Published online May 16, 2024. doi: 10.12998/wjcc.v12.i14.2438
Revised: March 13, 2024
Accepted: April 3, 2024
Published online: May 16, 2024
Processing time: 94 Days and 17.3 Hours
Autoimmune pancreatitis (AIP) is a rare form of autoimmune-mediated pancrea
The chief complaint was yellowing of the body, eyes and urine for 21 d. The pa
In patients with pancreatic swelling, the possibility of AIP should be considered.
Core Tip: Autoimmune pancreatitis (AIP) has a low global incidence. Patients with pancreatic swelling need to differentiate between general pancreatitis and AIP. We report a clinical case and review the diagnosis of AIP in terms of clinical symptoms, physical examinations, laboratory examinations, and imaging examinations. To improve clinicians' ability to diagnose AIP will reduce misdiagnosis and increase the effectiveness of treatment.
- Citation: Zhang BY, Liang MW, Zhang SX. Type one autoimmune pancreatitis based on clinical diagnosis: A case report. World J Clin Cases 2024; 12(14): 2438-2444
- URL: https://www.wjgnet.com/2307-8960/full/v12/i14/2438.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i14.2438
Autoimmune pancreatitis (AIP) is a rare autoimmune-mediated pancreatitis with an incidence of about 10.1/100000[1], whose pathogenesis is still unclear and is effectively treated with corticosteroids. However, AIP is difficult to differentiate from general pancreatitis or pancreatic cancer. It is important to improve the diagnosis and differentiation ability of cli
A 43-year-old Chinese man suffered from yellowing of the skin and mucous membranes of the eyes for 21 d.
A 43-year-old male patient was admitted on July 3, 2023, without any obvious cause. He had yellow coloring of the body and eyes, yellow urine, yellow stool, no itching of the skin, no anorexia, no nausea and vomiting, no chills, no fever, and no abdominal pain. On July 22, 2023, the patient went to the outpatient clinic of the Shunde Hospital of the Guangzhou University of Traditional Chinese Medicine in Guangzhou for diagnosis and treatment. His liver function was checked at the emergency clinic: Alanine aminotransferase 324.0 U/L, alkaline phosphatase 660.39 U/L, γ-glutamyl transferase 1173.96 U/L, total bilirubin 129.60 μmol/L, conjugated bilirubin 50.0 μmol/L, unconjugated bilirubin 27.10 μmol/L, and δ-bilirubin 52.50 μmol/L. White blood cell count was 13.61 × 109/L, absolute neutrophil count was 8.14 × 109/L. Hepatitis A antibody IgM, and five hepatitis B indicators were normal. Ultrasound showed a suspected solid focal lesion in the head of the pancreas (the nature of which was to be determined), dilatation of the upper portion of the common bile duct and intraductal bile ducts, and enlargement of the pancreas. Polyene phosphatidylcholine was given to protect the liver after symptomatic treatment, but without symptom relief. On July 24, 2023, the patient was hospitalized for further diag
The patient denied past medical history.
The patient reported no significant abnormalities in his personal or family history.
Vital signs were stable, the whole body of the skin and mucous membranes showed moderate yellow stain, and the sclera showed mild yellow stain. The whole abdomen was flat, no gastrointestinal type and peristaltic wave, no epigastric pul
There were no significant abnormalities in carcinoembryonic antigen, carbohydrate antigen 19-9 (CA19-9), α-fetoprotein, hepatitis C immunoglobulin G (IgG) antibody, autoimmune liver disease quantitative test, autoimmune liver disease antibody spectrum, and hepatitis E test.
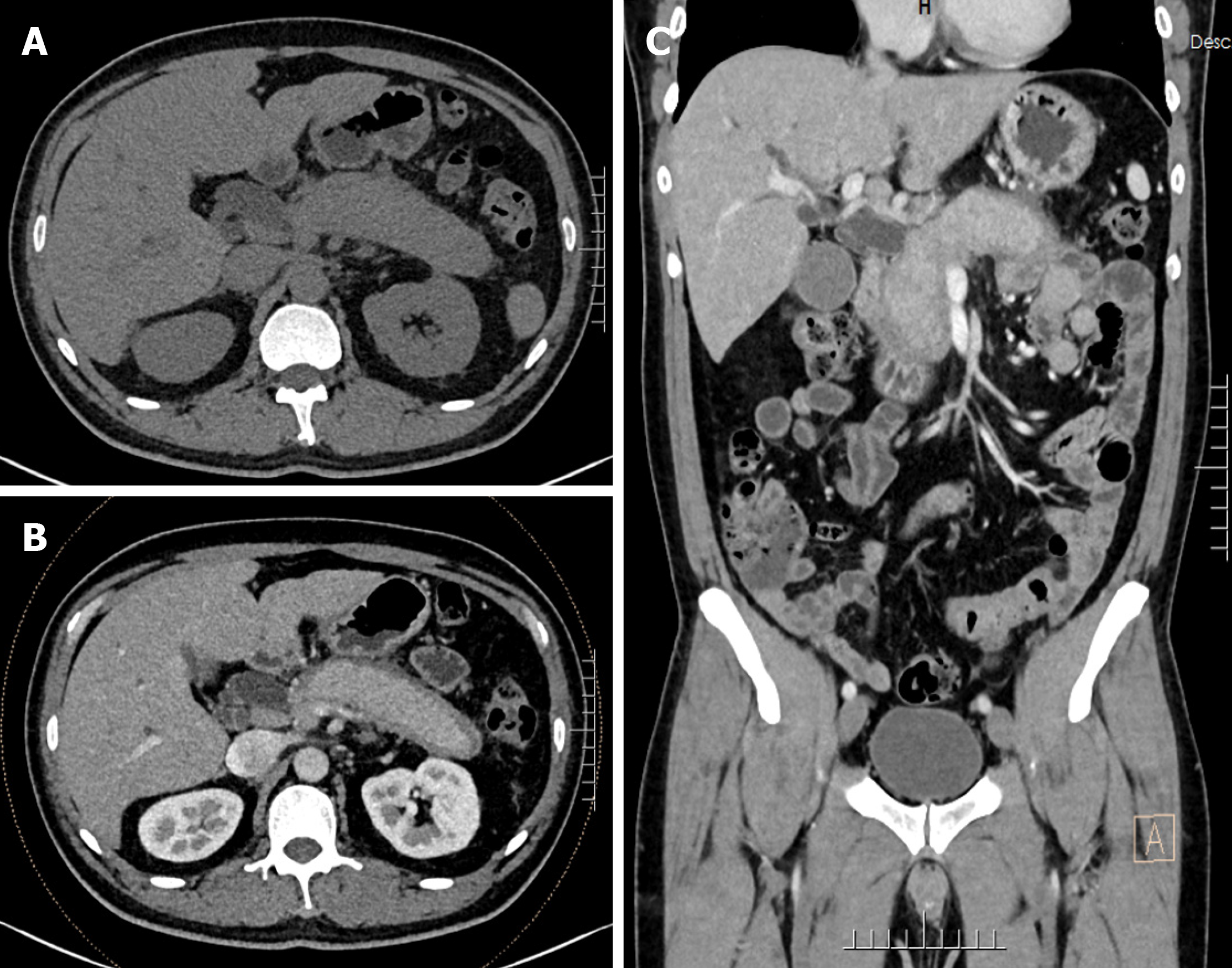
Computed tomography (CT) plain scan, CT enhanced scan and reconstruction of the whole abdomen showed: pancreatic swelling and peripancreatic oozing (inflammatory changes suggestive of AIP); and dilated intra- and extrahepatic bile ducts. Cholecystitis and prostate calcification were considered.
At the time of admission, CT plain scan and enhanced scan of the whole abdomen showed swollen pancreas, and pericardium-like low density shadow at the edge with sheath-like changes. CT enhanced scan showed that the pancreatic ducts were not dilated, and peripancreatic oozing. There was no dilatation of the pancreatic ducts and some peripancreatic exudation. The intra- and extrahepatic bile ducts were dilated and the gallbladder was slightly enlarged (Figure 1).
The patient was positive for hepatic schistosomiasis IgG antibody. Magnetic resonance cholangiopancreatography (M
Combining the patient's history, signs, laboratory examinations, and imaging examinations, the final diagnosis was: AIP, IgG4-related cholangitis, acute cholecystitis and hepatic impairment.
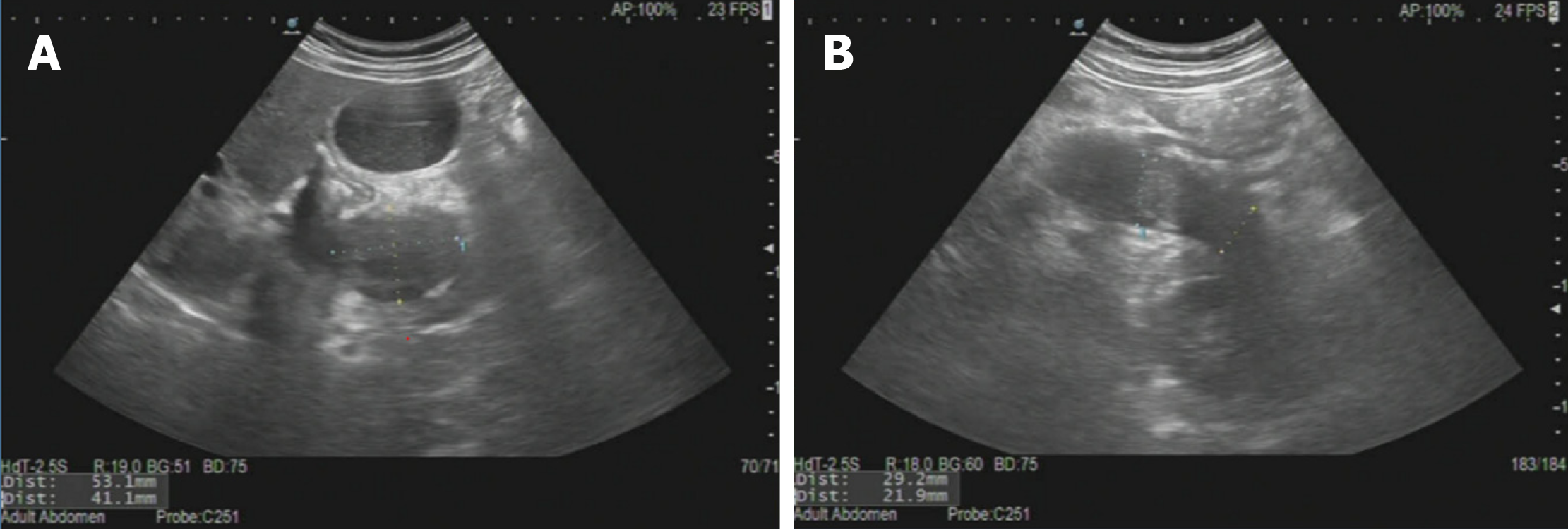
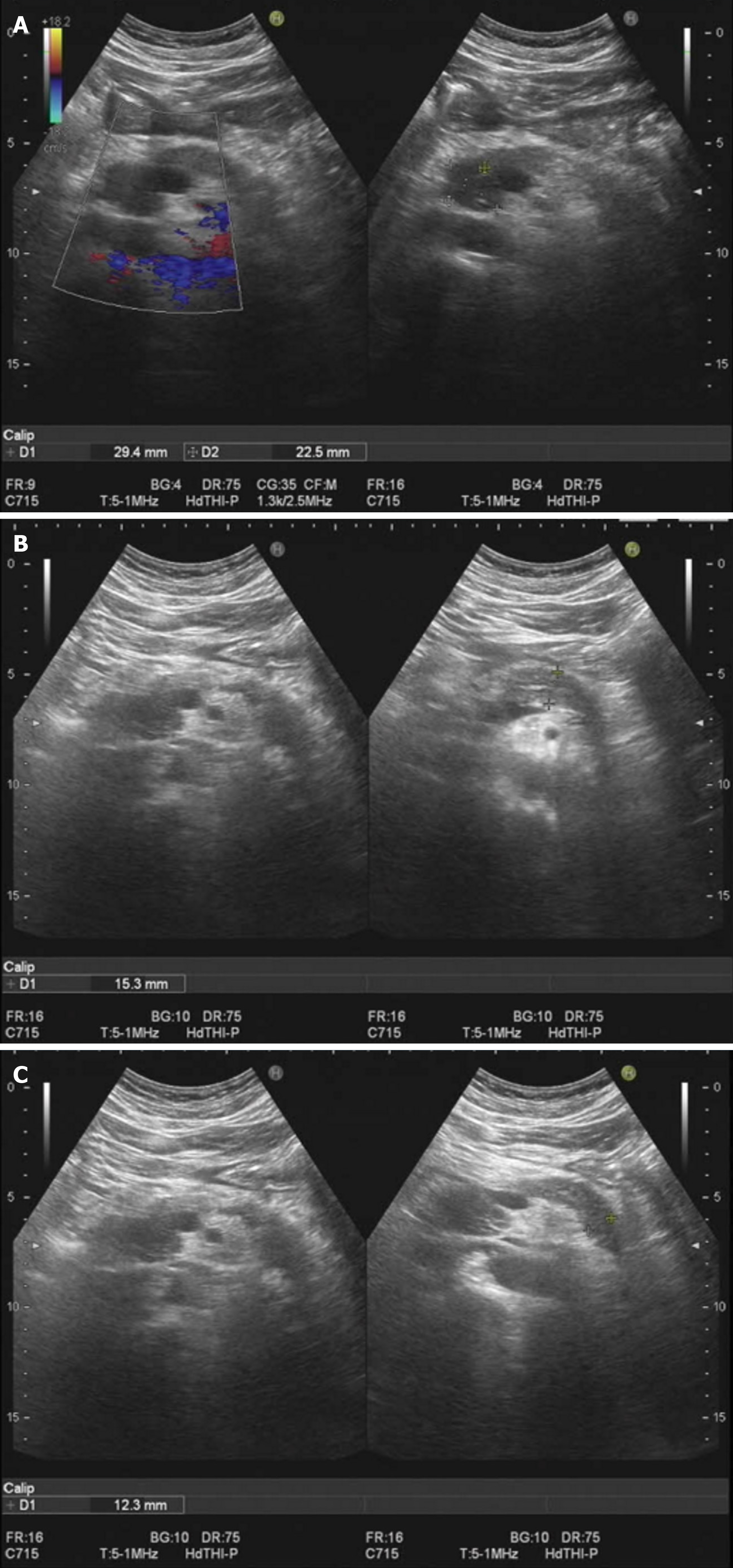
On July 29, 2023, the patient initiated hormonal shock therapy (prednisone acetate tablets, 45 mg/d), and was discharged from the hospital on August 2, 2023. He also received hepatoprotective therapy (diammonium glycyrrhizinate enteric-coated capsules 150 mg tid, bicyclol tablets 50 mg tid). The bicyclol tablets were changed to glucuronolactone tablets (0.2 g tid) on August 10. Ursodeoxycholic acid capsules were added on August 24 (250 mg qd), and the dose of prednisone acetate tablets was adjusted to 35 mg/d. The dose of prednisone acetate tablets was adjusted further to 25 mg/d, 15 mg/d and 10 mg/d on September 9, September 23 and October 7, respectively. The treatment was maintained until now. The results of laboratory tests during treatment were significantly improved (Table 1). Ultrasound comparison before and after treatment is shown in Figures 2 and 3.
| Item/time | July 24, 2023 | July 26, 2023 | July 29, 2023 | August 1, 2023 | August 20, 2023 | October 7, 2023 | Reference point |
| WBC (× 109/L) | 10.98 | 12.04 | 12.71 | 14.50 | 14.70 | 12.01 | 3.50-9.50 |
| ALT (U/L) | 231.80 | 210.80 | 172.10 | 27.50 | 24.70 | 9.00-50.00 | |
| AST (U/L) | 138.20 | 117.90 | 66.80 | 19.90 | 23.00 | 15.00-40.00 | |
| TBIL (μmol/L) | 69.40 | 31.60 | 24.10 | 16.70 | 10.70 | 0.00-26.00 | |
| DBIL (μmol/L) | 59.50 | 29.40 | 23.80 | 10.10 | 4.10 | 0.00-8.00 | |
| IgG4 (g/L) | 14.70 | 10.80 | 0.03-2.01 |
The patient's skin mucosa and eye color returned to normal. Liver function and bilirubin returned to normal. IgG4 gradually approached the normal range. The size of the pancreas was close to normal in imaging examination. The patient was recommended to undergo repeat pancreatic ultrasound elastometry.
AIP is a rare form of autoimmune-mediated pancreatitis, which is distributed worldwide, with a predominance of type 1. The clinical presentation is mainly obstructive jaundice and epigastric pain. The pathogenesis of the disease is still un
We searched for cases in the past 5 years in PubMed with "(autoimmune pancreatitis) and (case report)". In accordance with the 2010 International Association of Pancreatic Diseases (IAPD) diagnostic criteria for AIP, 50 relevant case reports were selected, totaling 51 cases of AIP (Table 2).
| Autoimmune pancreatitis (n = 51) | |
| Sex | |
| Male | 39 (76) |
| Women | 12 (24) |
| Age (yr) | |
| < 50 | 15 (30) |
| 50-60 | 19 (37) |
| > 60 | 17 (33) |
| Clinical symptom | |
| Jaundice | 33 (65) |
| Abdominal pain | 22 (43) |
| Wasting away, fever etc. | 10 (20) |
| Not have | 4 (8) |
| Laboratory tests | |
| CA19-9 | |
| Ascend | 14 (27) |
| Normalcy | 12 (24) |
| Not mentioned | 25 (49) |
| IgG4 | |
| Ascend | 45 (88) |
| Normalcy | 4 (8) |
| Not mentioned | 2 (4) |
| Swollen parts of the pancreas | |
| Head of pancreas | 30 (59) |
| Tail of pancreas | 7 (14) |
| The whole pancreas | 14 (27) |
| Diagnostic methods | |
| Surgical resection | 14 (27) |
| Puncture biopsy | 27 (53) |
| Clinical diagnosis | 10 (20) |
The male-to-female ratio was 3.25:1 (39:12) in all age groups. Jaundice and epigastric pain predominated in 33 (65%) and 22 (43%) of the patients, and the tumor index CA19-9 was elevated or normal in a similar proportion of the laboratory tests, while the serological IgG4 test was elevated in 45 (88%) patients. Imaging showed swelling of the head of the pancreas in 30 cases, the tail of the pancreatic body in seven, and the entire pancreas in 14. About 53% of AIP cases were diagnosed primarily by puncture biopsy, but 14 (27%) patients were eventually diagnosed surgically with AIP.
Type 1 AIP is common in middle-aged and older men, and the 50–60 years' age group is common, with no specific clinical manifestations, but mostly obstructive jaundice, epigastric pain and other symptoms. The fifty-one cases of AIP in the literature review were diagnosed with a single symptom of obstructive jaundice, which may be related to the fact that the biliary tract has the highest rate of involvement in AIP.
Serum IgG4 is an important and specific serological marker for type 1 AIP[2], and the IgG4 in the patient diagnosed in our hospital was 14.7 g/L, which was > 7 times the upper limit of normal value. In the literature, 88% of the patients had elevated IgG4, which means that it is an important diagnostic indicator for AIP. In addition, CA19-9 may be elevated in patients with AIP, and should be further considered for malignant pancreatic cancer. Some studies have shown that diagnosis of AIP can be improved by combining serum IgG4 (> 280 mg/dL) and CA19-9 (< 85.0 U/mL)[3], and the simultaneous detection of IgG4 and CA19-9 is of practical value in identifying pancreatic cancer.
The typical imaging manifestation of AIP on CT and MRI is diffuse enlargement of the pancreas, which resembles a sausage, commonly known as the sausage sign. Not all cases of AIP have this manifestation. In the present case, ultra
Histopathological examination is important in the diagnosis of AIP. Type 1 AIP is characterized by a large number of lymphoplasmacytic cells infiltrating around the pancreatic ducts, accompanied by tissue-matted fibrosis, and immunohistochemical staining reveals > 10 IgG4-positive cells per high-power field. Pancreatic tissue can be obtained by minimally invasive and surgical methods, and minimally invasive puncture biopsy, which accounted for about half of the 51 cases reported in the literature. The latest Japanese guideline[4] recommends the collection of histopathological specimens by endoscopic-ultrasound-guided, fine-needle aspiration. Surgical removal of pancreatic tissue is invasive, and AIP was diagnosed in this way in 27% of patients reported in the literature, which often occurs when puncture biopsy is negative and pancreatic cancer is highly suspected, making the accuracy of puncture biopsy important.
Type 1 AIP has no characteristic clinical manifestations, and the imaging manifestations are atypical or similar to those of pancreatic cancer. Therefore, it is easy to misdiagnose, and its diagnosis mainly relies on detection of serological IgG4 levels. Short-term diagnostic treatment with small doses of hormones has a role in identification of AIP, but diagnostic confirmation mainly relies on pathology. However, there are many clinical situations in which pathology is unavailable; for example, if the patient refuses to have a biopsy, as in our case. The cases reported in the literature included some pregnant women with AIP. Pregnant women are a special population that is unsuitable for surgery and biopsy, and these patients require physicians to have sufficient familiarity with AIP and vigilance, and accurate and rapid diagnosis can avoid aggravating the patient's burden and lead to early recovery. At present, the most widely recognized diagnostic standard is that issued by IAPD in 2011[5], which classifies the diagnosis into confirmed and suspected after comprehensive assessment of the diagnostic basis from five aspects, and can provide clinical diagnostic guidance for physicians.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Negreanu L, Romania S-Editor: Zheng XM L-Editor: A P-Editor: Xu ZH
| 1. | Löhr JM, Vujasinovic M, Rosendahl J, Stone JH, Beuers U. IgG4-related diseases of the digestive tract. Nat Rev Gastroenterol Hepatol. 2022;19:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 2. | Nista EC, De Lucia SS, Manilla V, Schepis T, Pellegrino A, Ojetti V, Pignataro G, Zileri Dal Verme L, Franceschi F, Gasbarrini A, Candelli M. Autoimmune Pancreatitis: From Pathogenesis to Treatment. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Chang MC, Liang PC, Jan S, Yang CY, Tien YW, Wei SC, Wong JM, Chang YT. Increase diagnostic accuracy in differentiating focal type autoimmune pancreatitis from pancreatic cancer with combined serum IgG4 and CA19-9 levels. Pancreatology. 2014;14:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Okazaki K, Kawa S, Kamisawa T, Ikeura T, Itoi T, Ito T, Inui K, Irisawa A, Uchida K, Ohara H, Kubota K, Kodama Y, Shimizu K, Tonozuka R, Nakazawa T, Nishino T, Notohara K, Fujinaga Y, Masamune A, Yamamoto H, Watanabe T, Nishiyama T, Kawano M, Shiratori K, Shimosegawa T, Takeyama Y; Members of the Research Committee for IgG4-related Disease supported by the Ministry of Health, Labour, Welfare of Japan, Japan Pancreas Society. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2020. J Gastroenterol. 2022;57:225-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M, Notohara K, Okazaki K, Schneider A, Zhang L; International Association of Pancreatology. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1059] [Article Influence: 75.6] [Reference Citation Analysis (0)] |