HQ, Chen SM, Deng YQ. Giant Cell Tumor and Giant Cell Reparative Granuloma of Bone of the Head: CT and MR Imaging Findings. Comb Chem High Throughput Screen. 2023;26:1180-1185. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Ma Y, Xu W, Yin H, Huang Q, Liu T, Yang X, Wei H, Xiao J. Therapeutic radiotherapy for giant cell tumor of the spine: a systemic review. Eur Spine J. 2015;24:1754-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Kadowaki M, Yamamoto S, Uchio Y. Late malignant transformation of giant cell tumor of bone 41 years after primary surgery. Orthopedics. 2012;35:e1566-e1570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Hoch B, Inwards C, Sundaram M, Rosenberg AE. Multicentric giant cell tumor of bone. Clinicopathologic analysis of thirty cases. J Bone Joint Surg Am. 2006;88:1998-2008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Deventer N, Budny T, Gosheger G, Rachbauer A, Puetzler J, Theil JC, Kovtun D, de Vaal M, Deventer N. Giant cell tumor of bone: A single center study of 115 cases. J Bone Oncol. 2022;33:100417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Liu W, Chan CM, Gong L, Bui MM, Han G, Letson GD, Yang Y, Niu X. Malignancy in giant cell tumor of bone in the extremities. J Bone Oncol. 2021;26:100334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Ning XJ, Lun DX, Hu YC. Morphological features and surgical management of giant cell tumor of bone. Zhonghua Guke Zazhi. 2016;36:1335-1340. [DOI] [Full Text] |
| 11. | Deng QX, Zhou T, Pan AZ, Yang GY. Study on the Correlation between Imaging Features and Pathological Stage of Giant Cell Tumor of Long Bone. Zhongguo CT He MRI Zazhi. 2022;20:169-171, 176. [DOI] [Full Text] |
| 12. | Chen L, Shi XL, Zhou ZM, Qin LD, Liu XH, Jiang L, Zhang QJ, Ding XY. Clinical Significance of MRI and Pathological Features of Giant Cell Tumor of Bone Boundary. Orthop Surg. 2019;11:628-634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 13. | O'Connor W, Quintana M, Smith S, Willis M, Renner J. The hypermetabolic giant: 18F-FDG avid giant cell tumor identified on PET-CT. J Radiol Case Rep. 2014;8:27-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Shen ZH, Xu BX, Guan ZW, Lei JX, Yang H, Xu XD, Liu YZ. PET/CT in the diagnosis of giant cell tumor. Zhongguo Linchuang Yixue Yingxiang Zazhi. 2017;6:438-441. [DOI] [Full Text] |
| 15. | Simpson E, Brown HL. Understanding osteosarcomas. JAAPA. 2018;31:15-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |