Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2050
Peer-review started: December 23, 2023
First decision: February 24, 2024
Revised: March 7, 2024
Accepted: March 28, 2024
Article in press: March 28, 2024
Published online: April 26, 2024
Processing time: 114 Days and 19.5 Hours
The severity of nonalcoholic fatty liver disease (NAFLD) and lipid metabolism are related to the occurrence of colorectal polyps. Liver-controlled attenuation parameters (liver-CAPs) have been established to predict the prognosis of hepatic steatosis patients.
To explore the risk factors associated with colorectal polyps in patients with NAFLD by analyzing liver-CAPs and establishing a diagnostic model.
Patients who were diagnosed with colorectal polyps in the Department of Gastroenterology of our hospital between June 2021 and April 2022 composed the case group, and those with no important abnormalities composed the control group. The area under the receiver operating characteristic curve was used to predict the diagnostic efficiency. Differences were considered statistically signi
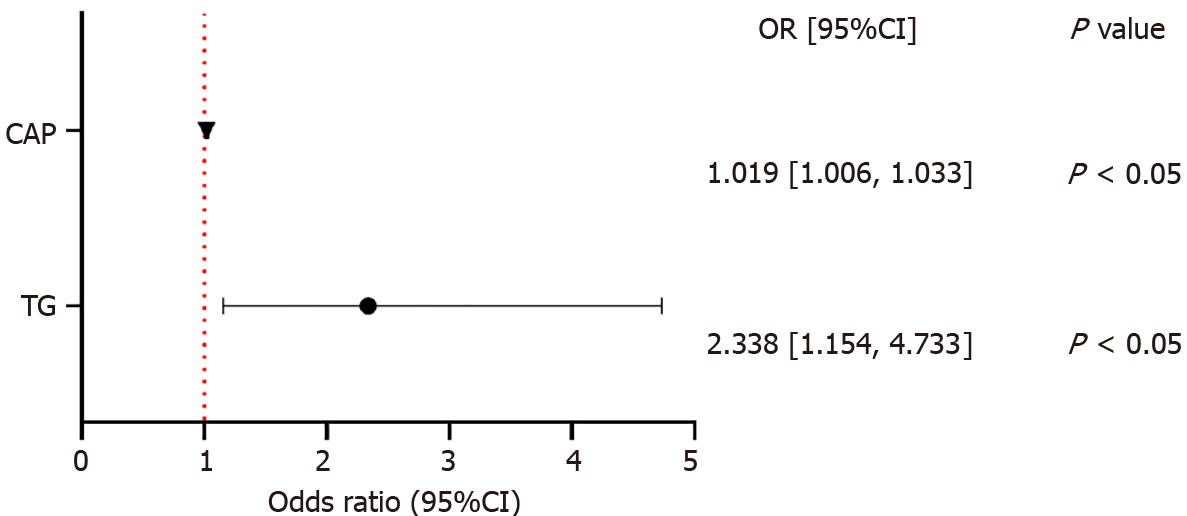
The median triglyceride (TG) and liver-CAP in the case group were significantly greater than those in the control group (mmol/L, 1.74 vs 1.05; dB/m, 282 vs 254, P < 0.05). TG and liver-CAP were found to be independent risk factors for colorectal polyps, with ORs of 2.338 (95%CI: 1.154–4.733) and 1.019 (95%CI: 1.006–1.033), respectively (P < 0.05). And there was no difference in the diagnostic efficacy between liver-CAP and TG combined with liver-CAP (TG+CAP) (P > 0.05). When the liver-CAP was greater than 291 dB/m, colorectal polyps were more likely to occur.
The levels of TG and liver-CAP in patients with colorectal polyps are significantly greater than those patients without polyps. Liver-CAP alone can be used to diagnose NAFLD with colorectal polyps.
Core Tip: This study was designed to explore the risk factors associated with colorectal polyps in patients with nonalcoholic fatty liver disease (NAFLD) by analyzing liver-controlled attenuation parameters (liver-CAPs) and establishing a diagnostic model. We found that the triglyceride (TG) and liver-CAPs in patients with colorectal polyps were significantly greater than those in patients without colorectal polyps. When the liver-CAP was greater than 291 dB/m, colorectal polyps were more likely to occur. Additionally, no difference was observed in the diagnostic efficacy or specificity between liver-CAP and TG+CAP. Liver-CAP alone can also be used to diagnose NAFLD patients with colorectal polyps.
- Citation: Wang L, Li YF, Dong LF. Transient elastography with controlled attenuation parameter for the diagnosis of colorectal polyps in patients with nonalcoholic fatty liver disease. World J Clin Cases 2024; 12(12): 2050-2055
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2050.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2050
The global incidence of colorectal cancer, a malignant tumor, has significantly increased in recent years[1-3]. Colorectal polyps are precursors of malignant colorectal tumors whose pathogenesis involves multiple factors[1,4-7], including abnormal lipid metabolism and fatty liver[4-16]. Nonalcoholic fatty liver disease (NAFLD) is considered the main cause of chronic liver disease in most patients[17], and liver-controlled attenuation parameters (liver-CAPs) have been established to predict the prognosis of hepatic steatosis patients[13,17]. This study retrospectively analyzed liver-CAPs, lipid metabolism, and other indicators in NAFLD patients with colorectal polyps to investigate the correlation between liver-CAP, lipid metabolism, and colorectal polyps in NAFLD patients and to establish a diagnostic model.
Patients who were diagnosed with colorectal polyps and who underwent electronic colonoscopy at the Department of Gastroenterology of our hospital between June 2021 and April 2022 were selected as the case group. Patients without important abnormalities during the same period were selected as the control group.
The inclusion criteria for the patients were as follows: (1) Over 18 years old; (2) had undergone electronic enteroscopy during hospitalization; and (3) had NAFLD based on a liver elasticity test.
The exclusion criteria for the patients included the following: (1) Incomplete bowel preparation or colon examination for various reasons; (2) history of inflammatory bowel disease, intestinal tuberculosis, familial adenomatous polyposis, melanosis of the colon, colorectal cancer, intestinal lymphoma, or other intestinal diseases; (3) prior liver diseases other than alcoholic fatty liver diseases, such as viral liver disease, autoimmune liver disease, genetic metabolic liver disease, or cirrhosis; (4) history of malignant tumor, metabolic syndrome, chronic kidney disease, severe infection, or other systemic diseases; and (5) use of drugs such as lipid-regulating drugs, hormones, or immunosuppressants.
After admission, general data, medical history, liver-CAP, TG, total cholesterol (TC), low-density lipoprotein (LDL), and other indicators were collected. Colonoscopies were performed by qualified senior physicians. All participants were tested for liver-CAP using FibroScan 502 Touch (Echosens). The cutoff values for the degree of lipidosis diagnosed with hepatic CAP ≥ 11%, ≥ 34%, and ≥ 67% were 238, 259, and 292 dB/m, respectively.
SPSS (version 26.0) and GraphPad (version 8.0.1.244) were used for the statistical analysis of all the data. The median values and both the 25th and 75th percentiles were associated with continuous variables. Frequencies and percentiles were associated with categorical variables. Continuous variables were compared between groups using the independent t test or Mann-Whitney U test. Multivariate analysis was performed by logistic regression, and the area under the receiver operating characteristic curve (AUROC) was used to evaluate the diagnostic efficacy. The Jorden index was calculated to obtain the cutoff value. The DeLong method was used to compare the diagnostic efficiency among the models. Differences were considered statistically significant when P < 0.05. The statistical review of the study was performed by Li-Feng Dong from Beijing ChuiYangLiu Hospital.
The study was approved by the Human Ethics Committee of Beijing ChuiYangLiu Hospital. The requirement for informed consent from patients was waived (No. 2024-002KY).
Based on the inclusion criteria, 120 patients (76 males, accounting for 63%) were included in the case group, and 52 patients (26 males, accounting for 50%) were included in the control group. There were no statistically significant differences in terms of sex ratio, age, body mass index, TC, or LDL between the two groups. The median TG concentration in the case group was significantly greater than that in the control group (mmol/L, 1.74 vs 1.05, P < 0.05). The level of liver-CAP in the case group was significantly greater than that in the control group (dB/m, 282 vs 254, P < 0.05) (Table 1).
| Parameters | Case group | Control group | P value |
| Number of Patients | 120 | 52 | NS |
| Male patients (%) | 76 (63) | 26 (50) | NS |
| Age (yr) | 58 (48, 64) | 53 (46, 62) | NS |
| BMI (kg/m2) | 25.58 (23.97, 28.12) | 25.01 (22.98, 26.85) | NS |
| TC (mmol/L) | 4.81 (3.99, 314) | 4.86 (4.05, 5.13) | NS |
| LDL-C (mmol/L) | 3.03 (2.38, 3.77) | 3.08 (2.14, 3.42) | NS |
| TG (mmol/L) | 1.74 (1.14, 2.38) | 1.05 (0.74, 1.45) | < 0.05 |
| CAP (dB/m) | 282 (247, 314) | 254 (235, 282) | < 0.05 |
Logistic multivariate analysis and forest map description were used to analyze TG and liver-CAP levels (Figure 1). TG and liver-CAP were identified as independent risk factors for colorectal polyps, with ORs of 2.338 (95%CI: 1.154–4.733) and 1.019 (95%CI: 1.006–1.033), respectively (P < 0.05).
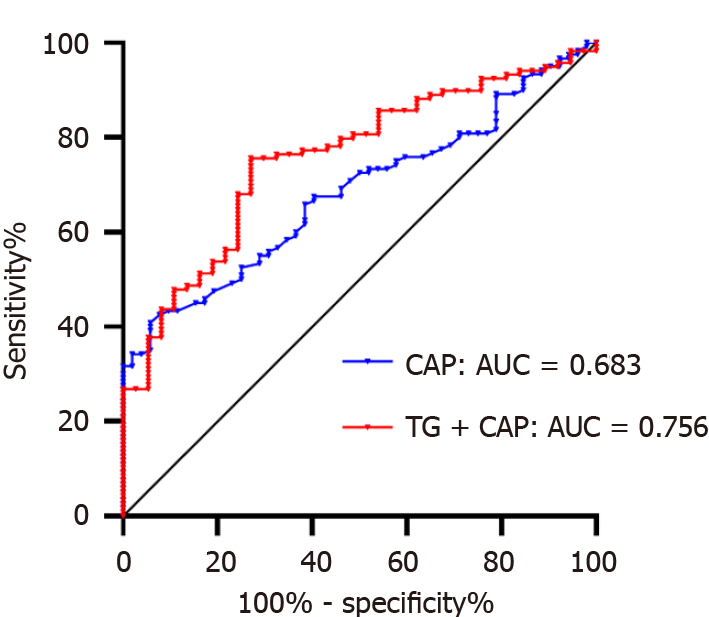
Receiver operating characteristic (ROC) analysis was performed on liver-CAP and TG+CAP samples (Figure 2). The diagnosis of colorectal polyps with liver-CAP had an AUROC of 0.683, a sensitivity of 0.408, a specificity of 0.942, and a cutoff value of 291 dB/m. When the liver-CAP was greater than 291 dB/m, the probability of developing colorectal polyps increased. The diagnosis of colorectal polyps with TG+CAP had an AUROC of 0.756, a sensitivity of 0.731, a specificity of 0.694, and a cutoff value of 0.704. Taken together, the prediction probability was calculated to be 0.704.
The DeLong method was used to compare the diagnostic efficacy of liver-CAP and TG+CAP (Table 2). No difference was observed between liver-CAP and TG+CAP in diagnosing colorectal polyps in NAFLD patients, despite the lower AUROC of liver-CAP than that of TG+CAP. Hence, the diagnostic efficacy in both groups was considered the same.
| Models compared | Z value | P value |
| CAP and TG+CAP | -1.815 | NS |
Colorectal polyps gradually develop into colorectal cancer, which can substantially impact quality of life and reduce the survival rate of patients without early intervention. Therefore, early detection and treatment of colorectal polyps are essential for improving the prognosis of colorectal cancer patients and their quality of life[1,2,4-6].
Many studies have reported that NAFLD is a risk factor for colorectal polyps[4-16]. For example, the detection rate of hyperplastic polyps in the NAFLD group was significantly greater than that in the control group, and NAFLD was associated with an increased risk of hyperplastic polyps[18]. Domestic studies have also validated that colorectal adenomatous polyps are positively correlated with NAFLD[16].
Shear wave quantified ultrasound diagnosis of liver function—liver elasticity examination—is a clinically established noninvasive method for liver evaluation. Likewise, liver-CAP can determine the prognosis of patients with hepatic steatosis and fatty liver[17].
In this study, the occurrence of colorectal polyps was predicted by the liver-CAP in patients with NAFLD. Some studies have reported that the level of liver-CAP in patients with colorectal polyps was significantly greater than that in patients with noncolorectal polyps[13], and we have also confirmed it in this study. Additionally, this study demonstrated that the diagnostic efficacy of TG+CAP was relatively similar to that of liver-CAP alone.
Compared with TG+CAP, liver-CAP had greater specificity in diagnosing colorectal polyps in NAFLD patients (0.942 vs 0.694) and was simple, rapid, and noninvasive. The findings of this study could assist patients and attending physicians in better managing fatty liver, as we reported that liver-CAPs greater than 291 dB/m increased the likelihood of developing colorectal polyps. This threshold value is close to the threshold value of liver elasticity for the diagnosis of severe steatosis (292 dB/m) and could be a promising alternative diagnostic option for patients.
In this work, the correlations between colorectal polyps, liver-CAP, and lipid metabolism in patients with NAFLD were analyzed, and ROC analysis indicated that colorectal polyps were more common in patients with NAFLD when liver-CAP was greater than 291 dB/m or when the TG+CAP index was greater than 0.704. In this study, liver-CAP was used as an indicator to predict the occurrence of colorectal polyps. Compared with those of the TG+CAP group, the AUROC (0.683) and sensitivity (0.408) in the liver-CAP group were lower, but the specificity was greater (0.942). Additionally, the diagnostic efficiency of liver-CAP alone was relatively similar to that of TG+CAP. However, further studies with larger sample sizes are still needed to verify the findings of this study.
There were several limitations to this study. This was a cross-sectional and retrospective study, and the sequence and specific time points at which fatty liver, lipid metabolism, and colorectal polyps occurred could not be distinguished. In addition, the small sample size and the place of residence of the enrolled patients could have affected the research results. Hence, it is necessary to expand the sample size and develop a prospective scheme to further verify the findings of this study.
In summary, this study revealed the correlation of both fatty liver-CAP and TG with the occurrence of colorectal polyps. In clinical practice, the findings of this study could facilitate diagnosis and treatment of colorectal polyps as well as the provision of better informed advice to patients, such as dietary plans and lifestyle information. Overall, the findings of this study could improve our understanding of colorectal polyps and fatty liver, enable detection of colorectal polyps as early as possible, and help to reduce the degree of fatty liver degeneration encountered in patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Shabrawi MH, Egypt S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Wu CX, Fu C, He J, Gu K, Gong YM, Zheng RS, Wang SM, Chen R, Zhang SW, Shi Y. [Analysis of incidence and mortality of colorectal cancer in China]. Zhongguo Aizheng Zazhi. 2020;30:241-245. [DOI] [Full Text] |
| 2. | Yang MH, Rampal S, Sung J, Choi YH, Son HJ, Lee JH, Kim YH, Chang DK, Rhee PL, Rhee JC, Guallar E, Cho J. The prevalence of colorectal adenomas in asymptomatic Korean men and women. Cancer Epidemiol Biomarkers Prev. 2014;23:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55828] [Article Influence: 7975.4] [Reference Citation Analysis (132)] |
| 4. | Wang X, Guo H, Tang Y, Chen L, Wang X. Establishment and evaluation of a nomogram predicting risks of missed diagnoses of colorectal polyps. BMC Gastroenterol. 2022;22:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Guo HH, Peng B, Xiong ZE, Xi JL, Zhou C, Tan J. [Clinical characteristics and risk factors of colorectal adenoma]. Jiezhichang Gangmen Waike. 2021;27:564-567+571. |
| 6. | Lee K, Kim YH. Colorectal Polyp Prevalence According to Alcohol Consumption, Smoking and Obesity. Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Zhu Y, Dai F, Peng Q. [Influential Factors and Correlation Analysis of Blood Lipid Levels in Patients with Gastrointestinal Polyps]. Yixue Xinxi. 2019;32:79-82. |
| 8. | Liu MR, Dai F, Peng Q. [Relationship between severity of liver fibrosis and colorectal adenomatous polyp in nonalcoholic fatty liver disease]. Zhongguo Zhongxiyi Jiehe Xiaohua Zazhi. 2022;30:36-41. |
| 9. | Gao JY, Zeng QX, and Zhang FC. [Correlation analysis between clinical features of colorectal adenomatous polyps and metabolic fatty liver]. Fujian Yiyao Zazhi. 2022;44:69-71. |
| 10. | Hao J. [Prevanlence of fatty liver disease and its correlation with colorectal adenoma in physical examination population]. Yanan Daxue. 2021;. |
| 11. | Chuan LX, Chang J, Zhao JH, Li LH, Yang XY, Yu D. [Association between nonalcoholic fatty liver disease and colorectal adenomatous polyps]. Linchuang Gandanbing Zazhi. 2020;36:1299-1303. |
| 12. | Zhang P, Li B, Han Y. [Risk factors of colorectal polyps in elderly patients and its correlation with fatty liver]. Shiyong Laonian Yixue. 2019;33:697-700. |
| 13. | Zhang X, Cui YX, Hu DD, Jiang XJ. [Analysis of Correlation of Lipid Metabolism and Controlled Attenuation Parameter of Liver With Colorectal Polyps]. Weichangbingxue. 2018;23:741-743. |
| 14. | Wang S, Fang F, Ye L, Chen J, Lu H, Yang MF, Wang FY. [Analysis of Correlation Between Nonalcoholic Fatty Liver Disease and Colorectal Polyps]. Weichangbingxue. 2018;23:75-77. |
| 15. | Xu Y. [Clinical study on the correlation between nonalcoholic fatty liver disease and gastrointestinal polyps]. Shanghai Jiaotong Daxue. 2017;. |
| 16. | Yang W, Yu XH, Wang YZ, Yang ZJ. [Colorectal adenomatous polyps and non-alcoholic fatty liver disease]. Zhonghua Ganzangbing ZAzhi 2014; 22: 66-68. |
| 17. | National Workshop on Fatty Liver and Alcoholic Liver Disease, Chinese Society of Hepatology; Chinese Medical Association; Fan JG, Wei L, Zhuang H. [Guidelines of prevention and treatment for nonalcoholic fatty liver disease: a 2018 update]. Linchuang Gandanbing Zazhi. 2018;34:947-957. [DOI] [Full Text] |
| 18. | Mahamid M, Abu-Elhija O, Yassin T, Nseir W. Advanced Hepatic Fibrosis in Fatty Liver Disease Linked to Hyperplastic Colonic Polyp. Can J Gastroenterol Hepatol. 2017;2017:2054871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |