Published online May 6, 2024. doi: 10.12998/wjcc.v12.i13.2286
Peer-review started: January 23, 2024
First decision: January 31, 2024
Revised: February 6, 2024
Accepted: March 27, 2024
Article in press: March 27, 2024
Published online: May 6, 2024
Processing time: 92 Days and 20.7 Hours
Ulcerative colitis (UC) and systemic lupus erythematosus (SLE) are both systemic immunoreactive diseases, and their pathogenesis depends on the interaction between genes and environmental factors. There are no reports of UC with SLE in China, but six cases of SLE with UC have been reported in China. The combi
A female patient (30 years old) came to our hospital due to dull umbilical pain, diarrhea and mucous bloody stool in August 2018 and was diagnosed with UC. The symptoms were relieved after oral administration of mesalazine (1 g po tid) or folic acid (5 mg po qd), and the patient were fed a control diet. On June 24, 2019, the patient was admitted for treatment due to anemia and tinnitus. During hospitalization, the patient had repeated low-grade fever and a progressively decreased Hb level. Blood tests revealed positive antinuclear antibody test, positive anti-dsDNA antibody, 0.24 g/L C3 (0.9-1.8 g/L), 0.04 g/L C4 (0.1-0.4 g/L), 32.37 g/L immunoglobulin (8-17 g/L), and 31568.1 mg/24 h total 24-h urine protein (0-150 mg/24 h). The patient was diagnosed with SLE involving the joints, kidneys and blood system. Previously reported cases of SLE were retrieved from PubMed to characterize clinicopathological features and identify prognostic factors for SLE.
The patient was discharged in remission after a series of treatments, such as intravenous methylprednisolone sodium succinate, intravenous human immunoglobulin, cyclophosphamide injection, and plasma exchange. After discharge, the patient took oral prednisone acetate tablets, cyclosporine capsules, hydroxychloroquine sulfate tablets and other treatments for symptoms and was followed up regularly for 1 month, after which the patient's condition continued to improve and stabilize.
Core Tip: The association between ulcerative colitis (UC) and systemic lupus erythematosus (SLE) is a rare phenomenon. We first diagnosed a patient with coexisting UC and SLE with refractory autoimmune hemolytic anemia. Combined with the analysis of the cases indexed in PubMed, plasma exchange (PE) has been reported as a promising strategy for treating refractory autoimmune hemolytic anemia. The patient was successfully treated and maintained stable conditions through PE and continuous treatment with cyclophosphamide and hydroxychloroquine. Therefore, personalized treatment is currently the best approach.
- Citation: Chen DX, Wu Y, Zhang SF, Yang XJ. Refractory autoimmune hemolytic anemia in a patient with systemic lupus erythematosus and ulcerative colitis: A case report. World J Clin Cases 2024; 12(13): 2286-2292
- URL: https://www.wjgnet.com/2307-8960/full/v12/i13/2286.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i13.2286
Considerable overlap occurs between various autoimmune rheumatic diseases, either from the beginning of the illness or at any point during the disease course. This may pose a considerable diagnostic challenge. Systemic lupus erythematosus (SLE) is a chronic, potentially severe, frequently disabling autoimmune disease with multiorgan involvement and typically a waxing and waning course. SLE is an immune complex-mediated disorder common in women of reproductive age group and is often considered the prototypical autoimmune disease. SLE can affect virtually every organ, including the gastrointestinal system, but most commonly, patients present with skin rashes, arthritis, oral ulcers, photosensitivity, renal, serositis, neurologic and hematologic disorders[1]. In contrast to other autoimmune diseases, such as inflammatory bowel disease (IBD), is a chronic idiopathic gastrointestinal disorder that includes ulcerative colitis (UC) and Crohn’s disease (CD). Almost one fourth of IBD patients suffer from extra-intestinal manifestations, including sacroilitis or spondylitis, non-deforming peripheral arthritis, erythema nodosum, episcleritis, pyoderma gangrenosum, sclerosing cholangitis and thromboembolic events. The coexistence of the IBD and SLE is rare. The coexistence of clinical features of both diseases in a patient represents a diagnostic challenge.
Autoimmune hemolytic anemia (AIHA) is an autoimmune disorder characterized by the production of autoantibodies against erythrocytes and can be attributed to several factors, such as infections, medications, certain malignancies and autoimmune diseases[2]. Steroid or steroid combination with immunoglobulin (IG) is the mainstay of AIHA treatment[3]. Moreover, plasma exchange (PE), such as steroid-resistant or steroid-dependent AIHA, has been reported as a promising strategy for treating refractory AIHA[4,5]. However, the effect of PE on refractory AIHA in patients with multiple coexisting autoimmune diseases has not been evaluated. Here, we report refractory steroid-resistant AIHA in a patient with coexisting UC and SLE who was successfully treated with PE.
A 30-year-old Chinese woman presented to the gastroenterology department with a complaint of fatigue and tinnitus for 1 wk.
Symptoms started 1 wk before presentation with recurrent fatigue and tinnitus, without systemic joint pain or fever.
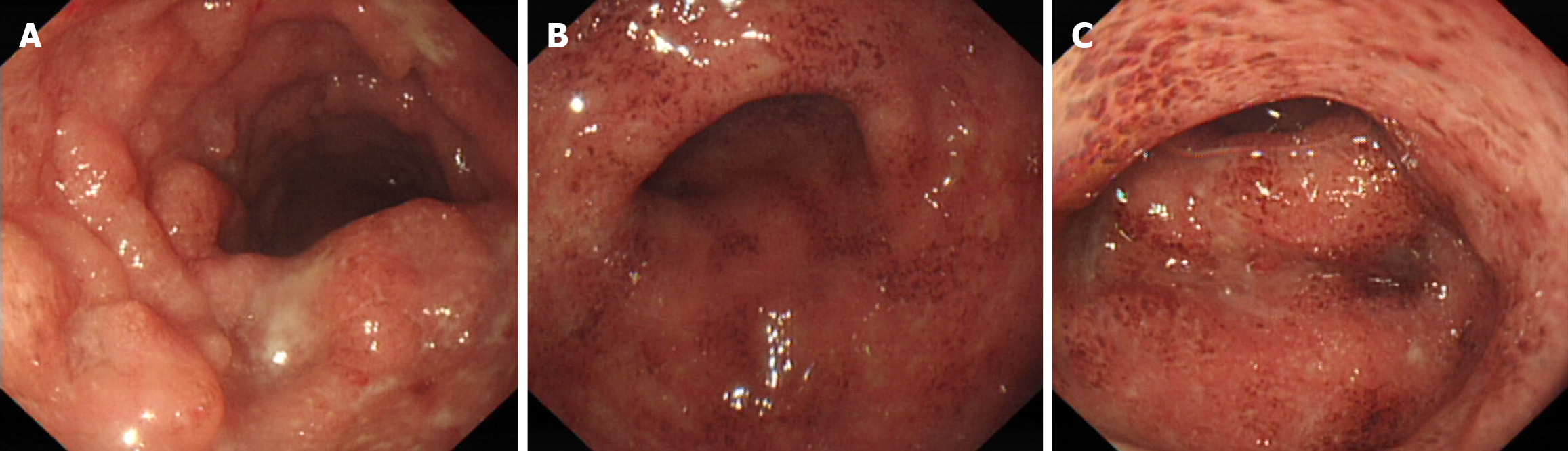
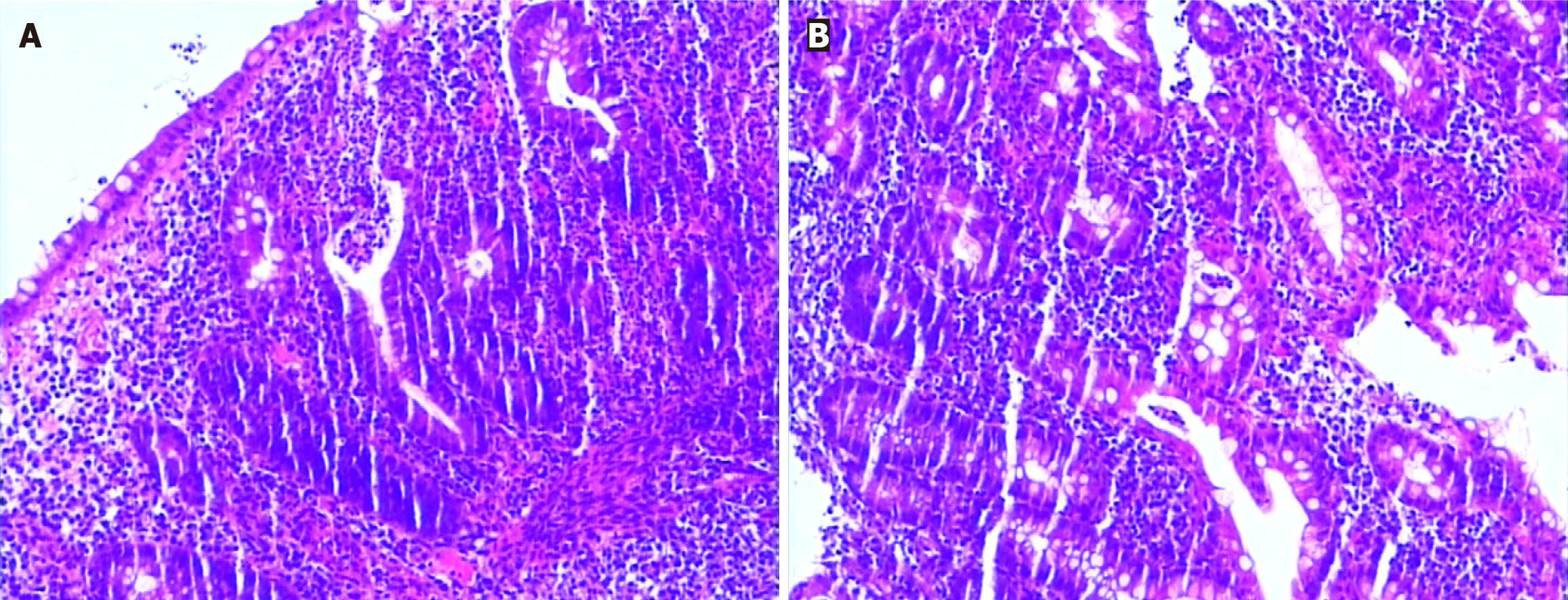
A 30-year-old female came to our hospital on August 26, 2018, due to "dull pain around the umbilicus complicated with viscous bloody stools", and underwent electronic colonoscopy, which suggested "diffuse erosion and multiple superficial ulcers of the rectum, sigmoid colon and descending colon mucosa" (Figure 1). Pathology revealed "diffuse lymphocytic infiltration of (sigmoid colon) mucosa, visible cryptitis, cryptal abscess, irregular surface epithelium, and distortion of cryptal structure" (Figure 2), and UC (type E2) was diagnosed. Routine blood tests revealed a hemoglobin level of 69 g/L and a serum iron concentration of 2.9 μmol/L, and the stool sample was "white blood cell (WBC) 2 +/HP, red blood cell (RBC) 2-5/HP, OB +, pus cell 2 +/HP", and antinuclear antibody-negative or anti-Sm negative. After admission, the patient was given mesalazine sustained-release granules (1 g, po, tid), enteral nutrition powder, iron saccharate and other symptomatic treatment, after which the disease condition improved; after discharge, the patient was orally administered mesalazine sustained-release granules (0.5 g, po, tid) and folic acid tablets to control the disease condition and had 1-2 stools/d, without mucus or purulent bloody stool; on January 14, 2019, routine blood tests showed a hemoglobin level of 122 g/L. The drug was stopped spontaneously in February 2019 without recurrence of the disease.
The patient denied any family history of disease involving the immune system.
On physical examination, the vital signs were as follows: Body temperature, 36.7 °C; blood pressure, 102/62 mmHg; heart rate, 92 beats/min; and respiratory rate, 19 breaths/min. Furthermore, the patient’s face, skin and eyelid membrane were pale, without malar erythema.
Relevant laboratory data can be displayed in the diagnosis and treatment process.
Combined with the patient’s medical history, the patient was diagnosed with SLE involving the joints, kidneys and blood system.
Combined with the patient’s medical history, the patient was diagnosed with SLE involving the joints, kidneys and blood system.
In June 2019, the patient was readmitted to our medical center because she presented with fatigue and tinnitus for 1 wk. When she was presented to our hospital, she had no abdominal or digestive symptoms, such as abdominal pain, diarrhea or bloody stool, and she was experiencing only fatigue and tinnitus. The physical examination was unremarkable, except for a body temperature of 37.7 °C. Laboratory findings revealed a decrease in blood cells (1.67 × 1012/L, normal range: 3.8-5.6 × 1012/L) and a decrease in hemoglobin (62 g/L), as did the normal WBCs, platelet count, mean corpuscular volume and mean hemoglobin concentration. The fecal occult blood test was negative, and the concentration of serum iron was normal. However, strongly positive direct anti-human globulin and indirect anti-human globulin results were identified. Moreover, abdominal ultrasonography revealed splenomegaly. Therefore, AIHA was considered. Subsequently, the immunological results, including positive antinuclear antibody test results (1:100, 1:320 and 1:1000), positive anti-dsDNA antibody, positive SS-A antibody and decreased complement component C3 (0.24 g/L, normal range 0.9-1.8 g/L) and C4 (0.04 g/L, normal range 0.1-0.4 g/L), were verified. Based on these findings, concomitant SLE was also diagnosed.
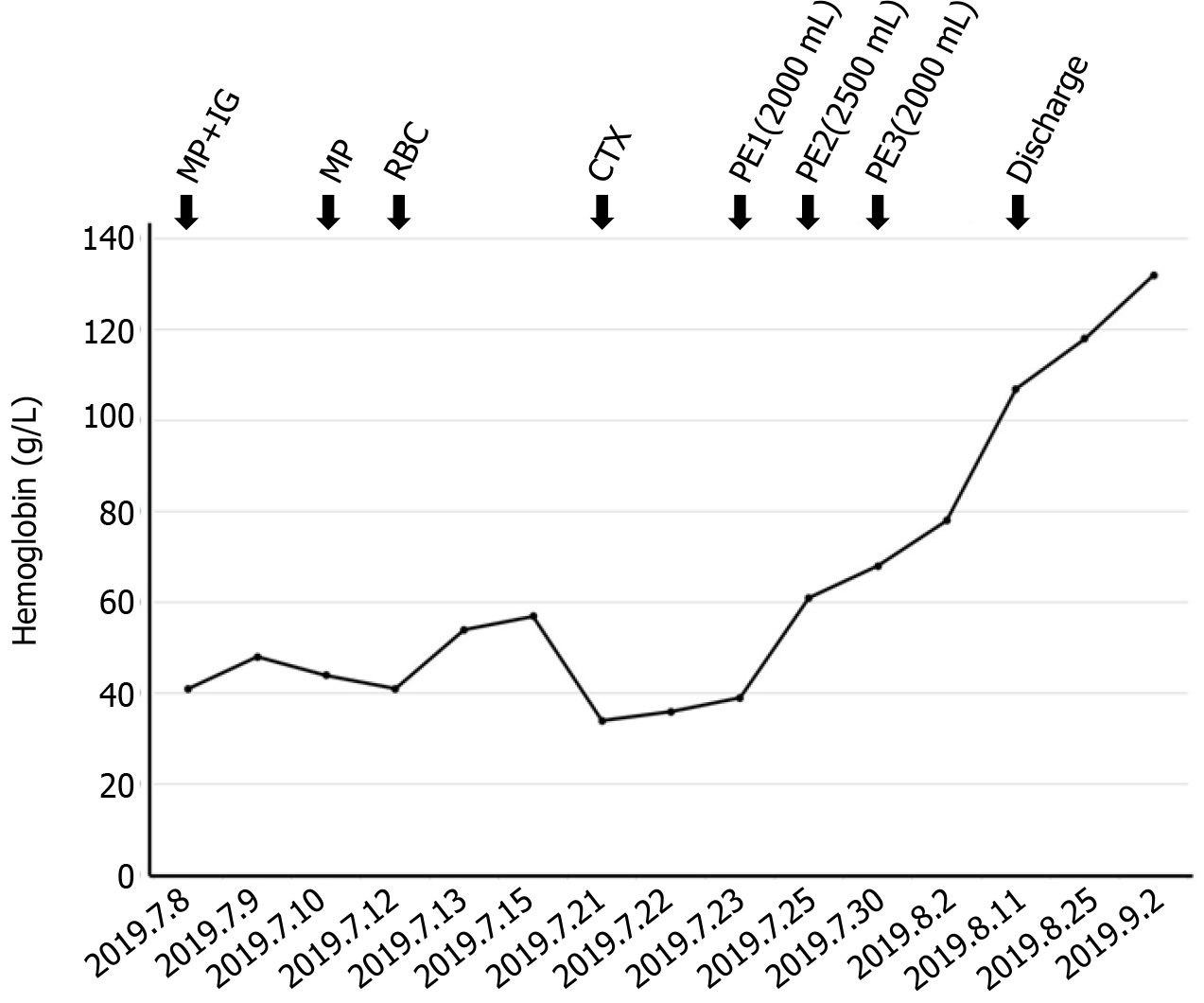
The clinical response to therapy is shown in Figure 3. The patient was initially treated with intravenous methylprednisolone (MP) at a dose of 500 mg/d plus intravenous IG at a dose of 20 g/d. Due to the minimal clinical improvement of anemia after high-dose MP in combination with IG therapy, we treated her with intravenous cyclophosphamide (CTX) at a dose of 0.2 g/d after treatment with MP (80 mg/d). Moreover, minimal transfusion of RBCs was performed. Unfortunately, the anemia still did not improve. PE has been used to treat refractory AIHA[3,4]. To improve refractory AIHA, PE was administered on July 24, July 26 and July 29. Hemoglobin was significantly increased following PE therapy. We conclude that PE therapy successfully controlled severe hemolysis. On August 2, 2019, her hemoglobin level was 78 g/L, and the patient was hospital discharged. At the outpatient follow-up, one month after her last session of PE, her hemo
Currently, the patient takes cyclosporine capsules (100 mg/d) and hydroxychloroquine sulfate tablets (0.4 g/d), and her condition is stable without related complications. The follow-up laboratory data are shown in the Supplementary Figure.
UC and SLE are both systemic immunoreactive diseases, and their pathogenesis depends on the interaction between genes and environmental factors. There are no reports of UC with SLE in China, but six cases of SLE with UC have been reported in China. There are sporadic reports from abroad of preceding SLE with later UC development; there are also cases of preceding UC with later SLE development[6,7]. The combination of these two diseases is likely related to the presence of immune and genetic defects in the pathogenesis of both diseases.
Although the patient’s history was nonextensive and the diagnosis was confirmed, the treatment process was extremely difficult, and the selection of PE, which quickly stabilized the patient’s condition, was one of the most valuable points of this case report. AIHA is a common feature of SLE. However, AIHA is a relatively rare IBD that develops in 0.2%-1.7% of patients with UC[8]. Notably, to our knowledge, no case of AIHA in patients with coexistent IBD and SLE has been reported. Currently, the confirmation of AIHA is primarily based on the direct antiglobulin test detecting autoantibodies and/or complement agents on the surface of RBCs[9]. Strongly positive results for direct anti-human globulin and indirect anti-human globulin were identified in this patient. Therefore, the diagnosis of AIHA was exact for the patient. The backbone of treatment in AIHA is based on corticosteroid therapy, which induces remission from autoantibody production in approximately 80% of patients[10]. PE therapy is used in many autoimmune disorders, such as amyopathic dermatomyositis, dermatomyositis, SLE during pregnancy and lupus enteritis, to decrease the antibody burden, which contributes to acute crises[11-14]. PE can effectively remove pathogenic substances, such as auto
The pathogenesis of UC is multifactorial and involves genetic predisposition, epithelial barrier defects, dysregulated immune responses, and environmental factors. Extraintestinal manifestations can occur in approximately one-third of patients with UC[16]. Histological findings include distortion of the crypt architecture, crypt shortening, increased lymphocytes and plasma cells in the lamina propria (basal plasmacytosis), mucin depletion, and Paneth cell metaplasia[17,18]. Treatments for UC include 5-aminosalicylic acid drugs, steroids, and immunosuppressants. However, oral ulcers are a cardinal feature of SLE. Studies show that 0.2% to 5.8% of patients with SLE are affected by lupus enteritis[19]. The median age of onset was 34 years, and symptoms typically appear, on average, 34.3 months after the diagnosis of SLE; 85% of the patients were females. The three principal pathologic and pathophysiologic components of lupus enteritis include lupus mesenteric vasculitis, intestinal pseudo-obstruction, and protein-losing enteropathy. Other observational studies have shown a prevalence of UC in patients with SLE of 0.4%, which is comparable to that of general population controls[20]. Treatments for SLE include steroids, CTX, azathioprine, mycophenolate mofetil, and (less frequently) hydroxychloroquine and immunosuppressants. A meta-analysis revealed a significant association between miRNA-499 gene polymorphisms and autoimmune diseases, such as Behcet’s disease, rheumatoid arthritis (RA), SLE and UC[21]. Aynacıoğlu believed that Midkine is involved in the onset and progression of autoimmune rheumatic diseases, including RA, SLE, and Sjögren’s syndrome and other autoimmune conditions such as multiple sclerosis[22]. However, a two-sample Mendelian randomization study revealed a negative causal effect of SLE on overall incidence of IBD and UC in European populations but not between SLE and CD[23]. In contrast, there was no causal relationship between SLE and IBD in East Asian populations. We consider these two autoimmune disorders to share certain common features.
In this case, the initial attack of UC involved only the intestine. After enteral nutrition powder (Ansu) was given to replace the diet at the time of initial treatment, along with mesalazine supplementation, abdominal pain, diarrhea and mucous bloody stool were rapidly relieved. This may be due to the action of environmental factors on the susceptibility gene. With the participation of antigens such as intestinal bacteria or food, the intestinal immune system is initiated, causing the excessive and continuous development of the intestinal immune inflammatory response. Since then, the systemic immune response of patients may be abnormally activated, which causes an immune response in systemic multisystem connective tissue on the basis of possible immune and genetic defects and adverse factors. Many published reports suggest that cyclosporine can also be used for the treatment of some patients with UC to alleviate this condition. This patient received maintenance treatment with cyclosporine and hydroxychloroquine. Cyclosporine has immunosuppressive effects, while hydroxychloroquine has anti-inflammatory and immunomodulatory effects. Therefore, we speculate that this is also the reason why UC and SLE can be relieved in patients without the use of steroid hormones. UC and SLE are both immune system diseases. However, the specific underlying mechanism is unclear and needs to be further explored and studied by rheumatologists.
The association between UC and SLE seems to be rare, and it is not fully clear whether this association is due to a common physiopathology. On the other hand, for the first time, we report refractory steroid-resistant AIHA in a patient with coexisting UC and SLE who was successfully treated with PE. Moreover, we provide a reference treatment strategy for similar patients. Doctors should make a positive and accurate diagnosis and provide humane care and treatment because patients have experienced both mental and physical problems.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dauyey K, Kazakhstan S-Editor: Liu H L-Editor: A P-Editor: Zhao S
| 1. | Trapani S, Rubino C, Simonini G, Indolfi G. Gastrointestinal and hepatic involvement in paediatric systemic lupus erythematosus. Clin Exp Rheumatol. 2021;39:899-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Barcellini W, Fattizzo B. The Changing Landscape of Autoimmune Hemolytic Anemia. Front Immunol. 2020;11:946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 3. | Heisel MA, Ortega JA. Factors influencing prognosis in childhood autoimmune hemolytic anemia. Am J Pediatr Hematol Oncol. 1983;5:147-152. [PubMed] |
| 4. | Anani W, Wucinski J, Baumann Kreuziger L, Gottschall J, Karafin M. Therapeutic plasma exchange in refractory warm autoimmune hemolytic anemia. Transfusion. 2017;57:1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Damlaj M, Séguin C. Refractory autoimmune hemolytic anemia in a patient with DiGeorge syndrome treated successfully with plasma exchange: a case report and review of the literature. Int J Hematol. 2014;100:494-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Khan BA, Saleem N, Hassan D, Kiani S, Haneef M. Coexistence of Lupus Nephritis, Ulcerative Colitis, and Communicating Hydrocephalus: A Report of a 21-Year-Old Male. Case Rep Nephrol. 2022;2022:1079300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 7. | Mansour HE, Arafa SG, Shehata WA. Systemic lupus erythematosus with inflammatory bowel disease-ulcerative colitis: case report. Lupus. 2018;27:1198-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Giannadaki E, Potamianos S, Roussomoustakaki M, Kyriakou D, Fragkiadakis N, Manousos ON. Autoimmune hemolytic anemia and positive Coombs test associated with ulcerative colitis. Am J Gastroenterol. 1997;92:1872-1874. [PubMed] |
| 9. | Wheeler CA, Calhoun L, Blackall DP. Warm reactive autoantibodies: clinical and serologic correlations. Am J Clin Pathol. 2004;122:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Lechner K, Jäger U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116:1831-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 11. | Yamagata A, Arita M, Tanaka A, Tokioka F, Yoshida T, Nishimura K, Ishida T. Therapeutic plasma exchange for clinically amyopathic dermatomyositis (CADM) associated with rapidly progressive interstitial pneumonia. J Clin Apher. 2020;35:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Shirakashi M, Nakashima R, Tsuji H, Tanizawa K, Handa T, Hosono Y, Akizuki S, Murakami K, Hashimoto M, Yoshifuji H, Ohmura K, Mimori T. Efficacy of plasma exchange in anti-MDA5-positive dermatomyositis with interstitial lung disease under combined immunosuppressive treatment. Rheumatology (Oxford). 2020;59:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 13. | Thomas A, Smithhart C, Chopra M, Jayan A, Ali F, Doughem K, Bhatt A, Scoon J, Larson S. Severe Lupus Enteritis Complicated by Intractable Gastrointestinal Hemorrhage. ACG Case Rep J. 2023;10:e01188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Zhang F, Zhang BY, Fan R, Cheng T, Hu XR, Liu YQ, Cen X, Bu YJ, Cao JP, Chen FW, Chen JW. Clinical efficacy of plasma exchange in systemic lupus erythematosus during pregnancy. Immun Inflamm Dis. 2023;11:e1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Deng J, Zhou F, Wong CY, Huang E, Zheng E. Efficacy of therapeutic plasma exchange for treatment of autoimmune hemolytic anemia: A systematic review and meta-analysis of randomized controlled trials. J Clin Apher. 2020;35:294-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Vavricka SR, Brun L, Ballabeni P, Pittet V, Prinz Vavricka BM, Zeitz J, Rogler G, Schoepfer AM. Frequency and risk factors for extraintestinal manifestations in the Swiss inflammatory bowel disease cohort. Am J Gastroenterol. 2011;106:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 378] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 17. | Kornbluth A, Sachar DB; Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-23; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 942] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 18. | Magro F, Langner C, Driessen A, Ensari A, Geboes K, Mantzaris GJ, Villanacci V, Becheanu G, Borralho Nunes P, Cathomas G, Fries W, Jouret-Mourin A, Mescoli C, de Petris G, Rubio CA, Shepherd NA, Vieth M, Eliakim R; European Society of Pathology (ESP); European Crohn's and Colitis Organisation (ECCO). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis. 2013;7:827-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 466] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 19. | Koo BS, Hong S, Kim YJ, Kim YG, Lee CK, Yoo B. Lupus enteritis: clinical characteristics and predictive factors for recurrence. Lupus. 2015;24:628-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Shor DB, Dahan S, Comaneshter D, Cohen AD, Amital H. Does inflammatory bowel disease coexist with systemic lupus erythematosus? Autoimmun Rev. 2016;15:1034-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Kong X, Diao S, Xu H, Sun J, Ma B. Association between miRNA-499 gene polymorphism and autoimmune diseases: A meta-analysis. PLoS One. 2022;17:e0266265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 22. | Aynacıoğlu AŞ, Bilir A, Tuna MY. Involvement of midkine in autoimmune and autoinflammatory diseases. Mod Rheumatol. 2019;29:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Xie W, Jiang H, Chen Y, Zhang H, Song Y, Yu Z, Gu H, Xu H, Han S, Li S, Liu N. Association between systemic lupus erythematosus and inflammatory bowel disease in European and East Asian populations: a two-sample Mendelian randomization study. Front Immunol. 2023;14:1199896. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |