Published online May 26, 2024. doi: 10.12998/wjcc.v12.i15.2636
Revised: February 14, 2024
Accepted: April 7, 2024
Published online: May 26, 2024
Processing time: 104 Days and 22.3 Hours
Brain metastases (BM) are very rare in gastric adenocarcinoma (GaC), and patients with BMs have a higher mortality rate due to stronger tumor aggressiveness. However, its pathogenesis remains unclear. Genetic testing revealed cellular-mesenchymal epithelial transition factor receptor (MET) amplification. Therefore, treatment with savolitinib, a small molecule inhibitor of c-Met, was selected.
A 66-year-old woman was diagnosed with advanced GaC 6 months prior to presentation due to back pain. Cerebellar and meningeal metastases were ob
BM is very rare in advanced GaC, especially in meningeal cancer, that is characterized by rapid disease deterioration. There are very few effective treatment options available; however, technological breakthroughs in genomics have provided a basis for personalized treatment. Furthermore, MET amplification may be a key driver of BM in gastric cancer; however, this conclusion requires further investigation.
Core Tip: Common metastatic sites for gastric adenocarcinoma (GaC) occur in the lymph nodes, liver, and lungs. Additionally, brain metastases (BM) are rare. Interestingly, mesenchymal epithelial transition factor receptor (MET) amplification in GaC is rare, and its role in tumor metastasis remains unclear. Several previous reports have described the association between MET amplification and BM with GaC. This case highlights the unique phenomenon of rapid BM in advanced GaC after first-line treatment and the rapid short-term reduction of cerebellar metastases after the use of savolitinib. Therefore, it is hypothesized that MET amplification may be a key driver of BMs in GaC.
- Citation: Weng ZY, Huang WY, Shi BK, Pan JJ. Role of savolitinib in advanced gastric adenocarcinoma with meningeal carcinomatosis and cerebellar metastasis: A case report. World J Clin Cases 2024; 12(15): 2636-2641
- URL: https://www.wjgnet.com/2307-8960/full/v12/i15/2636.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i15.2636
The 2020 Global Cancer Statistics show that gastric cancer ranks fifth in global cancer incidence, with a prevalence of approximately 5.6%[1]. Advanced gastric adenocarcinoma (GaC) is the most predominant pathological type of gastric cancer, and is a multifactorial and heterogeneous disease. Approximately 1/3 of GaC patients have distant metastasis at the time of diagnosis; these patients have higher mortality rates due to stronger tumor aggressiveness[2]. Common metastatic sites of GaC include the peritoneum, lymph nodes, liver, and lungs. However, only 1%-2% of advanced GaC patients develop intracranial metastases and meningeal carcinomatosis (MC)[3].
A patient with advanced GaC harboring mesenchymal epithelial transition factor receptor (MET) amplification, intracranial metastases, and MC treated with savolitinib tablets is presented in this case study. The patient demonstrated rapid shrinkage of intracranial tumors in the short term.
A 66-year-old female presented to the emergency department due to frequent generalized seizures occurring within a week and persistent drowsiness.
In November 2023, the patient developed dizziness, headache, walking instability, tinnitus, and hearing loss. A head magnetic resonance imaging (MRI) indicated meningeal thickening and occupation of the cerebellum and occipital bone. The patient underwent lumbar puncture, which revealed an increase in cerebrospinal fluid (CSF) pressure of > 400 mm H2O, indicating a rapid disease progression, seizures developed more gradually.
In November 2022, the patient underwent gastroscopy with no abnormality. In May 2023, the patient was treated at another hospital for lumbar pain, and lumbar MRI was performed. Abnormal vertebral signals were considered for tumor metastasis. After further examination by positron emission tomography/computed tomography and gastroscopy, the patient was diagnosed with advanced GaC with multiple metastases to the abdominal lymph nodes and lumbar vertebrae. In June 2023, the patient was treated with the regimen of Candonilimab + oxaliplatin and capecitabine (CAPEOX). After two courses of treatment, the patient showed elevated troponin and aminotransferase levels. Considering the adverse immune reactions, the above drugs were discontinued and gamma globulin and methylprednisolone were administered to suppress the immune system. In September 2023, the patient was administered another two courses of CAPEOX chemotherapy because of disease progression.
The patient denied any family history of tumors or other notable medical history.
The patient’s vital signs were as follows: Body temperature, 38 °C; blood pressure, 170/94 mmHg; heart rate, 95 beats/min; respiratory rate, 18 breaths/min. When receiving treatment, the patient was in a shallow coma, with left conjunctival congestion, and she demonstrated signs of mydriasis.
Laboratory investigations revealed normocytic anemia (hemoglobin, 66 g/L), elevated leukocyte (14 × 109/L), and elevated C-reactive protein (28.48 mg/L). Tumor markers, including carcinoembryonic antigen (56.2 ng/mL) and carbohydrate antigen 199 (139.4 U/mL), were above the reference range. Genetic testing of CSF and peripheral blood revealed an increased MET copy number and genetic mutations at the TP53p.R213 Locus.
Gastric mucosal pathology: Less differentiated adenocarcinoma, differentiated grades II–III, and mixed type Lauren classification.
The immunohistochemical results of the Gastric mucosal are as follows: Claudin18.2 (40% +); Her-2 (0); Met (90%); MLH (+); MSH2 (+); MSH6 (+); programmed death 1 (tumor-, interstitial 5% +); programmed death-ligand 1 (PD-L1) (tumor-, interstitial 5% +); PMS (+); TROP2 (100% +++); Ki67 (90% +); EBER (-).
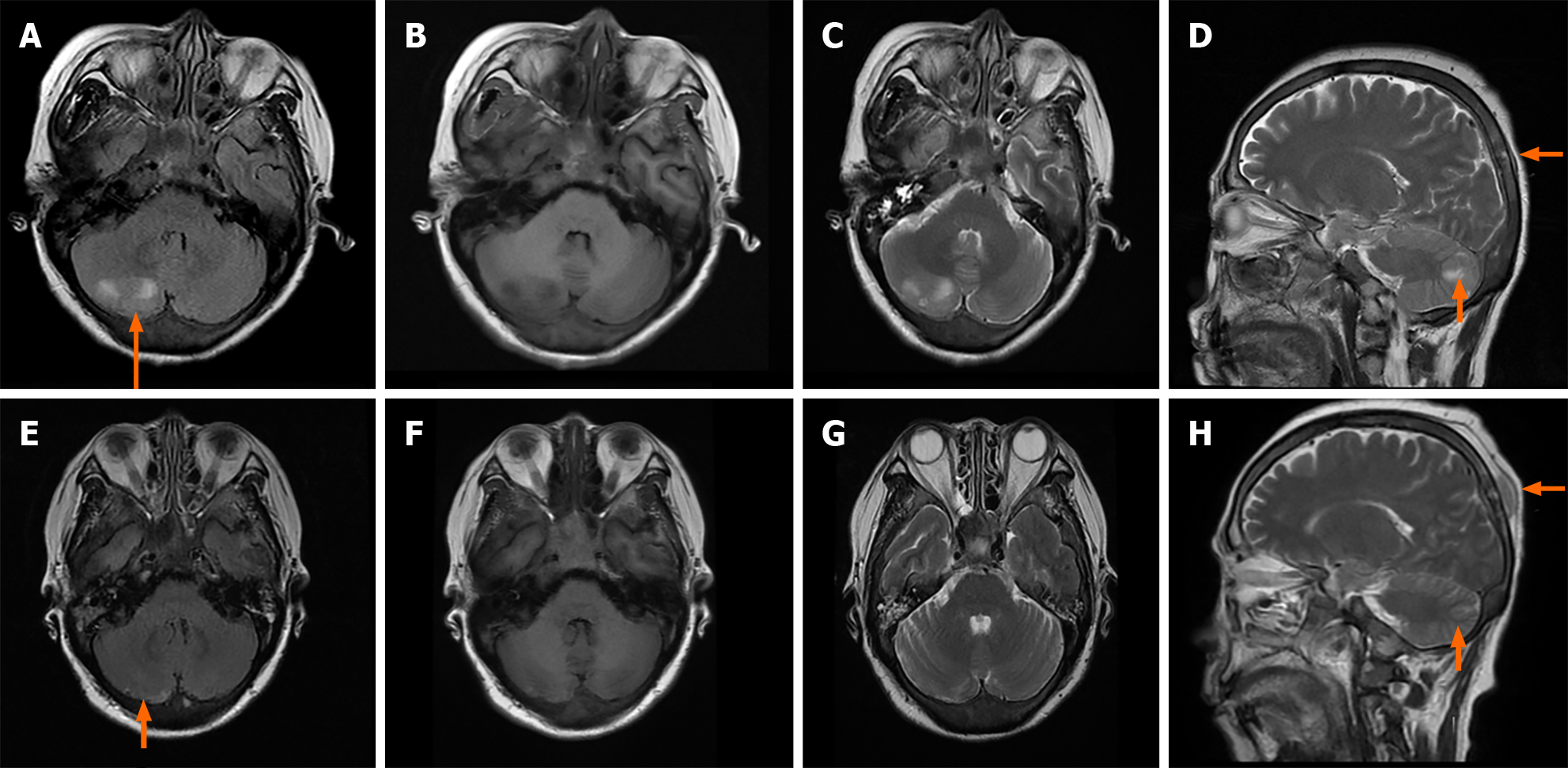
MRI revealed thickening of the meninges and abnormal diffuse signals in the skull. A mass on the right cerebellar showed mixed-signal intensity. In December 2023 there was a decrease in the lesions, but the top right nodules increased (Figure 1).
The GaC invaded the surrounding tissues and caused pelvic implant metastasis accompanied by multiple metastases to the bone, liver, cerebellum, and meninges. The patient was diagnosed with GaC (cT4NxM1 IV) and was comatose at the time of admission.
Considering the patient's poor health and comatose state with intracranial metastases and MC, the tumor was rapidly transferred after chemotherapy. After discussion, savolitinib tablets were administered to treat this patient.
After 4 d of oral administration of savolitinib, the patient regained autonomous consciousness and her lower limb muscle strength returned to grade IV after 2 wk. After one month of treatment, with the help of her family, she was able to walk normally. During hospitalization, MRI was used to examine the patient's brain, and the results showed that the cerebellar tumor had shrunk significantly, and the CSF pressure had returned to normal levels. After one month of treatment with savolitinib and a small-molecule MET kinase inhibitor, the patient's brain tumor was rapidly controlled in the short term, extending the patient's survival time.
Three major biomarkers, namely, HER2, PD-L1, and deficient mismatch repair/microsatellite instability-high group (dMMR/MSI-H), have been identified in advanced GaC to guide the main pathway of treatment decisions, and anti-angiogenesis large and small molecule targeting drugs can also be considered as second-line and above treatment. The accurate understanding of GaC has improved substantially over time, with considerable advancements made in treatment methods using molecule targeting drugs as compared to the era of chemotherapy. Despite this progress, there is still a gap between current clinical practices and ideal outcomes[4-6]. In reality, the proportion of patients with HER2-positive, PD-L1 CPS ≥ 5%, dMMR/MSI-H targets are small, thus they fall into a “high specific, low coverage” population. Although immune checkpoint inhibitors are used regardless of PD-L1 expression levels, their efficacy is limited with “wide coverage and low specificity”. Therefore, the examination of biomarkers to screen for beneficial traits and avoid ineffective treatments is required.
The MET gene is a proto-oncogene that encodes epithelial-mesenchymal transformation factor (c-Met), a receptor of hepatocyte growth factor. Under physiological conditions, the MET signaling pathway is involved in embryonic development and tissue regeneration, whereas in tumors, activation of the MET pathway can induce tumor occurrence, invasion, and metastasis[7]. Savolitinib is a selective MET inhibitor that prevents MET activation by disrupting the MET signal transduction pathway in an adenosine triphosphate competitive manner, resulting in the inhibition of tumor cell growth[8]. The proportion of MET amplification is low, constituting 4%–6% of GaC[9]. Furthermore, MET amplification includes two forms: primary amplification, defined as the activated MET signal at the time of tumor diagnosis, and secondary amplification, which refers to MET amplification detected after tumor treatment[10]. Patients with primary amplification have worse prognosis than those with secondary amplification[11]. Notably, GaC with MET amplification often appears more aggressive and advanced in the real world[12,13].
The most interesting aspect of this case was the rare intracranial metastasis and meningeal cancer. Therefore, to determine the factors associated with GaC brain metastases (BM), a comprehensive cohort study was carried out[14]. The results showed that BMs occurred less frequently in patients with antrum/pylorus tumors, and that patients with bone and lung metastases were more likely to develop BMs. However, this phenomenon is rare in liver metastases. In addition, there was a /significant negative correlation between BMs and age. Octogenarians had significantly fewer BMs compared to patients aged 60–69 years. BMs are very rare in GaC, and the clinical data of the 11 patients are summarized in Table 1. A review of these data revealed that the age of these patients was generally higher, mainly related to the target of HER2. Further, 10 of the 11 patients opted for radiation therapy and surgical resection as their treatment choice.
| Ref. | Case No. | Age/sex | BM | Therapy | Target point | Ending | Survival (month) |
| Cinar et al[19] | 1 | 55/M | + | Gamma-knife; Irinotecan + trastuzumab | HER2 | Dead | 1 |
| Cinar et al[19] | 2 | 60/M | + | Radiotherapy; Trastuzumab + capecitabine + carboplatin; Clinical trial | HER2 | Dead | 24 |
| Katayanagi et al[20] | 3 | 77/M | + | X-knife + surgical resection | Unknown | PR | > 12 |
| Kitayama et al[21] | 4 | 76/M | + | Paclitaxel + surgical resection + radiation therapy | Unknown | PR | 5 |
| Sakurai et al[22] | 5 | 78/F | + | Radiation therapy | Unknown | Dead | 4 |
| Rino et al[23] | 6 | 63/M | + | Irinotecan + cisplatin + radiation therapy | Unknown | PR | No specified |
| Rino et al[23] | 7 | 78/M | + | Irinotecan + cisplatin + Cyber-knife | Unknown | PR | No specified |
| Kostoglou et al[24] | 8 | 69/F | + | Radiation therapy + surgical resection | HER2 | Dead | 4 |
| Ahn et al[25] | 9 | 38/M | + | Stereotactic radiosurgery + anti-PD-1 | Unknown | PR | > 26 |
| Kosco et al[26] | 10 | 73/F | + | Radiation therapy + surgical resection + pharmacological intervention | Unknown | CR | > 26 |
| Murakami et al[27] | 11 | 65/M | + | Supportive care | Unknown | Dead | 1 |
It has been reported that patients with HER2+ GaC may have an increased risk of developing BM; however, the role of MET amplification in BM is unclear[15]. According to the well-known “seed and soil” hypothesis, tumor cells can survive and thrive only after adapting to the brain microenvironment microenvironment[16]. Pericytes, brain microvascular endothelial cells, and astrocytes are the three major components of blood–brain barrier[17]. For cancer cells to grow in the brain, they must acquire two key properties: overcoming the tumor-killing ability of glial cells and altering the brain microenvironment to create a suitable environment for tumor cells to survive. Activation glial cells and altering the brain microenvironment to create a suitable environment for tumor cells to survive. Activation of the c-Met signaling pathway can induce the secretion of interleukin (IL) 8 and CXCL1, which promotes the adhesion of tumor cells to brain endothelial cells and stimulates the formation of micro vessels. In addition, this signaling pathway secretes additional IL1β, causing astrocytes to secrete the ligand hepatocellular growth factor (HGF) of c-MET. This vicious feed-forward c-Met/HGF loop generates a favorable microenvironment for metastatic cells astrocytes to secrete the ligand HGF of c-MET. This vicious feed-forward c-Met/HGF loop generates a favorable microenvironment for metastatic cells[18]. However, this conclusion was reached during a search for breast cancer. In this case, the patient developed BM in the short term and achieved partial remission quickly after administration of savolitinib. Therefore, it is speculated that intracranial tumor subsets may be driven mainly by c-MET amplification.
BM are rare in advanced GaC, casing a rapid deterioration in these patients, especially in MC. There are very few effective treatment options available; however, technological breakthroughs in genomics have provided a basis for personalized treatment. Therefore, MET amplification may be a key driver of BMs in gastric cancer; however, this conclusion requires further investigation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C, Grade C, Grade C
Novelty: Grade A, Grade B, Grade C
Creativity or Innovation: Grade A, Grade B, Grade C
Scientific Significance: Grade B, Grade B, Grade C
P-Reviewer: Adam CA, Romania; Haddadi S, Algeria S-Editor: Che XX L-Editor: A P-Editor: Yu HG
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64648] [Article Influence: 16162.0] [Reference Citation Analysis (176)] |
| 2. | Huang L, Jansen L, Balavarca Y, Verhoeven RHA, Ruurda JP, Van Eycken L, De Schutter H, Johansson J, Lindblad M, Johannesen TB, Zadnik V, Žagar T, Mägi M, Bastiaannet E, Lagarde SM, van de Velde CJH, Schrotz-King P, Brenner H. Decreasing resection rates for nonmetastatic gastric cancer in Europe and the United States. Clin Transl Med. 2020;10:e203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 3. | Huang L, Jansen L, Verhoeven RHA, Ruurda JP, Van Eycken L, De Schutter H, Johansson J, Lindblad M, Johannesen TB, Zadnik V, Žagar T, Mägi M, Lagarde SM, Bastiaannet E, van de Velde CJH, Schrotz-King P, Brenner H. Largely varying patterns and trends of primary cancer-directed resection for gastric carcinoma with synchronous distant metastasis in Europe and the US: a population-based study calling for further standardization of care. Ther Adv Med Oncol. 2021;13:17588359211027837. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 4. | Muro K, Chung HC, Shankaran V, Geva R, Catenacci D, Gupta S, Eder JP, Golan T, Le DT, Burtness B, McRee AJ, Lin CC, Pathiraja K, Lunceford J, Emancipator K, Juco J, Koshiji M, Bang YJ. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): a multicentre, open-label, phase 1b trial. Lancet Oncol. 2016;17:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 911] [Article Influence: 101.2] [Reference Citation Analysis (1)] |
| 5. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 6. | Sampera A, Sánchez-Martín FJ, Arpí O, Visa L, Iglesias M, Menéndez S, Gaye É, Dalmases A, Clavé S, Gelabert-Baldrich M, Poulsen TT, Kragh M, Bellosillo B, Albanell J, Rovira A, Montagut C. HER-Family Ligands Promote Acquired Resistance to Trastuzumab in Gastric Cancer. Mol Cancer Ther. 2019;18:2135-2145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | de Bono JS, Yap TA. c-MET: an exciting new target for anticancer therapy. Ther Adv Med Oncol. 2011;3:S3-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | Lee TS, Kim JY, Lee MH, Cho IR, Paik WH, Ryu JK, Kim YT, Lee SH. Savolitinib: A Promising Targeting Agent for Cancer. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Janjigian YY, Tang LH, Coit DG, Kelsen DP, Francone TD, Weiser MR, Jhanwar SC, Shah MA. MET expression and amplification in patients with localized gastric cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Guo R, Luo J, Chang J, Rekhtman N, Arcila M, Drilon A. MET-dependent solid tumours - molecular diagnosis and targeted therapy. Nat Rev Clin Oncol. 2020;17:569-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 233] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Guo L, Liu X, Li W, Ying J. MET overexpression, gene amplification and relevant clinicopathological features in gastric adenocarcinoma. Oncotarget. 2017;8:10264-10273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Peng Z, Zhu Y, Wang Q, Gao J, Li Y, Ge S, Shen L. Prognostic significance of MET amplification and expression in gastric cancer: a systematic review with meta-analysis. PLoS One. 2014;9:e84502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Fuse N, Kuboki Y, Kuwata T, Nishina T, Kadowaki S, Shinozaki E, Machida N, Yuki S, Ooki A, Kajiura S, Kimura T, Yamanaka T, Shitara K, Nagatsuma AK, Yoshino T, Ochiai A, Ohtsu A. Prognostic impact of HER2, EGFR, and c-MET status on overall survival of advanced gastric cancer patients. Gastric Cancer. 2016;19:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Huang L, Wang L, Shi Y, Zhao Y, Xu C, Zhang J, Hu W. Brain metastasis from gastric adenocarcinoma: A large comprehensive population-based cohort study on risk factors and prognosis. Front Oncol. 2022;12:897681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Limon D, Gal O, Gordon N, Katz L, Perl G, Purim O, Amit L, Stemmer SM, Kundel Y, Ben-Aharon I, Brenner B, Siegal T, Yust-Katz S. Brain metastasis in gastroesophageal adenocarcinoma and HER2 status. J Neurooncol. 2018;138:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Fidler IJ. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3231] [Cited by in RCA: 3325] [Article Influence: 151.1] [Reference Citation Analysis (0)] |
| 17. | Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3452] [Cited by in RCA: 3884] [Article Influence: 204.4] [Reference Citation Analysis (0)] |
| 18. | Xing F, Liu Y, Sharma S, Wu K, Chan MD, Lo HW, Carpenter RL, Metheny-Barlow LJ, Zhou X, Qasem SA, Pasche B, Watabe K. Activation of the c-Met Pathway Mobilizes an Inflammatory Network in the Brain Microenvironment to Promote Brain Metastasis of Breast Cancer. Cancer Res. 2016;76:4970-4980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 19. | Cinar P, Calkins SM, Venook AP, Kelley RK. A case series of patients with HER2-overexpressed primary metastatic gastroesophageal adenocarcinoma. Anticancer Res. 2014;34:7357-7360. [PubMed] |
| 20. | Katayanagi S, Arai K, Iwasaki Y, Takahashi K, Yamaguchi T, Matsumoto H, Miyamoto H. [A case of liver metastases from gastric cancer successfully treated with induced hypertensive chemotherapy]. Gan To Kagaku Ryoho. 2003;30:1722-1725. [PubMed] |
| 21. | Kitayama Y, Yoden Y, Okamoto N. [A case of effective paclitaxel therapy for gastric cancer with brain metastasis]. Gan To Kagaku Ryoho. 2006;33:981-984. [PubMed] |
| 22. | Sakurai K, Muguruma K, Murata A, Toyokawa T, Amano R, Kubo N, Tanaka H, Yashiro M, Maeda K, Ohira M, Hirakawa K. Early gastric cancer with suspected brain metastasis arising eight years after curative resection: a case report. BMC Res Notes. 2014;7:818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Rino Y, Sekino Y, Yamada T, Nakayama T, Arai H, Kanari M, Saeki H, Yukawa N, Wada N, Masuda M, Higashida T, Imada T. [Irinotecan+cisplatin and irradiation are effective for brain metastases of gastric cancer--two case reports]. Gan To Kagaku Ryoho. 2007;34:1095-1098. [PubMed] |
| 24. | Kostoglou A, Tzanakis N, Epaggelis I, Vlasis K, Skandalakis P, Filippou D. Solitary cerebellum metastasis from gastric adenocarcinoma. A rare case report. Int J Surg Case Rep. 2019;57:110-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 25. | Ahn MJ, Lee K, Lee KH, Kim JW, Kim IY, Bae WK. Combination of anti-PD-1 therapy and stereotactic radiosurgery for a gastric cancer patient with brain metastasis: a case report. BMC Cancer. 2018;18:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Kosco E, King N, Waack A, Hoyt A, Schroeder J. Gastric Cancer With Brain Metastasis: A Case Report. Cureus. 2023;15:e50040. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Murakami Y, Kobayashi T, Naruse Y, Watanabe F, Ishino A, Kanai R, Goto T, Saito K. Exclusive Cerebellar and Leptomeningeal Metastases from Early Gastric Cancer 14 Months after Proximal Gastrectomy: An Autopsy Case Report. NMC Case Rep J. 2019;6:65-70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |