Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2079
Revised: February 26, 2024
Accepted: April 2, 2024
Published online: April 26, 2024
Processing time: 156 Days and 18.8 Hours
Infections by non-tuberculous mycobacteria (NTM) have become more common in recent years. Mycobacterium canariasense (M. canariasense) was first reported as an opportunistic pathogen in 2004, but there have been very few case reports since then. Nocardia is a genus of aerobic and Gram-positive bacilli, and these species are also opportunistic pathogens and in the Mycobacteriales order. Conventional methods for diagnosis of NTM are inefficient. Metagenomic next-generation se
A 52-year-old woman presented with a productive cough and chest pain for 2 wk, and recurrent episodes of moderate-grade fever for 1 wk. She received antibiotics for 1 wk at a local hospital, and experienced defervescence, but the productive cough and chest pain persisted. We collected samples of a lung lesion and alveo
Etiological diagnosis is critical for patients with infectious diseases. mNGS can identify rare and novel pathogens, and does not require a priori knowledge.
Core Tip: Non-tuberculous mycobacteria (NTM) and Nocardia are opportunistic pathogens that can occur in immunocompromised patients who present with atypical clinical symptoms. Mycobacterium canariasense (M. canariasense) is a rare NTM species was first identified 20 years ago. We describe a patient with multiple lung nodules of unequal size with uneven internal density, and multiple small burrs at the edges of these lung lesions. The pathology results were inconsistent with malignancy, and metagenomic next-generation sequencing indicated overlapping infections of M. canariasense, Nocardia farcinica, and Candida parapsilosis. The anti-infective treatment was successful.
- Citation: Huang HY, Bu KP, Liu JW, Wei J. Overlapping infections of Mycobacterium canariasense and Nocardia farcinica in an immunocompetent patient: A case report. World J Clin Cases 2024; 12(12): 2079-2085
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2079.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2079
The non-tuberculous mycobacteria (NTM), also referred to as environmental mycobacteria, atypical mycobacteria, or anonymous mycobacteria, are ubiquitous species and potential causes of infectious diseases. Based on their growth characteristics determined from subculturing, the NTM are classified as rapidly growing mycobacteria (RGM; mature colonies in less than 7 d) or slowly growing mycobacteria (mature colonies in more than 7 d)[1]. Several RGM species have been identified as etiologic agents of bacteremia, especially in patients with low immunity, such as those with human immunodefciency virus (HIV) infections or malignant tumors. Mycobacterium canariasense (M. canariasense) is a rare species of RGM that is closely related to M. diernhofer[2,3], but has never been described separately in immunocompetent persons.
The genus Nocardia is also in the order Mycobacteriales, and includes at least 50 species that are aerobic Gram-positive bacilli which can invade the lungs, skin, or central nervous system, especially in immunocompromised persons. The sym
It can be difficult to diagnose infections by Mycobacterium and Nocardia from the conventional culture tests used in clinical practice, and delayed diagnosis may have serious adverse consequences. The rapid and efficient identification of the pathogen responsible for an infectious disease is a prerequisite for the effective treatment of these patients. Meta
A 52-year-old woman from Guangxi Zhuang Autonomous Region presented with productive cough and chest pain for 2 wk and recurrent episodes of moderate-grade fever for 1 wk.
The patient experienced a relapse of productive cough, persistent chest pain, and moderate-grade fever, but there were no chills, shivering, hemoptysis, or weight loss. She was diagnosed with pneumonia and treated with cefuroxime sodium (0.75 g/8 h) and levofloxacin (0.5 g/d) at a local hospital for 1 wk and experienced defervescence. However, the cough and chest pain persisted, and this affected her ability to work and study. The patient presented to another outpatient clinic for persistent cough and chest pain, with the possibility of malignancy unable to be excluded. Consequently, she presented at our hospital for further diagnosis and treatment.
This case had no specific history of past illness.
The patient had a history of exposure to sheep feces 1 wk before symptom onset. She had no history of using steroids or other medications, no smoking, no tuberculosis, no malignant tumors or immunosuppressive diseases, and was unaware of any contact with persons with mycobacterial infections. She also reported no relevant family history.
No abnormalities were detected in the physical examination.
Laboratory studies revealed leukocytosis, an elevated level of high-sensitivity C-reactive protein, and a high erythrocyte sedimentation rate (Table 1). After 5 d, multiple sets of blood and sputum cultures revealed no growth of bacteria or fungi. The culture of bacteria and fungi in bronchoalveolar lavage fluid (BALF), and acid-fast staining were also negative. An immunologic workup, which included HIV testing, was conducted based on suspicion of immunodeficiency, but all of the results were negative. Examination of autoantibodies and tumor markers also revealed no abnormalities. BALF and pulmonary nodular samples were subsequently sent for mNGS for the rapid identification of the causative pathogen(s). Sequencing results were compared with the sequences of bacteria in Gen Bank, and those with 100% agreement were accepted. Three days later, the pathogenic microbes in the nodular specimen were identified as N. farcinica, M. canariasense, and Candida parapsilosis (C. parapsilosis) (326 sequences), although the BALF only showed M. canariasense (Table 2).
| Variable | Reference range | On admission | Antibiotic treatment | |
| After 2-wk | After 1-month | |||
| Leukocytes (× 109/L) | 3.69-9.16 | 10.34 | 5.23 | 7.12 |
| Hemoglobins (g/L) | 113-151 | 130 | 130 | 123 |
| Platelets (× 109/L) | 100-300 | 439 | 286 | 100 |
| Neutrophiles (× 109/L) | 2-7.7 | 8.49 | 3.17 | 5.2 |
| Lymphocytes (× 109/L) | 0.8-4 | 1.39 | 1.53 | 1.4 |
| Monocytes (× 109/L) | 0.12-0.8 | 0.4 | 0.39 | 0.38 |
| ESR (mm/H) | 0-20 | 92.8 | 41.4 | 26.5 |
| hs-CRP (mg/L) | 0-3 | 32.48 | 0.92 | 0.51 |
| Specimen | Genus | Species | ||||
| Name | Sequence number | Relative abundance | Name | Sequence number | Cover degree | |
| Nodular | Nocardia | 1006 | 0.02% | Nocardia farcinica | 393 | 18.87% |
| Nodular | Mycobacterium | 9501 | 0.72% | Mycobacterium canariasense | 3630 | 6.89% |
| Nodular | Candida | 327 | 2.44% | Candida parapsilosis | 326 | 0.23% |
| Bronchoalveolar lavage fluid | Mycobacterium | 2260 | 42.34% | Mycobacterium canariasense | 2020 | 1.75% |
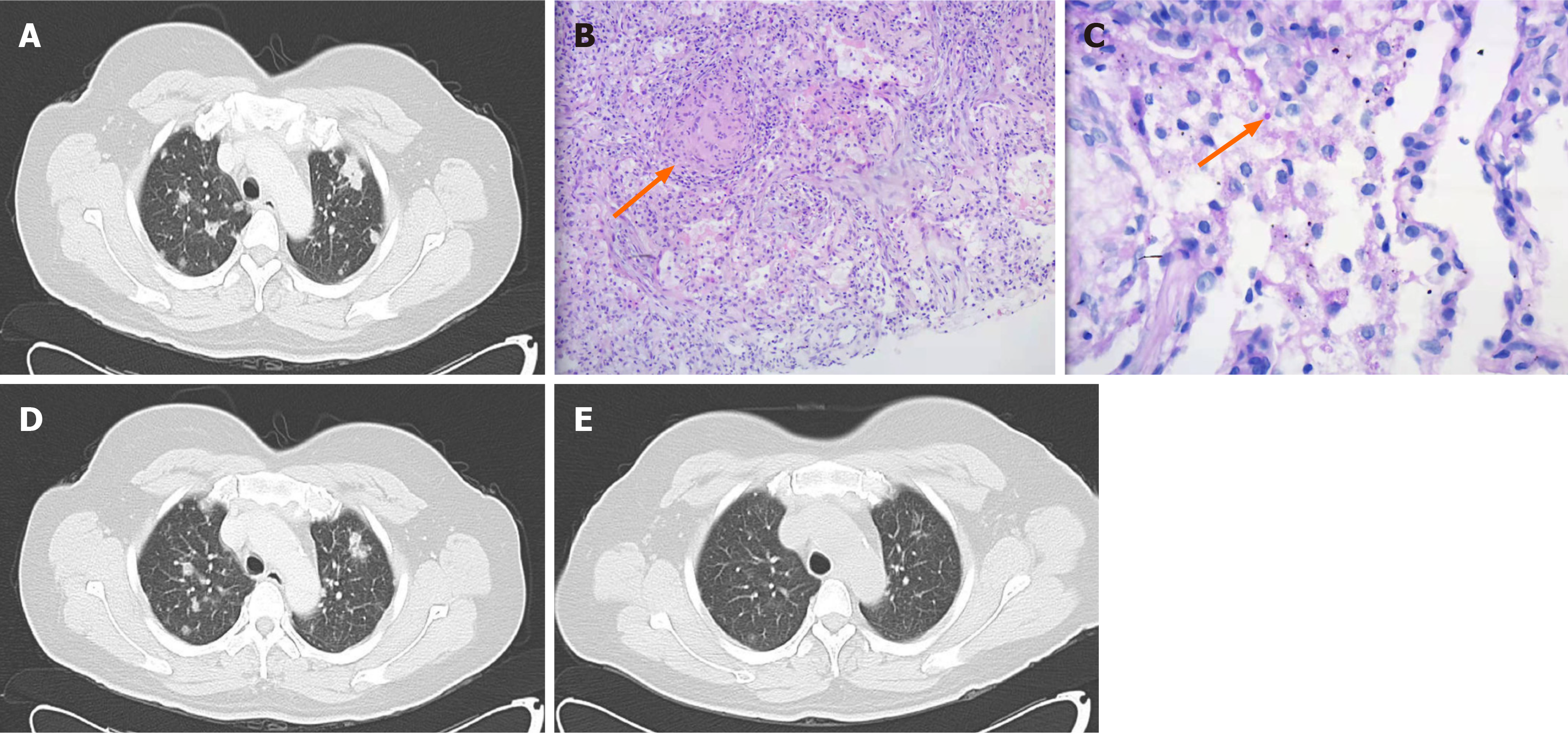
A chest computed tomography (CT) scan showed multiple nodules of unequal size with uneven internal density, and multiple small burrs at the edges of the lesions, suggesting the infection of both lungs, but not excluding the possibility of a tumor (Figure 1A). A pulmonary nodular biopsy was performed, and the results indicated no malignancy, but there was evidence of non-necrotizing granulomatous inflammation (Figure 1B) and a yeast-like corpuscle in the alveolar cavity (Figure 1C). These findings are consistent with fungal infection.
The patient had a history of cough and chest pain, but anti-infection treatment at a local hospital led to no significant resolution of these symptoms. As an inpatient at our hospital, a CT examination showed nodular lesions of the lungs, but did not exclude malignant tumor. However, the results from puncture biopsy of the injured part of the lung and pa
Upon admission and during the evaluation period, empirical intravenous treatment consisted of broad-spectrum an
After 2-wk of antibiotic treatment, CT reexamination showed that the pulmonary nodules were significantly reduced (Figure 1D). The mNGS also detected a sequence of C. parapsilosis, and pathological staining revealed a yeast-like cor
RGM are ubiquitous in the environment and commonly occur in water and soil. M. canariasense was described for the first time as the cause of a nosocomial infection in 17 patients during the period of January 2000 to September 2002 at a tertiary care hospital in the Canary Islands (Spain). This previous study reported that 15 of 17 patients had malignant diseases, and all of them had central venous catheters (CVCs) at the time of diagnosis[3]. Subsequent reports identified this species from clinical specimens, including blood, and domestic water samples[5,6]. All previously reported cases presented with malignant diseases, especially hematologic malignancies, and most of them had a history of CVC. To our knowledge, there has been no previous report of infection by M. canariasense in an immunocompetent patient. A retrospective review showed M. canariasense was considered the etiologic agent of bacteremia in 12 of 17 cases[2]. de Miguel-Martinez et al[7] also reported M. canariasense in an oncohematological patient who had a long-term central device. In general, M. canariasense is a NTM that is only rarely pathogenic. In Türkiye, 90 NTM strains obtained from four different centers only iden
Nocardia is ubiquitous in the environment and occurs worldwide as a saprophytic component in fresh water, saltwater, soil, dust, decaying vegetation, and decaying fecal deposits[11]. It is a Gram-positive bacillus, and has branching hyphae that are visible by microscopy. Nocardia infections are usually opportunistic and occur in immunocompromised hosts; infected immunocompetent patients usually develop localized cutaneous lesions. However, Beaman et al[12] found that 38 of 253 infected patients had none of predisposing factors contributing to opportunistic Nocardia infection. N. farcinica is related to Nocardia asteroides, accounts for 6.31% of Nocardia infections in China, mainly occurs in Gansu Province, and is considered the most virulent species of Nocardia[13]. Most Nocardia infections are pulmonary, and are usually attributed to inhalation of airborne spores or mycelial fragments from the environment. Dry, dusty, and windy conditions may fa
Identification of the etiology of an infectious disease plays an essential role in treatment of the patient, and laboratory culture is the traditional method for species identification. However, most patients with infections receive antibiotic treatment before sample collection, and this decreases the number of microbes and the sensitivity of culture. The mNGS technique is an unbiased method that can theoretically detect all kinds of pathogens, and is especially suitable for difficult and atypical infectious. Its main benefits are high sensitivity and rapid detection, and the results are less affected by prior use of antibiotics[15,16]. mNGS has a sensitivity rate that is approximately 15% higher than laboratory culture[16]. Less than 1% of the microorganisms identified by microscopy can be cultivated and characterized[17]. Our patient’s negative culture results from blood, sputamentum, and BALF specimens may be due to the prior use of antibiotics. On the other hand, some research suggests that cultures of isolates from patients with suspected Nocardia infections should be held in the clinical microbiology laboratory for at least 2 wk for examination. We only monitored the culture results for 5 d, and this could be a reason for the negative results.
M. canariasense is a rare species of RGM that can be grown on Lowenstein-Jensen medium after about 4 d of culture at 37 °C, and is susceptible to most antibiotics[18]. Thus, antibiotic administration prior to specimen acquisition may be the main reason for our negative culture results. A limitation in China is that patients must pay for mNGS testing. Thus, for economic reason, our patient did not receive mGNS testing of blood and fecal samples.
Previous susceptibility testing of M. canariasense showed it was highly susceptible to amikacin, cefoxitin, ciprofloxacin, moxifloxacin, trimethoprim sulfamethoxazole, imipenem, doxycycline, minocycline, and linezolid, but only had inter
In summary, this is the first report of a patient who had overlapping infections of M. canariasense and N. farcinica. In
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lomeli SM, Mexico S-Editor: Zheng XM L-Editor: A P-Editor: Xu ZH
| 1. | Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43:273-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 482] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Campos-Herrero MI, García D, Figuerola A, Suárez P, Campo C, García MJ. Bacteremia caused by the novel species Mycobacterium canariasense. Eur J Clin Microbiol Infect Dis. 2006;25:58-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Jiménez MS, Campos-Herrero MI, García D, Luquin M, Herrera L, García MJ. Mycobacterium canariasense sp. nov. Int J Syst Evol Microbiol. 2004;54:1729-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Piersimoni C, Scarparo C. Pulmonary infections associated with non-tuberculous mycobacteria in immunocompetent patients. Lancet Infect Dis. 2008;8:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Lecuona M, Abreu R, Rodríguez-Álvarez C, Castro B, Campos S, Hernández-Porto M, Mendoza P, Arias A. First isolation of Mycobacterium canariasense from municipal water supplies in Tenerife, Canary Islands, Spain. Int J Hyg Environ Health. 2016;219:48-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Grossman R, Adler A, Rubinstein M, Nissan I, Kaidar-Shwartz H, Dveyrin Z, Leshem E, Maor Y, Tau L, Rorman E. Emergence of Mycobacterium canariasense infections in central Israel. Eur J Clin Microbiol Infect Dis. 2022;41:501-504. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | de Miguel-Martinez I, Lorenzo-Garde L, Cañas-Hernandez F. [Bacteriemia due to Mycobacterium canariasense in an oncohematological patient with a long-term central device]. Enferm Infecc Microbiol Clin. 2014;32:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Gunaydin M, Yanik K, Eroglu C, Sanic A, Ceyhan I, Erturan Z, Durmaz R. Distribution of nontuberculous Mycobacteria strains. Ann Clin Microbiol Antimicrob. 2013;12:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Tagashira Y, Kozai Y, Yamasa H, Sakurada M, Kashiyama T, Honda H. A cluster of central line-associated bloodstream infections due to rapidly growing nontuberculous mycobacteria in patients with hematologic disorders at a Japanese tertiary care center: an outbreak investigation and review of the literature. Infect Control Hosp Epidemiol. 2015;36:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 10. | Sun Q, Yan J, Liao X, Wang C, Jiang G, Dong L, Wang F, Huang H, Wang G, Pan J. Trends and Species Diversity of Non-tuberculous Mycobacteria Isolated From Respiratory Samples in Northern China, 2014-2021. Front Public Health. 2022;10:923968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 11. | Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 783] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 12. | Beaman BL, Burnside J, Edwards B, Causey W. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 303] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 13. | Liu H, Lian L, Jiang Y, Huang M, Tan Y, Zhao X, Zhang J, Yu Q, Liu J, Dong H, Lu B, Wu Y, Wan K. Identification of Species of Nontuberculous Mycobacteria Clinical Isolates from 8 Provinces of China. Biomed Res Int. 2016;2016:2153910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Saubolle MA, Sussland D. Nocardiosis: review of clinical and laboratory experience. J Clin Microbiol. 2003;41:4497-4501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 15. | Han D, Li Z, Li R, Tan P, Zhang R, Li J. mNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. 2019;45:668-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 233] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 16. | Miao Q, Ma Y, Wang Q, Pan J, Zhang Y, Jin W, Yao Y, Su Y, Huang Y, Wang M, Li B, Li H, Zhou C, Li C, Ye M, Xu X, Li Y, Hu B. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin Infect Dis. 2018;67:S231-S240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 591] [Article Influence: 98.5] [Reference Citation Analysis (0)] |
| 17. | Torsvik V, Øvreås L. Microbial diversity and function in soil: from genes to ecosystems. Curr Opin Microbiol. 2002;5:240-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1196] [Cited by in RCA: 776] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 18. | Paniz-Mondolfi A, Ladutko L, Brown-Elliott BA, Vasireddy R, Vasireddy S, Wallace RJ Jr, Jakubiec W, Brecher S, Campbell S. First report of Mycobacterium canariasense catheter-related bacteremia in the Americas. J Clin Microbiol. 2014;52:2265-2269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Wei M, Wang P, Qu J, Li R, Liu Y, Gu L, Yang C. Identification and antimicrobial susceptibility of clinical Nocardia species in a tertiary hospital in China. J Glob Antimicrob Resist. 2017;11:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Cercenado E, Marín M, Sánchez-Martínez M, Cuevas O, Martínez-Alarcón J, Bouza E. In vitro activities of tigecycline and eight other antimicrobials against different Nocardia species identified by molecular methods. Antimicrob Agents Chemother. 2007;51:1102-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 21. | Jiao M, Deng X, Yang H, Dong J, Lv J, Li F. Case Report: A Severe and Multi-Site Nocardia farcinica Infection Rapidly and Precisely Identified by Metagenomic Next-Generation Sequencing. Front Med (Lausanne). 2021;8:669552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Daley CL, Iaccarino JM, Lange C, Cambau E, Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE, Guglielmetti L, Huitt GA, Knight SL, Leitman P, Marras TK, Olivier KN, Santin M, Stout JE, Tortoli E, van Ingen J, Wagner D, Winthrop KL. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 503] [Article Influence: 100.6] [Reference Citation Analysis (0)] |