Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2655
Revised: March 15, 2024
Accepted: April 9, 2024
Published online: May 26, 2024
Processing time: 96 Days and 1.4 Hours
High-grade B-cell lymphoma (HGBL) is an unusual malignancy that includes myelocytomatosis viral oncogene (MYC), B-cell lymphoma-2 (BCL-2), and/or BCL-6 rearrangements, termed double-hit or triple-hit lymphomas, and HGBL-not otherwise specific (HGBL-NOS), which are morphologically characteristic of HGBL but lack MYC, BCL-2, or BCL-6 rearrangements. HGBL is partially transformed by follicular lymphoma and other indolent lymphoma, with few cases of marginal zone lymphoma (MZL) transformation. HGBL often has a poor prognosis and intensive therapy is currently mainly advocated, but there is no good treatment for these patients who cannot tolerate chemotherapy.
We reported a case of MZL transformed into HGBL-NOS with TP53 mutation and terminal deoxynucleotidyl transferase expression. Gene analysis revealed the gene expression profile was identical in the pre- and post-transformed tissues, suggesting that the two diseases are homologous, not secondary tumors. The chemotherapy was ineffective and the side effect was severe, so we tried combination therapy including venetoclax and obinutuzumab. The patient tolerated treatment well, and reached partial response. The patient had recurrence of hepatocellular carcinoma and died of multifunctional organ failure. He survived for 12 months after diagnosis.
Venetoclax combined with obinutuzumab might improve the survival in some HGBL patients, who are unsuitable for chemotherapy.
Core Tip: Marginal zone lymphoma (MZL) are a group of B-cell malignant neoplastic diseases with the most common transformation to diffuse large B-cell lymphoma. Herein, we report one rare case of MZL converted to high-grade B-cell lymphoma, not otherwise specific (HGBL-NOS) and concomitant terminal deoxynucleotidyl transferase expression in a 63-year-old male for the first time. HGBL-NOS often has a poor prognosis. The current treatment advocates high-dose chemotherapy, but there is currently no effective treatment for patients who cannot tolerate chemotherapy. We have attempted targeted pharmacological combinations and achieved certain therapeutic effects, which may provide new treatment for these patients.
- Citation: Fan ZM, Wu DL, Xu NW, Ye L, Yan LP, Li LJ, Zhang JY. Transformation of marginal zone lymphoma into high-grade B-cell lymphoma expressing terminal deoxynucleotidyl transferase: A case report. World J Clin Cases 2024; 12(15): 2655-2663
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2655.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2655
Marginal zone lymphomas (MZLs) are a group of B-cell indolent malignant diseases with the possibility of transformation to aggressive lymphoma, often to diffuse large B-cell lymphomas (DLBCLs)[1]. MYC rearrangements have been detected in 5%-15% of DLBCLs, often with mutations in BCL-2 and/or BCL-6, which is defined as high-grade B-cell lymphoma (HGBL) according to the 2016 World Health Organization classification[2,3]. In HGBL cases, the MYC rearrangement is termed a double attack when it is mutated with BCL-2 or BCL-6 and a triple attack when it is mutated with BCL-2 and BCL-6, in addition to a specific subtype called HGBL-not otherwise specific (HGBL-NOS), which is morphologically characterized as a HGBL but lacks the genetic appearance of the MYC, BCL-2, or BCL-6 rearrangement[1]. Terminal deoxynucleotidyl transferase (TDT) is often considered a marker for immature cells and is of importance in the diagnosis of B-lymphoblastoma/leukemia. However, in recent reports, a small proportion of HGBL cases were positive for TDT[4]. We recently identified a patient with MZL who converted to HGBL-NOS and concomitant TDT expression. To our knowledge, this is the first case of HGBL-NOS from MZL.
A 63-year-old man came to the hospital because of bilateral swollen lymph nodes.
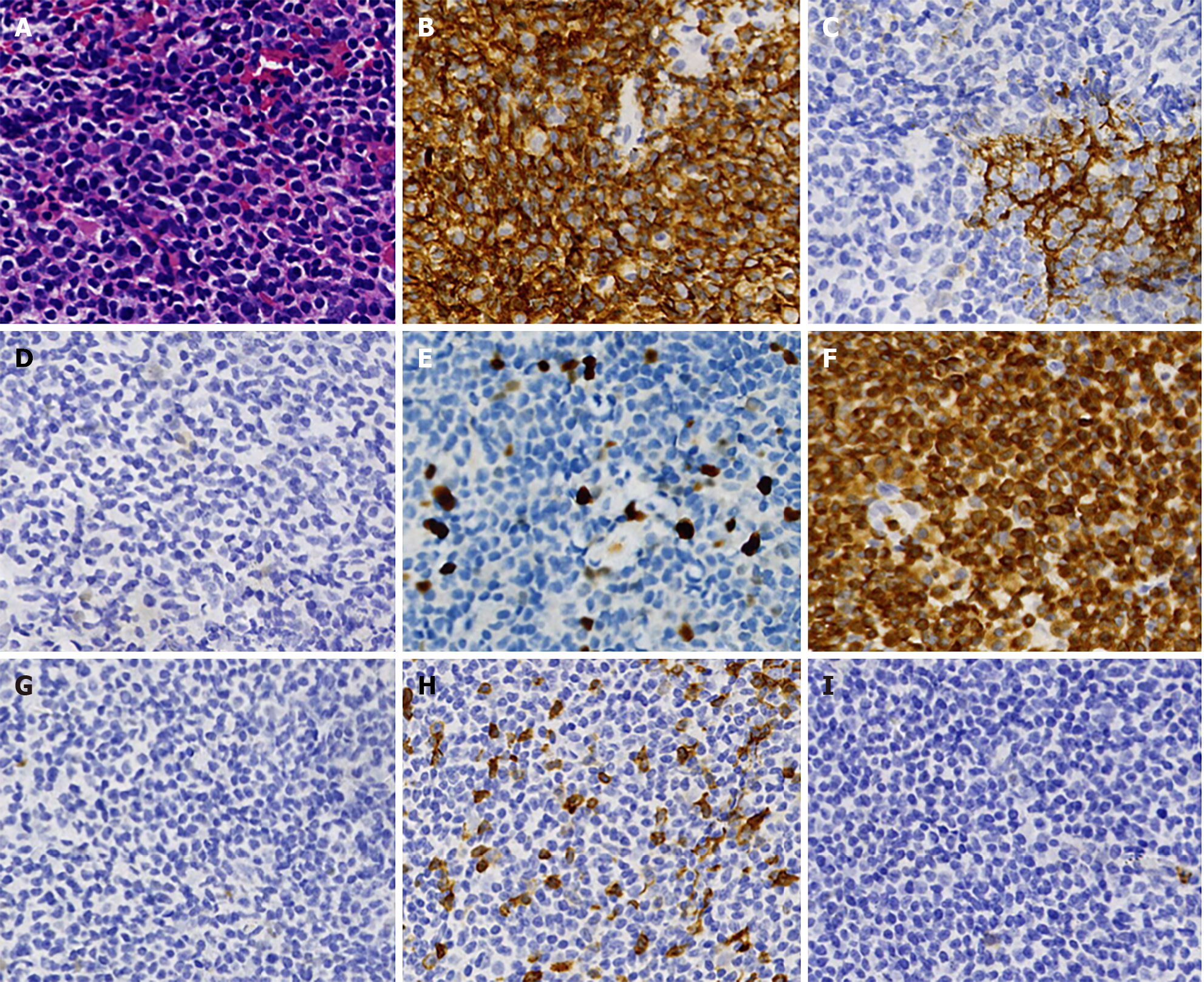
In April 2020, the patient received a pathological examination performed on a right cervical lymph node biopsy and immunohistochemical examination revealed that the infiltrating cells were positive for cluster of differentiation 20 (CD20), paired box 5 (PAX5), BCL-2, CD21, CD23, and Ki-67 staining of 10%, and were negative for CD10, BCL-6, CyclinD1, sry-related HMG-box 11 (SOX-11), CD3, and CD5 (Figure 1). The involvement of bone marrow was not found. Positron emission tomography (PET)-computed tomography (CT) scan demonstrated mildly enlarged bilateral cervical lymph nodes (SUVmax: 5.4), and cirrhosis.The patient was diagnosed with MZL (Ann Arbor stage IIA, IPI 1 score). The patient refused radiation therapy, so we gave him 2 cycles of rituximab, cyclophosphamide, doxorubicin, vindesine and prednisone (R-CHOP). The enlarged lymph nodes disappeared after 2 cycles; however, the toxicity of chemotherapy was severe, so we reduced the dose of the drug (R-minCHOP). The patient reached complete response after 2 cycles, then he stopped chemotherapy, and the disease was stable during the follow-up period.
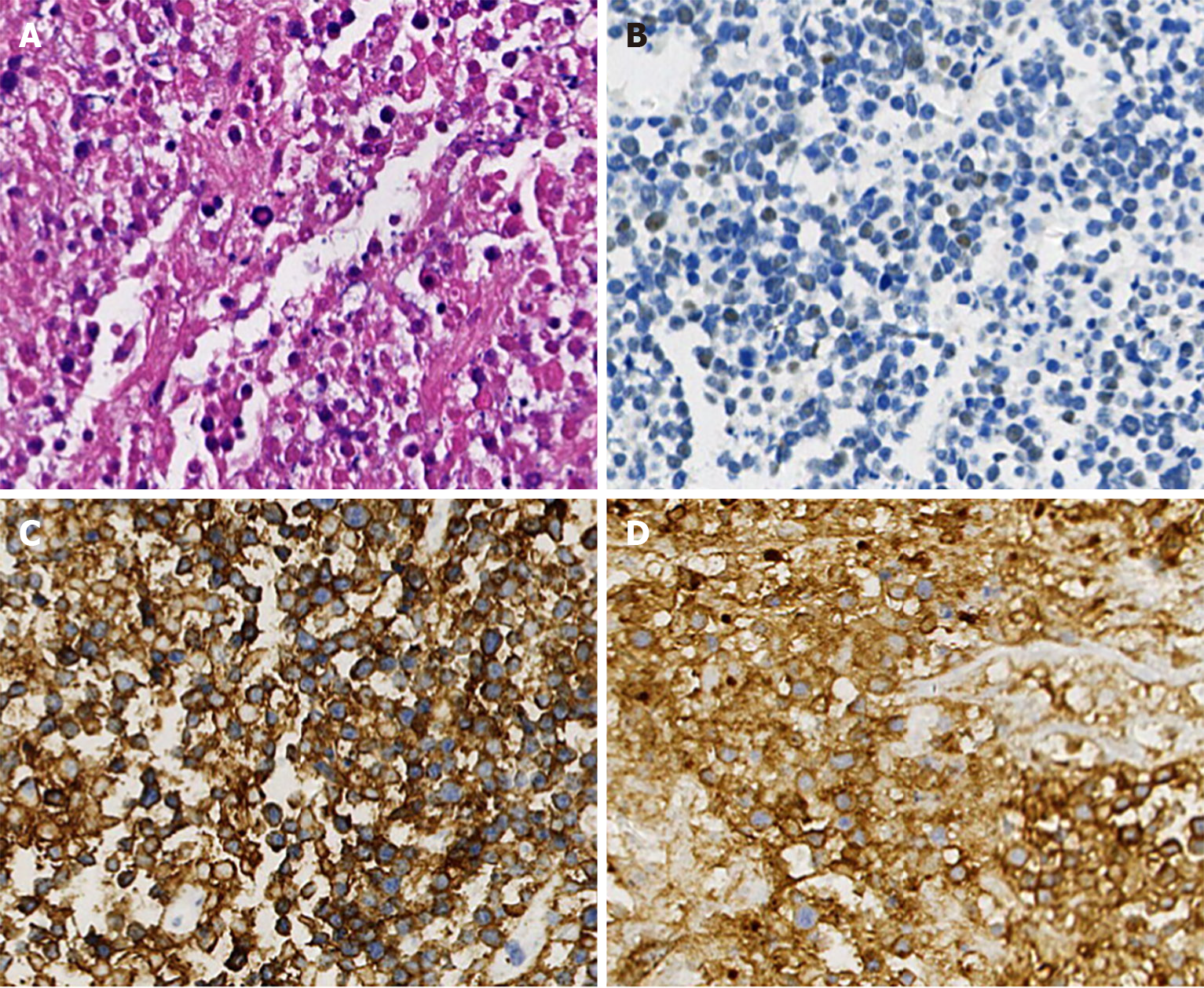
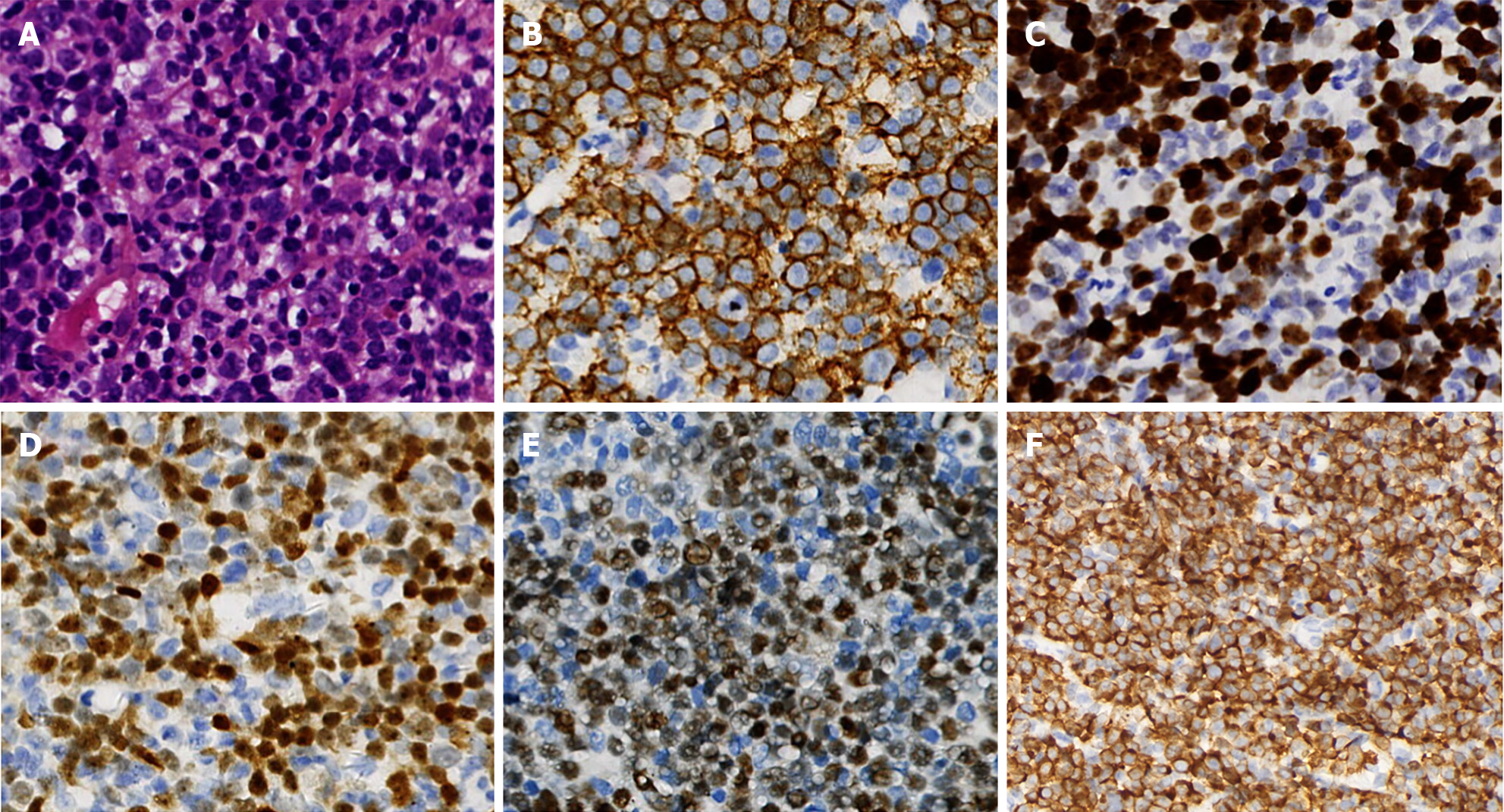
In April 2022, new lesions appeared, with notably enlarged lymph nodes on the right side of the neck. The patient then received a lymph node biopsy again. The lymph node was replaced by diffuse medium-to small tumor cells, with multifocal, and massive necrosis. Immunohistochemical examination showed that the cells were positive for CD20, BCL-2, CD10, and CD5, partially expressing TDT, but negative for BCL-6, CyclinD1, SOX-11, CD3, PAX5, CD21, and Ki-67 staining of approximately 30% (Figure 2). Therefore, the pathologic diagnosis of MZL with the possibility of transformation into HGBL was given. The PET-CT scans demonstrated that partial necrosis and multiple enlarged lymph nodes were observed on the right side of the cervix, with a large diameter of 2.2 cm (SUVmax: 7.6), and an enlarged lymph node in the right anterior aspect of the new ascending aorta, with a diameter of 0.9 cm (SUVmax: 7.2). Moreover, there was a new nodule in the liver measuring 2.9 cm × 2.0 cm (SUVmax 2.4), which was considered a recurrence of liver cancer. After the patient underwent hepatic artery chemoembolization and radiofrequency ablation of hepatocellular carcinoma, he received ultrasound-guided coarse-needle aspiration in the right cervical mass. Immunohistochemical staining showed that the neoplastic cells were positive for CD20, PAX5, CD5, TDT, BCL-2 (approximately 90%), CD10, C-MYC, CD7, CD43, MUM1, CD99 and Ki-67 staining of approximately 80% but negative for BCL-6, myeloperoxidase, CD34, CyclinD1, CD21, and CD3 (Figure 3). The CT scan showed the mass on the right side of the neck, measuring 6.4 cm × 4.0 cm.
The patient had a history of liver cancer and underwent surgical treatment in 2010.
The patient had no remarkable personal or family history.
Physical examination showed bilateral mildly enlarged lymph nodes in the neck.
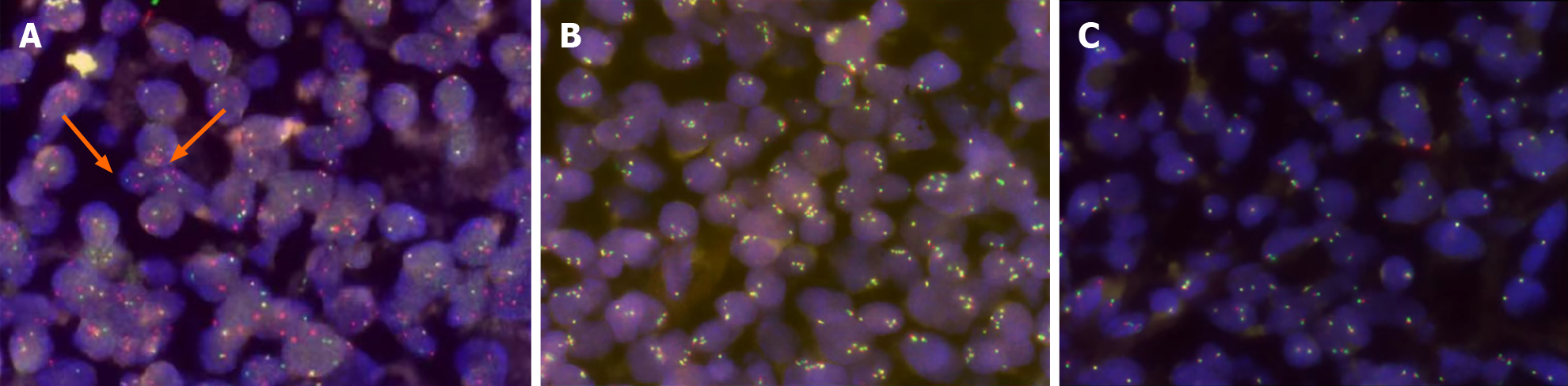
The involvement of bone marrow was not found. Routine laboratory tests, including peripheral blood cells, lactic dehydrogenase (168 U/L), liver, and renal function, were normal and the virus tests consisted of viral hepatitis B and epstein-barr virus were negative. The reexamination of bone marrow and routine laboratory tests were still normal, including lactate dehydrogenase (LDH) (195 U/L). Fluorescence in situ hybridization (FISH) analysis revealed positivity for the MYC gene but negativity for BCL-2 and BCL-6 (Figure 4). MYC/immunoglobulin heavy chain (IGH) rearran
In April 2020, a PET-CT scan demonstrated mildly enlarged bilateral cervical lymph nodes (SUVmax: 5.4), and cirrhosis.
In April 2022, a follow-up PET-CT scan showed that partial necrosis and multiple enlarged lymph nodes were observed on the right side of the cervix, with a large diameter of 2.2 cm (SUVmax: 7.6), and an enlarged lymph node in the right anterior aspect of the new ascending aorta, with a diameter of 0.9 cm (SUVmax: 7.2). Moreover, there was a new nodule in the liver measuring 2.9 cm × 2.0 cm (SUVmax: 2.4), which was considered a recurrence of liver cancer. And 2 months later, the CT scan showed the mass on the right side of the neck, measuring 6.4 cm × 4.0 cm.
Based on histological, immunohistochemical, and consultation findings, a final diagnosis of MYC-rearranged HGBL, NOS, stage II, and IPI2 with TDT expression was made.
We first diagnosed the patient as having B-lymphoblastic lymphoma because the atypical cells were positive for TDT and CD99. The tumor had been rapidly enlarging, so treatment was started immediately under a tentative diagnosis. The patient started on a combination of Gemox (oxaliplatin and gemcitabine) with lenalidomide, which was not effective. Then we changed it into BTK inhibitor (zanubrutinib), but the regimen was still ineffective. FISH analysis revealed positivity for the MYC gene but negativity for BCL-2 and BCL-6 (Figure 4). MYC/IGH rearrangement was confirmed by FISH examination. Due to the diagnostic dilemma, we undertook a pathology consultation with Gandi Li, Professor of Pathology at West China Hospital. Based on histological, immunohistochemical, and consultation findings, a final diagnosis of MYC rearranged HGBL, NOS, stage II, and IPI2 with TDT expression was made.
Subsequently, we given him a modified Hyper CVAD A regimen (cyclophosphamide, vincristine, mitoxantrone liposomal and dexamethasone) combined with obinutuzumab, when the diagnosis of HGBL-NOS was made. After two cycles of treatment, the cervical enhanced CT found the lesion was markedly reduced, measuring 1.7 cm × 3.3 cm. However, our patient developed myelosuppression followed severe infections and liver dysfunction. Depending on the previous response to therapy and drug-related toxicity we thought that conventional chemotherapy was inadequet. Therefore, we attempted to treat our patient with targeted combination therapies, and a new induction with obinutuzumab and venclexta was given.
Fortunately, the cervical mass disappeared after 2 cycles, and a partial response was achieved. The patient experienced no serious complications. Therefore, the treatment was continued, although the arch nodes of the ascending aorta were not reduced but were also in stable condition. However, the CT scan of the abdomen showed multiple lesions in the liver 6 months later. The patient also had fever, abdominal effusion, and pancytopenia, and we had to stop the therapy because of the severe situation. He was transferred to a local hospital for palliative care and died of multivisceral failure a few weeks later.
MZLs have the possibility of transformation to aggressive lymphoma, often to DLBCL. Conconi et al[5] retrospectively analyzed the clinical data of 373 patients with MZL, and the rate of pathological tissue transformation was 4%, of which 12 converted to DLBCL. Clinical characteristics at the time of initial MZL diagnosis that have been associated with the development of a transformation are elevated levels of LDH, advanced (III-IV) Ann Arbor stage, involvement of more than four nodal sites, a high follicular lymphoma international prognostic index score, history of cancer, and failure to achieve complete remission after first-line treatment[6]. The mechanism of transformation was still unclear. Some finds showed that KMT2D/MLL2, TP53, NOTCH2, and TRAF3 mutations may be associated with an increased risk of transformation[7]. In our patient, liver cancer and a history of chemotherapy, TP53 mutations, may confer a high translational risk and poor prognosis.
HGBL is an uncommonly aggressive B-cell malignancy that includes double-hit lymphoma (DHL), triple-hit lymphoma (THL) and HGBL-NOS[1]. HGBL-NOS are rare and heterogeneous, including tumors with Burkitt-like or blastoid morphology. However, there is some controversy regarding the diagnosis of this subtype, and the criteria for diagnosis are as follows: (1) Invasive mature B-cell lymphoma composed of medium-sized or blastocyte-like cells; and (2) Exclusion of other specific types of lymphoma[8]. Accurate classification requires evaluation by cytomorphology, immunohistochemistry, and FISH. In a pathologic study, they found that CD10, BCL-6 and BCL-2 were expressed in cases[9]. Another study described that among confirmed HGBLs, MYC-R was present in 46%, and the most common mutations involved KMT2D and TP53[10]. The Burkitt-like variant of HGBL-NOS was morphologically indistinguishable from BL but was negative for MYC rearrangement[9]. Blastoid HGBL, NOS, needs to be distinguished from B-lymphoblastic lymphoma, which always expresses TDT or CD34, whereas the emergence of MYC or BCL-2 rearrangement is rare[11]. TDT is considered an immature marker that can be expressed in both T-cell and B-cell malignancies[12]. We first diagnosed our patient as having B-cell lymphoblastic lymphoma (B-LBL). However, B-LBL often occurs preferentially among young individuals under 18 years of age[13], and the expression of CD20 is negative in most cases[14]. Depending on the clinical features of our patient, we consider HGBL-NOS to be a more appropriate diagnosis for the patient. Reports that TDT is expressed in HGBL are not uncommon, often in the patients with DHL or THL[15]. Some cases were transformations from follicular lymphoma or other indolent B-cell lymphomas, but cases of HGBL-NOS with TDT expression have not been described.
The traditional view is that HGBL has a poor prognosis, particularly in patients with DHL or THL. Currently, there are no reliable data on prognostic factors specific to HGBL-NOS. A study of 2383 DLBCLs found no significant difference in outcomes between DLBCL with or without a single-hit MYC-R[16]. Some neoplasms were diagnosed as DLBCL having striking biological similarities to HGBL, which are called molecular high-grade lymphomas. And the response to the R-CHOP regimen was poor, and the 3-year progression-free survival (PFS) was only 37% after treatment[17]. TP53 alteration is always considered an independent adverse prognostic factor[18]. HGBL, NOS, with concurrent MYC-R and TP53 alterations (deletion or mutation), often had complex karyotypes and dismal outcomes, which might further expand the molecular HGBL category[19].
As the first-line treatment, R-CHOP is still considered the preferred regimen for patients with early/limited-stage HGBL-NOS. The controversy of treatment in HGBL-NOS is whether intensified regimens are necessary. A study from Chinese cases showed that only 43% of patients achieved CR, and the disease relapsed soon after chemotherapy with R-CHOP. The median overall survival (OS) was 9 months, and there was no difference between DHL and MYC-R status[17]. These data suggest that R-CHOP may not be suitable for patients with advanced-stage HGBL, NOS, DHL or high-risk DLBCL, and we favor high-dose chemotherapy regimens. A study of patients with central nervous system involvement who received treatment with rituximab, cyclophosphamide, vincristine, doxorubicin, high-dose methotrexate/isocyclophosphamide, etoposide, and high-dose cytarabine showed that the 2-year PFS was 68%. However, toxicities were obvious, with grade 4 neutropenia in 88%[20]. Therefore, it is believed that high-dose chemotherapy may be appropriate for younger patients and in better general condition. However, in a recent retrospective analysis, only patients with advanced stage disease had significantly improved PFS with an intensified regimen, but the impact of a prolonged OS was small[21]. Therefore, further exploration of appropriate therapeutic options is still warranted. A trial of chimeric antigen receptor-T-cell therapy in high-risk DLBCLs, showed encouraging results (12-month OS 91%)[22]. Meanwhile, some researchers believe that combination therapy with targeted drugs can be attempted in these unfit patients[23]. A multicenter study of venetoclax in combination with bendamustine-rituximab in patients with relapsed/refractory non-Hodgkin’s lymphoma showed that the regimen is superior in the treatment of aggressive lymphoma compared with indolent lymphoma, suggesting that the efficacy of venetoclax may be related to the expression of BCL-2 in different subtypes[24].
In the case reported here, the patient converted from MZL to HGBL after repeated chemotherapy and was unable to tolerate high-intensity chemotherapy and hematopoietic stem cell transplantation. There are no good treatments for these patients. In conjunction with the data from previous studies, we initiated treatment with venetoclax in combination with obinutuzumab based on pathological immunohistochemical BCL-2 overexpression and CD20 positivity. The patient achieved a partial response, and chemotherapy was tolerable. Although the patient eventually died, we still believed that venetoclax may have the opportunity to improve survival in some patients with HGBL, especially in those with high BCL-2 expression. However,additional data are needed to determine the safety and efficacy of combination therapy between targeted drugs.
The authors would like to thank the patient and families for their cooperation in this study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Kawabata H, Japan S-Editor: Zheng XM L-Editor: A P-Editor: Yu HG
| 1. | Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4245] [Cited by in RCA: 5427] [Article Influence: 603.0] [Reference Citation Analysis (0)] |
| 2. | Niitsu N, Okamoto M, Miura I, Hirano M. Clinical features and prognosis of de novo diffuse large B-cell lymphoma with t(14;18) and 8q24/c-MYC translocations. Leukemia. 2009;23:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 176] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Snuderl M, Kolman OK, Chen YB, Hsu JJ, Ackerman AM, Dal Cin P, Ferry JA, Harris NL, Hasserjian RP, Zukerberg LR, Abramson JS, Hochberg EP, Lee H, Lee AI, Toomey CE, Sohani AR. B-cell lymphomas with concurrent IGH-BCL2 and MYC rearrangements are aggressive neoplasms with clinical and pathologic features distinct from Burkitt lymphoma and diffuse large B-cell lymphoma. Am J Surg Pathol. 2010;34:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 4. | Moench L, Sachs Z, Aasen G, Dolan M, Dayton V, Courville EL. Double- and triple-hit lymphomas can present with features suggestive of immaturity, including TdT expression, and create diagnostic challenges. Leuk Lymphoma. 2016;57:2626-2635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Conconi A, Franceschetti S, Aprile von Hohenstaufen K, Margiotta-Casaluci G, Stathis A, Moccia AA, Bertoni F, Ramponi A, Mazzucchelli L, Cavalli F, Gaidano G, Zucca E. Histologic transformation in marginal zone lymphomas†. Ann Oncol. 2015;26:2329-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Alderuccio JP, Zhao W, Desai A, Gallastegui N, Ramdial J, Kimble E, de la Fuente MI, Rosenblatt JD, Chapman JR, Vega F, Reis IM, Lossos IS. Risk Factors for Transformation to Higher-Grade Lymphoma and Its Impact on Survival in a Large Cohort of Patients With Marginal Zone Lymphoma From a Single Institution. J Clin Oncol. 2018;JCO1800138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Onaindia A, Medeiros LJ, Patel KP. Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod Pathol. 2017;30:1338-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Olszewski AJ, Kurt H, Evens AM. Defining and treating high-grade B-cell lymphoma, NOS. Blood. 2022;140:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Hüttl KS, Staiger AM, Richter J, Ott MM, Kalmbach S, Klapper W, Biesdorf AS, Trümper L, Rosenwald A, Ziepert M, Horn H, Ott G. The "Burkitt-like" immunophenotype and genotype is rarely encountered in diffuse large B cell lymphoma and high-grade B cell lymphoma, NOS. Virchows Arch. 2021;479:575-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Collinge BJ, Hilton LK, Wong J, Ben-Neriah S, Rushton CK, Slack GW, Farinha P, Cook JR, Ott G, Rosenwald A, Campo E, Amador C, Greiner TC, Raess PW, Song JY, Inghirami G, Jaffe ES, Weisenburger DD, Chan WC, Holte H, Beiske K, Fu K, Delabie J, Pittaluga S, Feldman AL, Savage KJ, Mungall AJ, Staudt LM, Steidl C, Rimsza LM, Morin RD, Scott DW. Characterization of the genetic landscape of high-grade B-cell lymphoma, NOS – an llmpp project. Hematol Oncol. 2021;39:157-159. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Moore EM, Aggarwal N, Surti U, Swerdlow SH. Further Exploration of the Complexities of Large B-Cell Lymphomas With MYC Abnormalities and the Importance of a Blastoid Morphology. Am J Surg Pathol. 2017;41:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Jaffe ES, Campo E, Harris NL, Pileri SA, Stein H, Swerdlow SH. Introduction and overview of the classification of lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC Press, 2017: 190-198. |
| 13. | Borowitz MJ, Chan JKC, Downing JR, Le Beau MM, Arber DA. B-lymphoblastic leukemia/Lymphoma, not otherwise specified (NOS). In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R, editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. Lyon: IARC Press, 2017: 200-202. |
| 14. | Bassan R, Maino E, Cortelazzo S. Lymphoblastic lymphoma: an updated review on biology, diagnosis, and treatment. Eur J Haematol. 2016;96:447-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Ok CY, Medeiros LJ, Thakral B, Tang G, Jain N, Jabbour E, Pierce SA, Konoplev S. High-grade B-cell lymphomas with TdT expression: a diagnostic and classification dilemma. Mod Pathol. 2019;32:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Rosenwald A, Bens S, Advani R, Barrans S, Copie-Bergman C, Elsensohn MH, Natkunam Y, Calaminici M, Sander B, Baia M, Smith A, Painter D, Pham L, Zhao S, Ziepert M, Jordanova ES, Molina TJ, Kersten MJ, Kimby E, Klapper W, Raemaekers J, Schmitz N, Jardin F, Stevens WBC, Hoster E, Hagenbeek A, Gribben JG, Siebert R, Gascoyne RD, Scott DW, Gaulard P, Salles G, Burton C, de Jong D, Sehn LH, Maucort-Boulch D. Prognostic Significance of MYC Rearrangement and Translocation Partner in Diffuse Large B-Cell Lymphoma: A Study by the Lunenburg Lymphoma Biomarker Consortium. J Clin Oncol. 2019;37:3359-3368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 17. | Li J, Liu X, Yao Z, Zhang M. High-Grade B-Cell Lymphomas, Not Otherwise Specified: A Study of 41 Cases. Cancer Manag Res. 2020;12:1903-1912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Song JY, Perry AM, Herrera AF, Chen L, Skrabek P, Nasr MR, Ottesen RA, Nikowitz J, Bedell V, Murata-Collins J, Li Y, McCarthy C, Pillai R, Wang J, Wu X, Zain J, Popplewell L, Kwak LW, Nademanee AP, Niland JC, Scott DW, Gong Q, Chan WC, Weisenburger DD. Double-hit Signature with TP53 Abnormalities Predicts Poor Survival in Patients with Germinal Center Type Diffuse Large B-cell Lymphoma Treated with R-CHOP. Clin Cancer Res. 2021;27:1671-1680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Deng M, Xu-Monette ZY, Pham LV, Wang X, Tzankov A, Fang X, Zhu F, Visco C, Bhagat G, Dybkaer K, Chiu A, Tam W, Zu Y, Hsi ED, You H, Huh J, Ponzoni M, Ferreri AJM, Møller MB, Parsons BM, Hagemeister F, van Krieken JH, Piris MA, Winter JN, Li Y, Xu B, Liu P, Young KH. Aggressive B-cell Lymphoma with MYC/TP53 Dual Alterations Displays Distinct Clinicopathobiological Features and Response to Novel Targeted Agents. Mol Cancer Res. 2021;19:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | McMillan AK, Phillips EH, Kirkwood AA, Barrans S, Burton C, Rule S, Patmore R, Pettengell R, Ardeshna KM, Lawrie A, Montoto S, Paneesha S, Clifton-Hadley L, Linch DC. Favourable outcomes for high-risk diffuse large B-cell lymphoma (IPI 3-5) treated with front-line R-CODOX-M/R-IVAC chemotherapy: results of a phase 2 UK NCRI trial. Ann Oncol. 2020;31:1251-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 21. | Zeremski V, McPhail ED, Habermann TM, Schieppati F, Gebauer N, Vassilakopoulos TP, Mougiakakos D. Treatment intensification might not improve survival in high-grade B-cell lymphoma with a concurrent MYC and BCL2 and/or BCL6 rearrangement: A retrospective, multicenter, pooled analysis. Hematol Oncol. 2023;41:776-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Neelapu SS, Dickinson M, Munoz J, Ulrickson ML, Thieblemont C, Oluwole OO, Herrera AF, Ujjani CS, Lin Y, Riedell PA, Kekre N, de Vos S, Lui C, Milletti F, Dong J, Xu H, Chavez JC. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: the phase 2 ZUMA-12 trial. Nat Med. 2022;28:735-742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 181] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 23. | Hamadani M, Radford J, Carlo-Stella C, Caimi PF, Reid E, O'Connor OA, Feingold JM, Ardeshna KM, Townsend W, Solh M, Heffner LT, Ungar D, Wang L, Boni J, Havenith K, Qin Y, Kahl BS. Final results of a phase 1 study of loncastuximab tesirine in relapsed/refractory B-cell non-Hodgkin lymphoma. Blood. 2021;137:2634-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 131] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 24. | de Vos S, Swinnen LJ, Wang D, Reid E, Fowler N, Cordero J, Dunbar M, Enschede SH, Nolan C, Petrich AM, Ross JA, Salem AH, Verdugo M, Agarwal S, Zhou L, Kozloff M, Nastoupil LJ, Flowers CR. Venetoclax, bendamustine, and rituximab in patients with relapsed or refractory NHL: a phase Ib dose-finding study. Ann Oncol. 2018;29:1932-1938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |