Published online Jun 6, 2024. doi: 10.12998/wjcc.v12.i16.2686
Revised: March 19, 2024
Accepted: April 19, 2024
Published online: June 6, 2024
Processing time: 169 Days and 15.7 Hours
The misuse and overuse of classic antifungals have accelerated the development of resistance mechanisms, diminishing the efficacy of established therapeutic pathways and necessitating a shift towards alternative targets. Despite this pressing need for new treatments, the antifungal drug pipeline has been largely stagnant for the past three decades, primarily due to the high risks and costs associated with antifungal drug development, compounded by uncertain market returns. Extensive research durations, special patient populations and rigorous regulatory demands pose significant barriers to bringing novel antifungal agents to market. In response, the “push-pull” incentive model has emerged as a vital strategy to invigorate the pipeline and encourage innovation. This editorial critically examines the current clinical landscape and spotlights emerging antifungal agents, such as Fosmanogepix, Ibrexafungerp, and Olorofim, while also unraveling the multifaceted challenges faced in new antifungal drug development. The generation of novel antifungals offers a beacon of hope in the battle against antimicrobial resistance, but it is premature to declare them as definitive solutions. Their future role hinges on thorough clinical validation, cost-effectiveness assessments, and continuous post-marketing surveillance. Only through strategic implementation and integration with market strategies we can transform the landscape of antifungal development, addressing both the resistance crisis and the treatment challenges.
Core Tip: The landscape of antifungal therapy has long been dominated by a handful of drug classes, namely azoles, polyenes, and echinocandins. Issues such as the development of resistance/tolerance, interactions and inherent toxicity, and narrow spectrum of activity have limited their therapeutic utility to clinicians. All these limitations underline the urgent need for novel approaches, with the pipeline for new antifungals having been relatively dry for about 30 years. Thankfully, the pharmaceutical landscape has recently shown promising signs of innovation regarding antifungal agents.
- Citation: Schinas G, Spernovasilis N, Akinosoglou K. Antifungal pipeline: Is there light at the end of the tunnel? World J Clin Cases 2024; 12(16): 2686-2691
- URL: https://www.wjgnet.com/2307-8960/full/v12/i16/2686.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i16.2686
While traditionally considered as secondary to bacterial and viral infections in both prevalence and severity, fungal infections have emerged as a significant global health threat[1]. Invasive fungal infections, in particular, have long been a subject of concern, but recent years have seen an alarming increase in both their incidence and complexity[2,3]. This newfound prominence may be attributable to multiple factors, including climate change, the increasing number of immunocompromised patients, advancements in medical interventions that disrupt normal flora, and the misuse of antifungal agents in certain settings, including agriculture, all of which create an environment fit for fungal proliferation, spread, and genetic evolution[4].
The escalating issue of antifungal resistance has further exacerbated the situation[5]. Most prominently the global spread of multidrug resistant strains of Candida glabrata and Candida auris, as well as the concurrent emergence of azole-resistant Aspergillus infections, have raised the stakes in the ongoing battle[6]. The emergence of these strains is not merely a regional concern but a global phenomenon, affecting both developed and developing nations[7]. Candida auris, for example, which was first identified in 2009 in Japan, has been responsible for numerous outbreaks in healthcare settings all around the world ever since[8]. Notably, it has exhibited increasing resistance to all three major classes of antifungals, significantly complicating hospitalizations and treatment outcomes[9]. According to the most recent estimates from the United States, this has led to associated mortality rates of up to 34%[10].
Despite the grim outlook and the heightened interest in the development of new antifungals, the existing arma
The landscape of antifungal therapy has long been dominated by a handful of drug classes, namely azoles, polyenes, and echinocandins. While these agents have been effective in treating a wide range of fungal infections, their limitations are becoming increasingly apparent. Issues such as the development of resistance/tolerance, interactions and inherent toxicity, and a narrow spectrum of activity have limited their therapeutic utility to clinicians[11,12]. Indeed, the most commonly employed antifungal agents, i.e., azoles and echinocandins, have shown declining efficacy due to widespread resistance[13,14] and limited clinical usage owing to their severe adverse effects, e.g., hepatotoxicity, nephrotoxicity and drug-drug interactions, making them less viable for long-term or combination options[12,15].
The challenge that antifungal infections pose is further compounded by the limited diagnostic tools available for early identification of fungal infections and resistance patterns. Conventional culture-based methods are especially time-consuming and may not be sensitive enough to detect emerging strains or identify resistance mechanisms. Molecular diagnostic tools, such as polymerase chain reaction, offer a reliable alternative in a variety of different scenarios. How
All these limitations underline the urgent need for novel approaches. With the pipeline for new antifungals having been relatively dry for about 30 years[16], the medical community is keenly awaiting the next generation of antifungal agents that can address the limitations of existing therapies and offer new avenues for treating resistant and novel fungal strains.
The pharmaceutical landscape has recently shown promising signs of innovation regarding antifungal agents. Recent advances in genomics and drug design have paved the way for the development of novel therapeutic targets, employing new mechanisms of action. Several promising drugs are currently in various stages of development, each with unique features that make them potential game-changers in antifungal therapy. Fosmanogepix, Ibrexafungerp, and Olorofim are among the most promising.
Fosmanogepix, which targets the Gwt1 enzyme, that is involved in the glycosylphosphatidylinositol biosynthetic pathway, has shown efficacy against a broad range of fungal pathogens, including multidrug-resistant strains. The recently published Phase 2 results indicate that Fosmanogepix is effective and well-tolerated for the first-line treatment of candidemia in non-neutropenic adults with a treatment success rate of 80% and an impressive 85% survival rate at 30 d follow-up[17]. Notably, approximately 48% of patients were transitioned to oral Fosmanogepix, indicating its potential for flexible administration. The ongoing Phase 3 trial aims to investigate the efficacy and safety of Fosmanogepix, compared to standard antifungal treatment, i.e., caspofungin and fluconazole, in adults with candidemia and/or invasive candidiasis.
Ibrexafungerp, a first-in-class triterpenoid antifungal, has completed Phase 3 trials, becoming the first non-azole agent approved by the United States Food and Drug Administration (FDA) for treating vaginal yeast infections caused by Candida spp., and is currently under investigation for invasive pulmonary aspergillosis (in combination with vori
Olorofim, another novel agent, targets rare mold infections by inhibiting dihydroorotate dehydrogenase in the pyrimidine synthesis pathway[18]. Of note, it has exhibited a favorable pharmacokinetic profile that allows for oral administration. It has been granted Qualified Infectious Disease Product and Breakthrough Therapy Designations for various invasive fungal infections from the FDA and according to the latest release from the drug sponsor, detailing the key findings of the Phase 2b open-label study, Olorofim demonstrated an acceptable benefit-risk profile with an overall success rate of 28.7% at day 42 and 27.2% at day 84 of follow-up. The all-cause mortality rates were 11.4% at day 42 and 15.8% at day 84 and the drug was generally well-tolerated, even in extended therapy exceeding 2 years[19]. A global Phase 3 trial is currently underway to compare treatment with Olorofim vs amphotericin B in patients with invasive aspergillosis[20].
Rezafungin, branded as Rezzayo, also deserves an honorable mention, despite its already established mechanism of action, as it was approved by the FDA on March 2023 for treating candidemia and invasive candidiasis in adult patients, particularly those with limited or no alternative treatment options. Its rather long half-life of approximately 133 h allows for less frequent intervals between doses, setting the basis for a once-weekly treatment regimen[21].
While these agents show considerable promise, it is essential to approach their potential with cautious optimism. The rigorous pharmacological evaluation, long-term safety data, and real-world effectiveness studies are yet to be conducted. Their true potential can only be assessed after extensive clinical trials that evaluate not just their efficacy but also their safety, tolerability, and cost-effectiveness.
While the development of new antifungal agents is certainly encouraging, it is crucial to acknowledge the myriad challenges and considerations that accompany their journey from the laboratory to clinical use. From early-stage research to clinical trials and regulatory approvals, the path to bringing a new antifungal agent to market is full of financial and logistical hurdles. The differences in legislations and regulatory approval processes between the United States and Europe add another layer of complexity for developers seeking global market entry.
However, common ground exists in that both the FDA and the European Medicines Agency (EMA) have stringent requirements for the approval of new antimicrobial agents. These requirements include not only extensive pre-clinical and clinical data on efficacy and safety but also post-marketing surveillance to monitor for adverse effects and the emergence of resistance. The rigorous nature of these requirements extends the drug development timeline and increases the financial burden, exceeding 10 years and $300 million according to some estimates[22], thereby acting as a deterrent for pharmaceutical companies.
Designing and conducting clinical trials for antifungal agents, in particular, presents unique challenges, primarily due to logistical considerations and the need for appropriate endpoints. To begin with, the relative rarity of certain invasive fungal infections necessitates multi-center trials to achieve adequate sample sizes[23]. Furthermore, the diversity of fungal pathogens' resistance patterns, the varying patient populations affected, and the different anatomical site of infection requires a multifaceted approach in trial design[24]. Ethical considerations, such as the use of placebo controls and/or other comparators, are especially complex due to the life-threatening potential of MDR strains and invasive species. What's more, the selection of clinically meaningful endpoints, like all-cause mortality or time to clinical reso
It is becoming increasingly evident that antifungal drug development is unattractive from an investment standpoint, particularly for large pharmaceutical companies. Over the past three decades, there has been a notable decline in the number of major pharmaceutical firms investing in antimicrobial research and development. Today, only about five companies maintain a significant investment in this area[25]. This reduction reflects the high risks and costs associated with drug development, compounded by the uncertain financial returns in the antimicrobial market.
In this challenging landscape, the concept of “push-pull” commercial incentives emerges as a strategy to facilitate the development and market entry of new treatment agents[26]. “Push” incentives, primarily provided by government and non-profit organizations, aim to stimulate early-stage research and development. These incentives can include funding, grants, and infrastructural support, helping to alleviate the financial risk associated with the initial stages of drug discovery and development. Among the most prominent examples of these incentives is the work done by Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator, a global non-profit partnership dedicated to accelerating antibacterial research. Other significant contributors include the Innovative Medicines Initiative in Europe, which collaborates with various stakeholders to accelerate the development of, and access to, innovative medicines. In the United States, the National Institute of Allergy and Infectious Diseases plays a similar role, offering grants and funding opportunities aimed at advancing research in infectious diseases, including antifungal resistance.
On the other hand, “pull” incentives, often implemented by regulatory bodies like the FDA and EMA, focus on creating a viable market for new drugs. These can include extended market exclusivity, patent extensions, and fast-track approval processes, which are designed to ensure a return on investment once the drug reaches the market. In the United States, for example, the Generating Antibiotic Incentives Now Act provides additional incentives such as the Qualified Infectious Disease Product Designation, offering extended exclusivity periods for drugs that target severe or life-threatening fungal infections[27]. Additionally, streamlined approval pathways are in place for antifungals intended for use in a limited and specific population of patients (limited population pathway). The European Union's approach emphasizes a unified procedure for drug approval and stresses multi-national collaboration. This strategy is beneficial for “pushing”, i.e., facilitating clinical trial conduction, while aligning with the “pull” aspect by facilitating market entry across multiple nations.
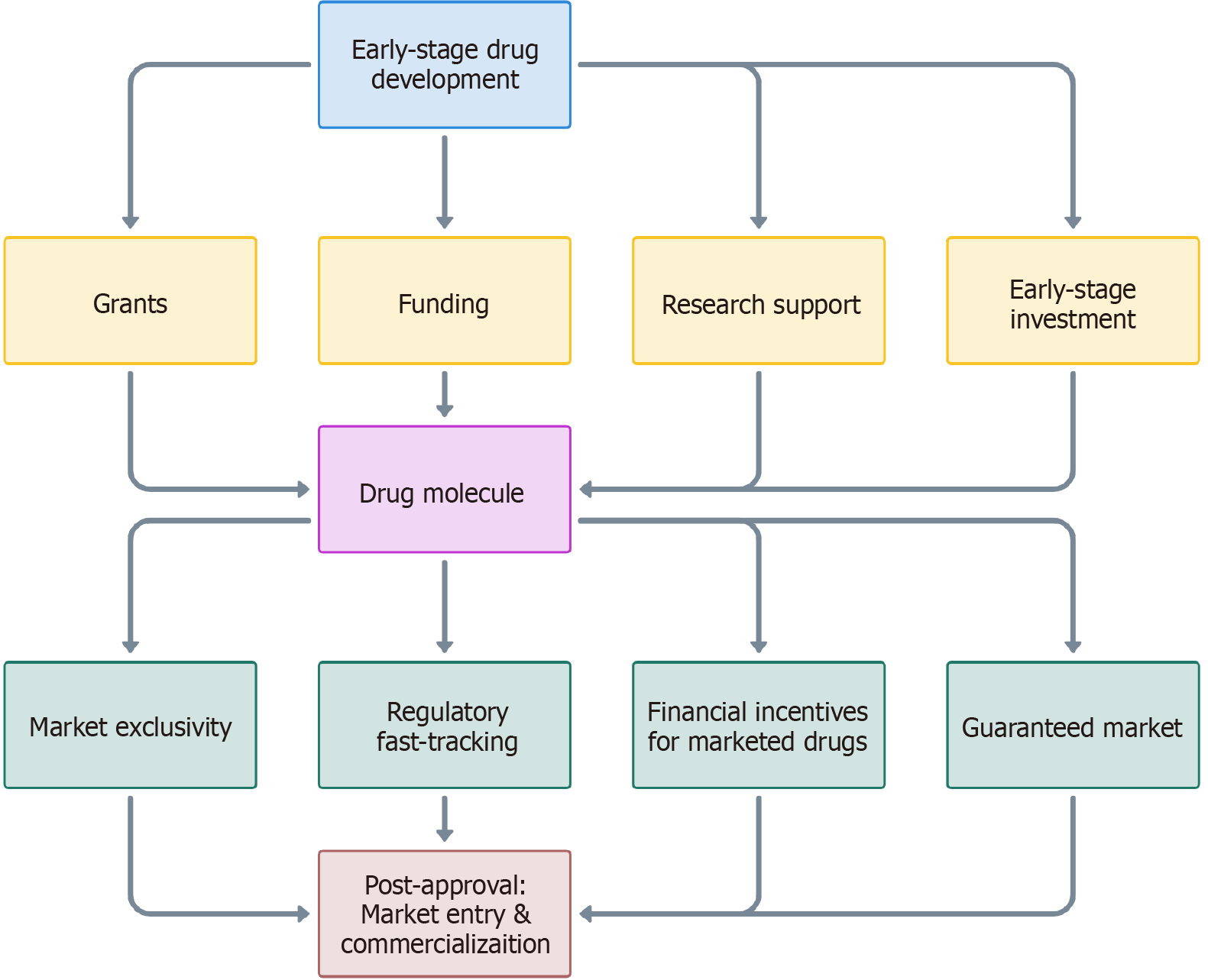
Together, these “push-pull” mechanisms create an environment that not only encourages the development of new antifungal drugs but also ensures their sustainability and accessibility post-approval. Figure 1 illustrates the journey of a drug from initial research through to market entry, highlighting the role of “push” incentives (yellow) in overcoming early-stage development challenges and “pull” incentives (green) in ensuring market viability and return on investment.
As we stand at the turning point of a new era in antifungal therapy, the question remains: Is there light at the end of the tunnel? While the development of promising new antifungal agents offers a glimmer of hope, it is crucial to temper our optimism with a realistic appraisal of the challenges ahead.
It is important to note that the success of these new agents cannot be gauged solely by their efficacy in clinical trials. Their real-world effectiveness must be rigorously evaluated through post-marketing surveillance and long-term follow-up studies, monitoring not only therapeutic outcomes but also adverse effects, drug-drug interactions, and the possible emergence of resistance. In addition, their potential clinical applications remain to be seen, as they are yet to be studied in diverse patient populations.
Moreover, the sustainability of these new antifungal therapies must be considered. While they may offer innovative mechanisms of action that circumvent existing resistance pathways, their long-term impact will be determined by their accessibility and the stewardship programs that accompany their use. Without robust antifungal stewardship programs and responsible prescribing practices, even the most promising new agents risk becoming another chapter in the story of failed therapies.
In conclusion, while the new candidates in the antifungal pipeline present innovative approaches to a complex and growing problem, they are not a panacea. They represent pieces of a much larger puzzle that includes not only drug development but also early diagnosis, comprehensive treatment plans, and global health policies. As we move forward, a multidisciplinary approach that involves clinicians, researchers, and policymakers will be crucial to fully illuminating the path ahead in the battle against fungal infections. By effectively leveraging existing incentives, we can better navigate the complexities of drug development, addressing both the pressing need for new antifungal agents and the practical realities of bringing these drugs to market. This approach not only stimulates innovation but also aligns with global health priorities, potentially transforming the landscape of antifungal therapy.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Cyprus
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Mohapatra S, India S-Editor: Zheng XM L-Editor: A P-Editor: Zhao S
| 1. | Parums DV. Editorial: The World Health Organization (WHO) Fungal Priority Pathogens List in Response to Emerging Fungal Pathogens During the COVID-19 Pandemic. Med Sci Monit. 2022;28:e939088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 2. | Bongomin F, Gago S, Oladele RO, Denning DW. Global and Multi-National Prevalence of Fungal Diseases-Estimate Precision. J Fungi (Basel). 2017;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1520] [Cited by in RCA: 1635] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 3. | Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4:165rv13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2510] [Cited by in RCA: 2974] [Article Influence: 247.8] [Reference Citation Analysis (0)] |
| 4. | Fisher MC, Alastruey-Izquierdo A, Berman J, Bicanic T, Bignell EM, Bowyer P, Bromley M, Brüggemann R, Garber G, Cornely OA, Gurr SJ, Harrison TS, Kuijper E, Rhodes J, Sheppard DC, Warris A, White PL, Xu J, Zwaan B, Verweij PE. Tackling the emerging threat of antifungal resistance to human health. Nat Rev Microbiol. 2022;20:557-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 516] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 5. | Lockhart SR, Chowdhary A, Gold JAW. The rapid emergence of antifungal-resistant human-pathogenic fungi. Nat Rev Microbiol. 2023;21:818-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 6. | McCarty TP, Pappas PG. Antifungal Pipeline. Front Cell Infect Microbiol. 2021;11:732223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Pfaller MA, Diekema DJ, Turnidge JD, Castanheira M, Jones RN. Twenty Years of the SENTRY Antifungal Surveillance Program: Results for Candida Species From 1997-2016. Open Forum Infect Dis. 2019;6:S79-S94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 521] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 8. | Du H, Bing J, Hu T, Ennis CL, Nobile CJ, Huang G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020;16:e1008921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 312] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 9. | Geremia N, Brugnaro P, Solinas M, Scarparo C, Panese S. Candida auris as an Emergent Public Health Problem: A Current Update on European Outbreaks and Cases. Healthcare (Basel). 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 10. | Benedict K, Forsberg K, Gold JAW, Baggs J, Lyman M. Candida auris‒Associated Hospitalizations, United States, 2017–2022. Emerg Infect Dis. 2023;29:1485-1487. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 26] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Chang YL, Yu SJ, Heitman J, Wellington M, Chen YL. New facets of antifungal therapy. Virulence. 2017;8:222-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 12. | Spernovasilis N, Kofteridis DP. Pre-Existing Liver Disease and Toxicity of Antifungals. J Fungi (Basel). 2018;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Bosetti D, Neofytos D. Invasive Aspergillosis and the Impact of Azole-resistance. Curr Fungal Infect Rep. 2023;1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 14. | Pristov KE, Ghannoum MA. Resistance of Candida to azoles and echinocandins worldwide. Clin Microbiol Infect. 2019;25:792-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 416] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 15. | Wiederhold NP. Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist. 2017;10:249-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 297] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 16. | Denning DW, Bromley MJ. Infectious Disease. How to bolster the antifungal pipeline. Science. 2015;347:1414-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 376] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 17. | Pappas PG, Vazquez JA, Oren I, Rahav G, Aoun M, Bulpa P, Ben-Ami R, Ferrer R, Mccarty T, Thompson GR, Schlamm H, Bien PA, Barbat SH, Wedel P, Oborska I, Tawadrous M, Hodges MR. Clinical safety and efficacy of novel antifungal, fosmanogepix, for the treatment of candidaemia: results from a Phase 2 trial. J Antimicrob Chemother. 2023;78:2471-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 18. | The Lancet Infectious Diseases. An exciting time for antifungal therapy. Lancet Infect Dis. 2023;23:763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 19. | F2G. F2G Announces Data from Phase 2b Study at ID Week 2022 Showing Positive Therapeutic Response in Patients with Invasive Fungal Infections Treated with Olorofim. [accessed 2023 Dec 11]. Available from: https://f2g.com/press-release/f2g-announces-data-from-phase-2b-study-at-id-week-2022-showing-positive-therapeutic-response-in-patients-with-invasive-fungal-infections-treated-with-olorofim/. |
| 20. | Olorofim Aspergillus Infection Study (OASIS). [accessed 2023 Dec 11]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/study/NCT05101187 ClinicalTrials.gov Identifier: NCT00417417. |
| 21. | Syed YY. Rezafungin: First Approval. Drugs. 2023;83:833-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 22. | Tillotson J, Tillotson GS. The Regulatory Pathway for Antifungal Drugs: A US Perspective. Clin Infect Dis. 2015;61 Suppl 6:S678-S683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 23. | Konsoula A, Tsioutis C, Markaki I, Papadakis M, Agouridis AP, Spernovasilis N. Lomentospora prolificans: An Emerging Opportunistic Fungal Pathogen. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Konsoula A, Agouridis AP, Markaki L, Tsioutis C, Spernovasilis N. Lomentospora prolificans Disseminated Infections: A Systematic Review of Reported Cases. Pathogens. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Reference Citation Analysis (0)] |
| 25. | Harbarth S, Theuretzbacher U, Hackett J; DRIVE-AB consortium. Antibiotic research and development: business as usual? J Antimicrob Chemother. 2015;70:1604-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Årdal C, Røttingen JA, Opalska A, Van Hengel AJ, Larsen J. Pull Incentives for Antibacterial Drug Development: An Analysis by the Transatlantic Task Force on Antimicrobial Resistance. Clin Infect Dis. 2017;65:1378-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Yasinskaya Y, Bala S, Waack U, Dixon C, Higgins K, Moore JN, Jjingo CJ, O'Shaughnessy E, Colangelo P, Botgros R, Nambiar S, Angulo D, Dane A, Chiller T, Hodges MR, Sandison T, Hope W, Walsh TJ, Pappas P, Katragkou A, Kovanda L, Rex JH, Marr KA, Ostrosky-Zeichner L, Sekine S, Deshpande M, Shukla SJ, Farley J. Food and Drug Administration Public Workshop Summary-Development Considerations of Antifungal Drugs to Address Unmet Medical Need. Clin Infect Dis. 2023;77:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |