INTRODUCTION
Respiratory infections are common and can lead to life-threatening conditions. The common respiratory infections can result in wheezing, respiratory distress, and difficulty in breathing in all aged people, and thus are the most common cause of hospitalization especially in children[1,2]. Respiratory infectious diseases have been known for their leading role in causing of morbidity and mortality, and thus are serious threat to global healthcare systems. A wide range of infections from bacteria to viruses are associated with respiratory conditions, and these diseases can be classified according to the type and nature of pathogen such as coronavirus disease 2019 (COVID-19) and H1N1 influenza are viral diseases, while tuberculosis is an example of respiratory bacterial infection disease[3]. Among these respiratory infectious diseases COVID-19 is typically accompanied by severe complications, and serious outcomes such as acute respiratory distress syndrome, pulmonary fibrosis, multiple organ failure, and septic shock[4,5].
Exposure to a different type of infections (both bacterial and viral) leads to the development of immune responses, which can prevent upcoming attacks of these infections. However, determination of immune response depends on the type and nature of infection. Although both viral and bacterial infection can induce immunity, serious infections can destroy the immune regulations and cause serious health conditions that can be a greater risk to life. One of the recent examples of such viral infections is severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes COVID-19 disease. It is widely known that the relationship between immunity and pathogens is complex and dynamic, however, researchers have largely focused on determining such relationships especially in the case of COVID-19. It is evident that the immune response is typically enacted by three components “systemic inflammation (soon after infectiousness), activation of the complement system (immune regulation or progression occurs) and formation of neutrophil extracellular traps (to destroy the infection)[6].
Millions of people across the globe have recently witnessed a serious pandemic of SARS-CoV-2 (causative agent of COVID-19 disease). Since the time of its first appearance, COVID-19 infection has infected millions of people and killed thousands of them. SARS-CoV-2 is a positive sense, enveloped, and single-stranded RNA virus (approximately 30 kb size). The entrance of the virus into host cells is critical for the disease development, which is indicated by the associated symptoms. Although, primary symptoms including pneumonia, cough, fatigue of COVID-19 are associated with the pulmonary system, the infection can harm multiple organ systems, including brain by causing neurological complications[7,8].
We know that respiratory infection induces inflammatory responses by increasing proinflammatory cytokines determined by the function and release of neutrophils, which varies with the type and level of infection. Similarly, the levels of myeloperoxidase, interleukin (IL)-6, IL-10, and p-selectin also are also the key indicators in such responses. Severe COVID-19 disease is characterized by cytokine storm or substantial pro-inflammatory response, which in turn increase the risk of multiorgan dysfunction increases[9]. In some cases, the expression of cytokines remains low in peripheral blood immune cells, where strong signatures induced by type I interferon-induced signatures indicate the action of other molecules despite inflammatory cytokines[10-12]. It is evident that proinflammatory cytokines generate in response to SARS-CoV-2 include TNF-α, interferon (IFN)-γ, monocyte chemotactic protein (MCP)-1/C-C motif chemokine ligand (CCL)-2, vascular endothelial growth factor, interleukins (especially IL-1Rα and IL-1β), basic fibroblast growth factor, granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage (GM)-CSF, macrophage inflammatory protein (MIP)-1α/CCL3, MIP-1β/CCL4, and induced protein (IP)-10/CXCL10[13]. Since SARS-CoV-2 induces cell death (infected cells) during viral replication, thus pyroptosis is enhanced in epithelial cells. Meanwhile, IL-1R induces proinflammatory cytokines (MIP-1β/CCL4, IL-6, IFN-γ, MCP-1/CCL2, IP-10/CXCL, and MIP-1α/CCL3) secretion. The aforementioned functions of COVID-19 infection can lead to the recruitment of activated macrophages to the infection site. In response to the release of cytokines inflammation is induced that in turn increases the risk of lung parenchyma destruction. Dysregulated IFN signaling can also modulate immunopathology, thereby enhancing the clearance of impaired viral entities from infected cells in target regions[13]. Furthermore, after the infection of SARS-CoV-2, CD4 and CD8 T cell responses are enhanced that causes the generation of virus-specific antibodies.
In the current review, we prominently discuss the immune responses and immune regulations in respiratory diseases with a specific focus on COVID-19. We elaborate the thrombosis process in COVID-19 disease and its utilization as drug target for specific treatment. We further discuss the importance and need of engineering of nanomedicines from different sources including antivirals, liposomes. Other biological materials as well inorganic materials. We also discuss how these drugs can be modified to make them further effective and efficient against respiratory conditions.
IMMUNE RESPONSES TRIGGERED RESPIRATORY INFECTIONS
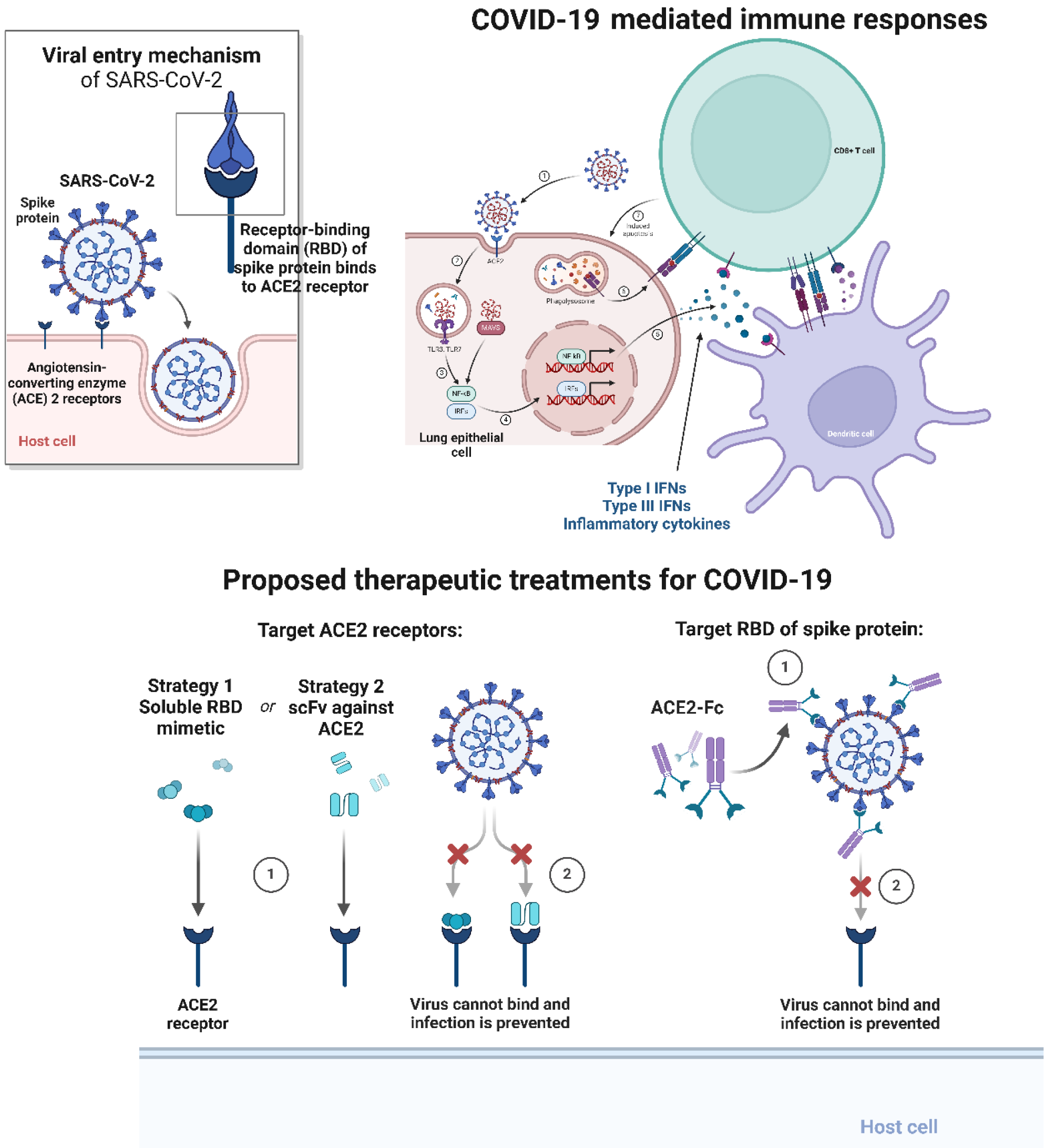
The immune responses are generated by the host immune system soon after the entrance and infectiousness of pathogen. In the case of SARS-CoV-2, which enters into human host cells with the help of specialized enzyme known as receptor angiotensin-converting enzyme 2 (ACE2) as well as serine protease TMPRSS2 for spike (S) protein priming[14,15]. The virus then multiplies and disseminates into the airway and affects a key regulatory pathway known as IFN type 1 antiviral pathway. This virus not only infects lungs but can also spread form lungs and disseminate to several tissues which can express ACE2[16,17]. Based on expression of ACE2, we conclude that SARS-CoV-2 can enter and infect alveolar cells, and neuronal and glial cells[18].
Macrophages
Macrophages are derived from monocytes which play role in inflammatory responses through regulation of FABP4, ficolin-1 (FCN1), and SPP1 in wide range of respiratory diseases. The most prominent molecular function of macrophages is the expression of genes that encode chemokines, inflammatory transcription factors, and peripheral monocyte-like markers[19]. These molecules also determine the level and state of disease i.e., from mild to severe. Such as in severe COVID-19 disease, researchers have detected alternative M2 macrophages, which can express profibrotic genes “TREM2, TGFB1, and SPP1”, and immunoregulatory genes “A2M and GPR3”. Overall, the molecular determinations associated with macrophages indicate that they are critical not only in inflammatory responses but also in pulmonary fibrosis, and related conditions[20,21].
Natural killer cells
In serious conditions of respiratory disease such as severe COVID-19 causes depletion of natural killer cells, thereby exhibiting an exhausted phenotype and increase the risk further. In the case of mild infection or mild symptomatic COVID-19 disease the natural killer cells are relatively less depleted as compared to those with severe symptoms. Moreover, antiviral cytotoxic CD56dim is depleted in ventilator-dependent patients, indicating that severe COVID-19 disease seriously harms immune functions[22,23]. However, it is yet to investigate that how peripheral natural killer cells get reduced; this reduction or depletion could be associated with cells trafficking to the infected lung tissues or cell death as indicated by the expansion of lung macrophages and release of associated molecules[24]. Researches indicate that COVID-19 infection causes upregulation of TP53 transcription and apoptosis pathways, which is convincing evidence that depletion of natural killer cells is associated with cell death[25].
Neutrophils
Neutrophils play key roles in disease progression and determination such that elevated neutrophil count is prognostic of COVID-19 infection mediated death and acute respiratory distress syndrome. The levels of neutrophil extracellular traps are increased in response to the severe infection of COVID-19. Neutrophil extracellular traps are DNA materials which form web in combination with oxidant enzymes extruded by neutrophils and antimicrobials to control infections[26]. They can increase the risk of or directly induce immunothrombosis as well as lung injury in COVID-19 patients[27,28]. Immunofluorescence based studies have largely reported that neutrophilic infiltrate with numerous Cit-H3+ and MPO+ neutrophils in the lungs, while extracellular DNA lattices with MPO and Cit-H3 have also been observed in patients with COVID-19 infection. In pulmonary blood vessels, neutrophils can get co-localized with platelets and increase the risk of microthrombi formation and development[29].
T cells
T cells contribute significantly to post COVID-19 infection immune responses, generated to control the infectiousness and prevention of severe symptoms[30]. Improved cytokine levels can reverse lymphopenia, which is prominent feature of COVID-19 disease. The reduction of CD4+ and CD8+ T cells in COVID-19 has been observed and confirmed by lymphocyte count. Since serum cytokine levels are inversely correlated with T-cell counts, thus T-cells can exacerbate the hyperinflammatory state in COVID-19 patients. Researchers have reported an increased pathogenic Th1 CD4+ T cells counts in COVID-19 patients, which can ultimately increase the expression of IFN-γ, IL-6, and GM-CSF[31]. Furthermore, Th1 cells produce proinflammatory cytokines, thereby inducing cytokine storm and potentiate lung damage. Dysregulation of T-regulatory cells can impair immune responses as indicated by the prominent role of these T-cells in immune homeostasis[32-34].
THROMBOSIS AND IMMUNE REGULATIONS IN COVID-19
Covid-19 triggers immune responses and activates inflammatory reactions soon after the viral entrance into the body[35,36]. Conventional thrombosis is developed by inflammation and complement activation, however, based on molecular level indications, researchers have reported unique involvement of NETosis in the case of COVID-19 pathophysiology[37]. The major components associated with processes are “activated platelets, tissue factor, and thrombin”, which contribute to thrombogenesis and are connected in a positive feedback loop[38]. Thrombin facilitates fibrinogen cleavage and fibrin is formed, which then forms thrombus after combining with activated platelets and red blood cells. Their upregulation is linked with thrombogenicity of the immune response while, breakdown of the blood clots the process known as thrombolysis is hindered[39,40]. Thrombolysis is typically facilitated by fibrin cleavage and formation of D-dimer by plasmin however, in patients with COVID-19 thrombolysis is hindered by upregulation of plasminogen activator inhibitor-1 as indicated by clinical studies[41]. Since thrombi causes pulmonary embolisms, thus clot-busting therapeutics should be adequately provided to treat COVID-19 symptoms. Thrombolytics (urokinase, streptokinase, and alteplase) stimulate plasmin followed by subsequent cleavage of fibrin, thereby degrading thrombus 18. Thus, these thrombolytics can be used against COVID-19 disease in severe cases. However, before going further several prominent limitations associated with thrombolytic treatment should be considered; such as the premature degradation of the thrombolytic enzymes by systemic proteolytic enzymes, and haemorrhagic risk[42].
ADMINISTRATION ROUTES OF NANOMATERIALS ENGINEERED FOR IMMUNE STIMULATION
Mucosal vaccines in nasal-associated lymphoid tissue, bronchial-associated lymphoid tissue, and intramuscularly administered vaccines are prominent processes to develop adaptive respiratory immune protection. Somatic or antigen presenting cells receive intramuscularly administered vaccines, where exogenously injected or endogenously expressed antigens activate immune cells including B cells, and CD8+ T cells in order to produce antibodies for protection from specific pathogen[34]. Considering the sensitivity and possible resistance by the immune system, antigen presentation should be carefully designed and administered to get maximum effectiveness from the designed vaccines administered into the host system. It is worth noting that intramuscularly administered vaccines do not have the ability to generate and propagate mucosal and systemic immune responses, thus mucosal immunization would be required. In this regard nasal mucosal vaccines could be suitable, however, they also face the barrier of the nasal cavity such as the surfaces of mucosal epithelial cells covered by cilia and submucosal glands produce mucus secreted. Thus, delivery of effective nasal vaccine requires the presentation of specified antigen in order to trigger a robust immune response. To overcome the problems and complications with conventional routes of administration, the vaccines can be delivered through advanced techniques most importantly with the use of nanocarriers. More specifically, the delivery of mRNA vaccines can be achieved by diverse nanocarriers for robust expression of the desired antigen and protection of mRNA from enzymatic degradation. Lipid-based carriers such as ionizable nanoparticles are prominently considered to deliver mRNA modalities[30]. Nevertheless, polymeric nanocarriers have other unique properties such as these are more versatile and flexible than lipid carriers.
NANOMEDICINES AND NANO IMMUNOTHERAPEUTIC FOR RESPIRATORY DISEASES
Thrombosis is a key regulating process in the case of COVID-19, which can a suitable target for several drugs especially nanomedicines. Nanoparticles are typically cleared by the spleen and liver. The chances of thrombosis in these organs are limited, which can reduce the efficacy and specificity of nanomaterials that target thrombolysis. To overcome this problem, use of micrometre-sized particles may be adequate, based on their ability of accumulation in the microvasculature of the lungs. However, microparticles with a size larger than 10 μm can occlude lung capillaries. On the other hand, localized accumulation of nanoparticles may be achieved by decorating nanoparticles with ligands, which have affinity for components of the thrombi. These components play a key role in COVID-19-induced thrombosis, therefore, decoration of nanoparticles with earlier discussed ligands ca be beneficial. However, the complex functional optimization of active targeting should be considered such that a previous study reported only 3.5% of proteins were successfully conjugated to a particle, which had an appropriate orientation for receptor recognition. In addition, surface density of the ligand can also affect targeting and therapeutic efficacy[43]. Considering these complications, researchers focused on improving the efficacy and targeting ability of nanomaterials. Several groups have utilized small peptides to target fibrin or activated platelets based on their low immunogenicity and high ease of scalability. To increase the effectiveness and targeting efficiency synergistic approaches by combining multiple ligands are preferred. Using ligands fibrin-binding peptides and platelet to decorate liposomes enhance thrombus-anchorage efficacy as compared to using one of the ligands. Researchers have observed that at high (beyond 5 mol%) total ligand density decreases the clot-anchorage capability of nanomaterials and increase their therapeutic efficacy, therefore, optimizing ligand conjugation is crucial for high targeting affinity and effectiveness[44,45]. It is also important to note that peptides often have poor proteolytic stability and lower affinity as compared to other antibodies. Therefore, researchers have studied the use of antibodies based on their favourable high affinity. In this regard, an earlier study reported increased accumulation of perfluorocarbon nanoparticle combined with anti-fibrin monoclonal antibody, at the thrombus[33].
Since optimization of the conjugation of the ligands is essential onto the nanoparticles, therefore some important steps must be considered while designing such nanoparticles; 1- to preserve affinity and warrant correct orientation, conjugation site of the linker should be considered, 2- Antibody’s density should be carefully considered for conjugation[46]. To benefit nanoparticles with inherent targeting capabilities, endogenously derived membranes are used, and designing nanoparticles this way can also provide extended pharmacokinetic profiles. To prevent potential (allogeneic) immune response, it is important to consider the source of platelet membrane before decorating the nanoparticles and using them further. Now, considering therapeutic agent is critical, as antagonist of platelet can compromise the targeting capability of an activated platelet member.
We know that neutrophils play a critical role in COVID-19 thrombotic pathophysiology, thus targeting neutrophils can be a suitable target for nanoparticles in order to treat COVID-19. Since the formation of thrombus is not needed for this targeting to be effective, therefore, this (neutrophil-targeting) approach is interesting and can be a promising strategy for early treatment. Moving on, a therapeutic strategy targeting with platelet-targeting peptide (DAEWVDVS) enabled binding to activated platelet–neutrophil complexes, which are highly prevalent in the case of COVID-19 infection. These observations indicate that this therapeutic strategy can be a promising approach against COVID-19-induced thrombosis.
TARGETING THROMBOSIS BY RESPONSIVE NANOMEDICINES IN RESPIRATORY DISEASES (COVID-19)
Responsive nanocarriers can release thrombolytics selectively on demand, and thus can be used for targeted therapy of thrombosis. A wide range of molecules have been studied to be effectively used against thrombosis, however, only a few approaches have been observed suitable or applicable in the case of COVID-19-induced thrombosis. Magnetic nanomaterials, other nanomedicines, which are responsive to external stimuli such as ultrasound require experts to handle, making the treatment process complicated. Therefore, it is crucial to develop drug delivery systems that are responsive to internal stimuli, as these systems appear to be more conducive to the treatment of COVID-19-induced thrombosis. The efficiency of this nanoparticle system against thrombosis is similar to that of free streptokinase. Moreover, this nanocarrier system contains notable clinical potential, due to the scalability and previous regulatory approval of liposomes. Thrombin is highly specific to acute thrombosis, and thus, off-target drug release from thrombin-sensitive nanoparticles may not be adequate. Since thrombin is a stimulant that cleaves of fibrinogen to fibrin, therefore, its sensitivity can be introduced through peptides incorporated into the system for mimicking its binding site on fibrinogen. However, it is imperative to know that surface loading int his system appears to have limited loading capacity, as well as there is a tendency for premature degradation by the attack of enzymes present in the system/environment. In addition, MnO2 particles acted as hydrogen peroxide scavengers, indicating that this therapeutic strategy can be promising option to treat COVID-19-induced thrombosis. Reactive oxygen species (ROS) are largely associated with thrombosis, and they also promote platelet activation and inflammation. Higher generation of ROS have been frequently reported in patients with COVID-19, therefore using antioxidants for the treatment of COVID-19 can improve the efficacy of anti COVID-19 drugs to quickly relieve symptoms. Therefore, these nanoparticles may also be effective if tested in the case of COVID-19 infection.
IMMUNOSUPPRESSANT AND ANTIVIRAL BASED NANOMEDICINES
Administration of therapeutics specified for suppression of infection through generating immune response can be an alternative to the use of thrombolytics to treat thrombosis induced by COVID-19 infection (Figure 1). Such approaches have always been considered central in treating different diseases including COVID-19. Antiviral agents including molnupiravir and paxlovid have received high recognition. Such virucidal and immunosuppressant based nanomedicines have been tested for treatment against COVID-19. In specific, virucidal nanoparticles appeared to be more effective in prevention of survival and replication of the virus[4,7,37]. Using nanomaterials with inherent virucidal properties is also an interesting approach such as using silver, and polylysine have been investigated. Nanoparticles based on these elements or molecules may disrupt integrity of SARS-CoV-2 as well as prevent them from entrance into endothelial cells as well as their replication in cells. Comparatively, glycyrrhizic acid nanoparticles showed limited efficacy, however, both polylysineand silver nanoparticles showed a positive correlation between their surface charge and antiviral activity. For optimal inhibition of the virus, silver nanoparticles showed size-dependent virucidal properties with 10 nm nanoparticles, while 100 nm nanoparticles were entirely ineffective[33,37]. Several groups that can utilize ACE2-expressing membranes to develop nanoconstructscan competitively bind and trap SARS-CoV-2. Furthermore, nanostructures generated by using these endogenously derived membranes show high biocompatibility, which can easily be core-loaded with therapeutics[33,37,47].
Figure 1 Viral entry activates immune responses in host cells, which can further be target using therapeutic agents or immunotherapy strategies for treating coronavirus disease 2019 infections.
ACE2: Angiotensin-converting enzyme 2; COVID-19: Coronavirus disease 2019; RBD; Receptor binding domain.
The aforementioned approaches based on antiviral drugs or immunosuppressants can prevent thrombogenesis, however, these cannot effectively remove pre-existing thrombi thus can only be used as preventive medicine. Therefore, dual-therapeutic nanomedicine containing antiviral or immunosuppressant nanoparticles in combination with thrombolytics should be developed and then can be utilized as a promising therapeutic strategy to treat COVID-19-induced thrombosis. Nevertheless, additional challenges should also be noted as these dual-therapeutic strategies can cause complications. In this regard the loading of therapeutics must be well optimized, and toxicity should be avoided along with ensuring therapeutic effects.
CONCLUSION
Identification of smart and efficient carriers and suitable therapeutic agent, and combine them to develop nanomedicine against COVID-19-induced thrombosis. These nanomedicines may improve patient outcomes, and reduce current challenges in clinical procedures, which mainly occur because of a lack of testing of novel viruses such asCOVID-19 infection, and less chances of translating current nanomedicines into clinical procedures. Although there is scarcity of detailed information on SARS-CoV-2 infection mediated immunothrombotic pathophysiology, researchers have known that COVID-19 mediated thrombosis varies drastically from conventional thrombosis. These observations indicate that animal models available for conventional thrombosis cannot be used as representative in the case of COVID-19 mediated thrombosis. Alternatively, models of immunothrombosis including sepsis models can be utilized, however, their relevance or suitability with pathophysiology of immunothrombosis mediated by COVID-19 must be confirmed. Other than the model, nanomedicine for COVID-19-induced thrombosis may face hurdles such as target specificity, irregular immune responses, off target effects, and complications in drug release. Therefore, advances are required in order to design translatable nanomedicines. The choice of nanomaterial is of critical importance, such as liposomes and iron oxide nanoparticles are thought to produce faster translatable systems, however certain drawbacks can come across including leakage and payload loading. Therefore, further search should be done to minimize these problems, and such nanomaterials could be greatly beneficial against COVID-19 disease. Nevertheless, artificial intelligence can also be considered and without any doubt, it can play an important role in selecting and designing antigen molecules, optimizing the composition and compatibility of nanoformulations, as well as exploring new nanocarriers.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, general and internal
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Sultana N, Bangladesh S-Editor: Liu JH L-Editor: A P-Editor: Zhao S