Published online Apr 26, 2024. doi: 10.12998/wjcc.v12.i12.2031
Peer-review started: December 30, 2023
First decision: January 16, 2024
Revised: February 7, 2024
Accepted: March 26, 2024
Article in press: March 26, 2024
Published online: April 26, 2024
Processing time: 107 Days and 19.2 Hours
Gluten ataxia and other central nervous system disorders could be linked to gluten enteropathy and related autoantibodies. In this narrative review, we focus on the various neuro-logical manifestations in patients with gluten sensiti
Core Tip: Crucial insights into the complex nexus between gluten enteropathy and neurological disorders underscore the significance of specific autoantibodies. Anti-gliadin, anti-transglutaminase 2, anti-glycine receptor, anti-glutamine acid decarboxylase, and anti-deamidated gliadin peptides antibodies emerge as pivotal biomarkers, linking conditions from the gluten spectrum to diverse neurological manifestations. The prevalence of these antibodies in patients with gluten enteropathy and associated neurological dysfunction offers a diagnostic compass. Furthermore, the transformative impact of a gluten-free diet on clinical outcomes highlights its therapeutic relevance.
- Citation: Velikova T, Vasilev G, Shumnalieva R, Chervenkov L, Miteva DG, Gulinac M, Priftis S, Lazova S. Autoantibodies related to ataxia and other central nervous system manifestations of gluten enteropathy. World J Clin Cases 2024; 12(12): 2031-2039
- URL: https://www.wjgnet.com/2307-8960/full/v12/i12/2031.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i12.2031
Ataxia (from the Greek "lack of order") is a spectrum of neurological symptoms characterized by a dysfunction of motor control affecting coordination and balance, occasionally also accompanied by cognitive impairment. Depending on the affected area of the nervous system, ataxia could be divided into cerebellar, sensory or vestibular and, depending on the causative factor, into sporadic, hereditary and acquired[1].
Celiac disease (CD) is a chronic, multisystemic, autoimmune condition caused by gluten consumption in genetically susceptible individuals. Almost 20 years ago, Fasano and Catassi[2] reported that for every one patient with gluten enteropathy with gastrointestinal symptoms, there are seven patients with extraintestinal manifestations. Moreover, it is assumed that the small bowel is no longer "the sole protagonist in gluten sensitivity"[3].
Conditions like dermatitis herpetiformis, gluten ataxia, etc., changed our perspective on CD. Although neurological disorders have been observed in patients with biopsy-proven CD[4]. Hadjivassiliou et al[5] demonstrated that gluten sensitivity, and gluten enteropathy particularly, can manifest as neurological dysfunction alone. The most prevalent neurological symptom of gluten enteropathy is ataxia (gluten ataxia)[6]. However, only a subset of individuals who present with neurological impairment due to gluten sensitivity will also have an enteropathy[7].
Additionally, idiopathic cerebellar ataxia (CA) (with a prevalence of 2%-15%)[8], peripheral neuropathy (1.5%-8%), some forms of epilepsy, migraine, attention/memory impairment, Guillain-Barre-like syndrome, chorea, myelopathy, mononeuritis multiplex, etc., could be neurological manifestations of CD[9,10].
The other individuals do not have histological signs of small intestinal damage but serological markers (serum autoantibodies) consistent with gluten enteropathy, a scenario similar to dermatitis herpetiformis. Genetic susceptibility may be tested in challenging cases because human leucocyte antigen (HLA) DQ2 is prevalent in up to 90% of CD patients[8].
The multisystem involvement in CD is probably due to the expression of transglutaminase (TG) isoforms, the main antigen for the disease, in many tissues and organs. The widespread localization of TG2 and TG3-6 includes skin, nervous system, pancreas, muscle, liver, joints, thyroid, etc., allowing for multiple damage in case of anti-TG production[11]. However, the pathogenesis of neurological involvement in gluten enteropathy is unclear and still discussed. Previous hypotheses focused on intestinal malabsorption and related vitamin deficiencies (i.e., folic acid, cyanocobalamin, vitamin E, thiamine, etc.).
Pathologic studies on the central nervous system (CNS) of patients with neurological CD, on the other hand, have recently revealed that immune-mediated processes can play a role by inducing neuronal damage and dysfunction[12]. In this vein, it has been revealed that circulating anti-neuronal antibodies (NA) of the IgG class target the central and enteric nervous systems (ENS) (CNS and ENS, respectively) in a considerable number of neurological CD patients[13].
An important aspect of the different causes of ataxia is the possibility of autoimmune-mediated damage of the cerebellum or its related structures by impaired cellular or cell-mediated immunity or humoral immunity with production of antibodies targeting the neuronal structures[14]. Autoimmune neuronal damage could occur in the setting of classical autoimmune diseases presenting with ataxia when the cerebellum is one of the multiple autoimmune targets, such as in multiple sclerosis (MS), Behçet syndrome, connective tissue disorders such as systemic lupus erythematosus, hypothyroidism, etc. The term immune-mediated CA (IMCA) is therefore used to describe primary or mainly pure CA when the cerebellum is the main autoimmune target. IMCA is divided into two main subtypes-triggered by another disease or condition (neoplasm, infection, gluten enteropathy) and not triggered by another disease or condition [anti-glutamine acid decarboxylase (GAD) ataxia]. When immune-mediated mechanisms are highly suspected but no serological markers are found, the disease is described as primary autoimmune CA[14-16].
Possible pathophysiological autoimmune-mediated mechanisms related to neuronal damage include both cell- and humoral-driven processes. Mechanisms such as deficits in immune tolerance or molecular mimicry have been linked to dysfunction of T- and B-cells and subsequent autoantibody production, cell cytotoxicity, exacerbation of local neural inflammation and cell death[1].
Studies in MS have shown that cell cytotoxicity has been linked to infiltration of CNS with Th1/Th17, CD8+ cells and macrophages and dysfunction of regulatory T cells resulting in increased production and secretion of cytokines leading to demyelination and cell death[17]. Cell-mediated cytotoxicity has also been suggested in some types of IMCA due to the increased CD8+ cells in the cerebrospinal fluid (CSF) and macrophage infiltrates in the neuronal structures[18].
An essential aspect of the pathophysiology of IMCA is the autoimmune response triggered by the formation of autoantibodies. The latter could be due to exposure to environmental factors or diseases and are directed against self-antigens, causing the formation of immune complexes, complement activation with subsequent cell migration, tissue damage and organ failure. Autoantibodies in IMCA have been described against nuclear, intracellular and extracellular antigens and have also been found in the CSF, suggesting an increased permeability and disruption of the blood-brain barrier[19].
Depending on the different subtypes of the IMCA, the neuropathological findings could include loss of Purkinje neurons with Bergmann gliosis, gliosis of the cerebellar granular neurons, inferior olivary nucleus neurons and deep cerebellar nuclei, variable inflammatory changes and perivascular lymphoid infiltrates[20].
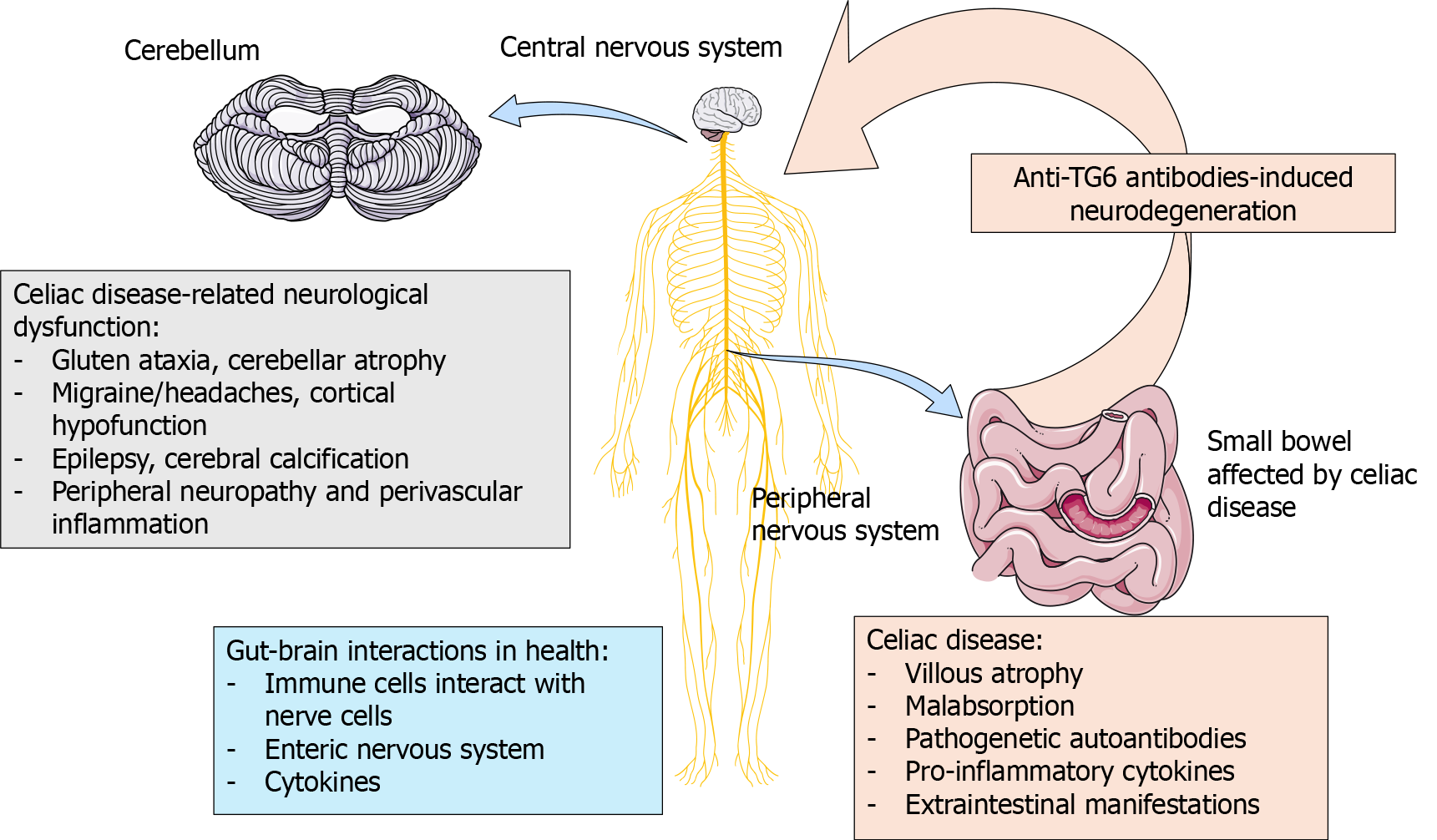
Some of the neurological manifestations in gluten enteropathy and the connection between gut and brain are shown in Figure 1.
Hadjivassiliou et al[21] investigated the prevalence of gluten-related autoantibodies in a group of patients with gluten ataxia, hypothesizing that conditions related to gluten sensitivity and especially gluten enteropathy may explain a large number of patients with sporadic idiopathic and familial ataxia (including spinocerebellar ataxia, Friedrich's ataxia, sporadic olivopontocerebellar atrophy-a cerebellar variant of multiple system atrophy). The prevalence of anti-gliadin antibodies (AGA) varied between 12%-41% depending on the type of ataxia. Furthermore, gluten enteropathy was diagnosed in 24% of tested patients. The authors suggested that gluten ataxia could be the sole most common trigger for sporadic idiopathic ataxia[3,21].
Bushara[8] also reviewed the presence of AGA in patients with gluten ataxia and other neurological manifestations in CD patients. They gathered information on the prevalence of AGA varying from 1.9% to 16.7% depending on the ataxia type. In the case of ataxia of unknown cause, the prevalence of AGA could be up to 41% in different studies. However, the antibodies were significantly higher in people with ataxia than in the general population[8].
It is worth mentioning that AGA could present in up to 50% of patients with non-celiac gluten sensitivity. In line with this, some of the symptoms from the gluten enteropathy repertoire could also be attributed to neurological manifestations (i.e., brain fog, limb numbness, headache, depression). However, it is not known whether AGA or other celiac-related antibodies cause these symptoms[22,23].
Further investigations of the team were focused on the different isoforms of TG. The authors suggested that TG6 and anti-TG6 antibodies may activate the immune system in patients with CD and neurological manifestations, such as idiopathic sporadic ataxia and peripheral neuropathy[24].
The authors reported that the prevalence of anti-TG6 antibodies was 32% in idiopathic sporadic ataxia, 73% in gluten ataxia, 32% in CD patients, and 5% in neurological controls, 4% in healthy controls. Interestingly, 42% of patients with gluten ataxia had enteropathy, and 51% of patients with ataxia had antibodies against TG6. Some patients were administered a gluten-free diet, and anti-TG6 antibodies were significantly lowered or undetectable after 1 year of treatment[24].
Still, whether the anti-NA to CNS/ENS may target or cross-react with TG6 or other isoforms is unclear[10]. Other investigators connected anti-TG antibodies to neurological and enteral damage, not just an epiphenomenon[12,25].
Sarrigiannis et al[26] hypothesized that brain hyperexcitability is a characteristic of patients with CD and neurological symptoms (i.e., cortical myoclonus, ataxia, and other dysfunctions). Furthermore, it was evidenced that these patients tend to develop refractory CD and are at risk of T cell lymphoma.
Recently, Ferlazzo et al[27] published similar results on the significance of anti-TG6 antibodies in epilepsy, cerebral calcifications and gluten-related disorders. They confirmed that anti-TG6 antibodies are biomarkers for gluten-related ataxia and neuropathy but not epilepsy[27].
The prevalence of anti-TG6 antibodies was estimated as follows: 11% in patients with CD, posterior cerebral calcifications and epilepsy; 22%-in patients with epilepsy and posterior cerebral calcifications but without CD; 0% in patients with focal epilepsy of unknown origin; 13.6%-in healthy subjects[27].
Stenberg et al[28] investigated celiac-related antibodies in children and adults with cerebral palsy, estimating a prevalence at about 36% for IgG AGA and 61% for IgA AGA, hypothesizing that poor growth in children with cerebral palsy could be associated with CD. About 7% were positive for anti-tTG2 IgA and 17.5%-for anti-deamidated gliadin peptides (DGP) antibodies[28,29].
When assessing the prevalence of anti-TG6 antibodies in patients with cerebral palsy, they found that 13% of patients with cerebral palsy and 6% of healthy controls were positive. Furthermore, the tetraplegic cerebral palsy subgroup had a significantly higher level (35%) than other groups. Additionally, the authors concluded that an early brain insult and inflammation could be prone to autoimmunity development, especially associated with anti-TG antibodies[30].
Stamnaes et al[31] speculate on the possible mechanisms of development and correlation between anti-TG6 antibodies and anti-TG2 IgA antibodies, which could explain the gluten enteropathy in patients with skin and CNS involvement.
We also know that TG6 expression is linked to neurogenesis in CNS in mice, with maturation and functional activity of the cerebellum and cerebral cortex[32].
It is now accepted that these antibodies are cross-reactive to different isoforms of TG, including TG2, TG3 and TG6. Furthermore, it was demonstrated that anti-TG antibodies from patients could induce ataxia-like defects in mice via intraventricular injection, revealing their pathogenetic potential[33]. The authors also reported the prevalence of anti-neural IgA/IgG antibodies in CD patients without neurological manifestations at 75%.
Also, Wang et al[34] and Li et al[35] conducted genetic investigations identifying mutations in the TG gene associated with autosomal dominant spinocerebellar ataxia development, suggesting the broad role of TG6 in cerebellar functioning[34,35].
However, the specificity of anti-TG6 IgA antibodies to gluten ataxia could be questioned since such antibodies were also found in patients with amyotrophic lateral sclerosis (15.3% vs 4.3% in healthy controls). Additionally, when tested for celiac-related HLA antigen alleles, 59.1% of seropositive patients were positive for celiac-related alleles. The authors concluded that amyotrophic lateral sclerosis could be associated with autoimmunity, gluten enteropathy, or sensitivity[36].
The most recent paper on anti-TG6 antibodies as a biomarker for gluten ataxia is by Sato and Nanri[37] (2017). Their review followed the presence of anti-TG6 antibodies in sporadic ataxia and also shared an experience with patients with a profound sensory disturbance that contributes to ataxia in contrast to the usually reported mild sensory disturbances[37].
Caio et al[10] investigated anti-NA in patients with CD based on the data for neurological manifestations at about 10% in these patients. They found anti-NA IgG to the CNS (at titer 1:50 to 1:400) in 21% of patients. Moreover, the prevalence of these antibodies was higher in patients with neurological dysfunction (49% vs 8%, P < 0.0001). Twenty-four percent of patients demonstrated ani-NA to the ENS, where 11/12 with antibody tire > 1:200 and severe constipation. Anti-NA to CNS and ENS were found in 7% and 5% of the healthy control group, respectively[10].
The authors conclude that anti-NA in patients with CD could be used as a marker for neurological dysfunction. Besides, this study also provides supportive data on the immune-mediated pathogenesis of CNS and ENS impairment and gut dysfunction related to gluten enteropathy, thus recommending celiac-related antibodies screening for patients with gluten ataxia peripheral neuropathy drug-resistant epilepsy. Caio et al[10] also discussed the role of anti-NA in damaging the morpho-functional integrity of the ENS, causing bowel dysmotility and altering secretion, which is linked to constipation. Moreover, sera of patients with CD containing anti-NA caused apoptosis and neuronal loss in neuronal cultures[12].
Similar to these results are those of Volta et al[13], who demonstrated that anti-NA are in low titers in patients with non-neurological CD, strengthening the association between the presence of anti-NA to CNS and neurological invol
Other antibodies related to neurological dysfunction in CD are those against glycine receptors. Kass-Iliyya et al[38] in their recent research (2021), discussed gluten enteropathy as a presentation of CNS hyperexcitability and cortical myoclonus, usually seen in refractory CD. This CNS hyperexcitability could be attributed to glycine receptor antibodies (GlyR-Abs) or, more frequently, GAD antibodies[38].
The authors previously documented a connection between gluten enteropathy and anti-GAD-associated ataxia, improved with a gluten-free diet. They were interspersed with finding a similar association with anti-GlyR-Abs. Usually, anti-GAD antibodies are associated with Stiff person syndrome, a rare autoimmune condition characterized by neuropsychiatric symptoms and axial muscle stiffness and spasms[39-41].
Furthermore, a gluten-free diet can lower the levels of anti-GAD antibodies and clinical improvement. This raised the hypothesis that gluten sensitivity is broad spectrum of conditions where anti-GAD-related neurological manifestations could occur[26,42].
Kass-Iliyya et al[38] also confirms that in the case of gluten sensitivity without enteropathy but with neurological involvement, antibodies against gliadin and TG6 could be the only positive biomarkers. In line with this, Manto et al[42] demonstrated with in vivo studies that anti-GAD antibodies inhibit GABA release leading to CA. Besides, antibodies to glycine receptors alter glycinergic neurotransmission in vitro[43]. Furthermore, it was shown that anti-GlyR-Abs related to neurological disease are a spectrum[44], and approximately one-fourth of all patients with these antibodies have other autoimmune disorders[40]. However, whether the gluten-free diet would benefit these patients is still unknown. Other authors suggested that the presence of these antibodies is an epiphenomenon rather than a pathogenetic mechanism. The other option is these antibodies are pathogenic- and the trigger is gluten sensitivity. Ashizawa et and Xia[45] suggested that a gluten-free diet in patients with CD protects against developing other autoimmune diseases in adulthood.
Ataxia is a clinical sign, not a specific disease, with diverse etiologies. Usually, imaging has a role in identifying cerebellar damage. Finding the exact site of the changes aids diagnosis-unilateral damage causes ipsilateral symptoms, while diffuse damage results in symmetrical ataxia. Limb ataxia is caused by lesions in the cerebellar hemispheres and impaired gait results from damage to the middle part of the cerebellum[45].
Ataxia can be congenital or acquired. Congenital ataxia is characterized by a smooth, chronic progression, while acquired ataxia has a more acute manifestation. Acquired ataxia can be caused by various inflammatory diseases such as cerebellitis or cerebellar abscess. Other causes of ataxia are systemic inflammatory diseases, vascular diseases such as ischemic or hemorrhagic infarction, or intoxication. Ataxia can also be caused by vitamin deficiency, various endocrinological diseases, paraneoplastic syndrome, neurodegenerative diseases and cerebellar tumors[45].
Diagnostic imaging plays a significant key role in the diagnosis of ataxia. Computed tomography (CT) and especially magnetic resonance imaging (MRI) are the methods of choice for detecting ischemic and hemorrhagic infarcts. The diffusion-weighted technique detects ischemic infarction up to 5 min after its onset, making the method indispensable in this regard. CT also immediately detects the hemorrhagic infarction. CT and MRI are the gold standard in diagnosing various cerebellar tumors and other tumors in the posterior cranial fossa with different origins[46].
In addition to acquired ataxia, imaging methods also play a role in diagnosing the congenital form of the disease[47]. The most common finding is atrophy of the cerebellum, which can be global or more pronounced in the vermis region. MRI, particularly T2 and fluid attenuated inversion recovery (FLAIR) sequences, is the preferred method for diagnosing cerebellar involvement. Characteristic findings are hyperintense lesions in the T2 sequence, most often in the deep cerebral white matter. Non-specific signs associated with ataxia are also the cortical atrophy of the cerebral hemispheres, expansion of the lateral ventricles, and the increased intensity of the frontal and parietal brain white matter. These changes are found in FLAIR sequences. Sometimes, atrophy of the cerebellum is combined with atrophy of the brainstem, with characteristic smoothing of the pons[47].
Spinocerebellar ataxia is a heterogeneous group of congenital ataxias, with over 28 subtypes described. A characteristic finding of this type of ataxia is the degeneration of the cerebellum and its tracts, as well as the brainstem, basal nuclei, cortex and peripheral nerves. Unfortunately, these findings are non-specific and do not always correspond to the severity of the disease. A modern diagnostic method is magnetic resonance morphography, which currently gives promising results[48,49].
Diffusion tensor imaging is an important sequence in detecting ataxia. A reduction in fractional anisotropy is most commonly found in the corticospinal and the pontine tract. In some cases, an increased mean diffusivity is also detected[50]. Magnetic resonance spectroscopy is another modern sequence used in ataxia. The most indicative markers are total N-acetyl aspartate (NAA), myoinositol (mI), glutamate/glutamine (Glx), and total creatine in the cerebellar hemispheres and NAA, mI and Glx in the pons. The most important change is the disturbance of the ratios between NAA and mI[51].
Gluten ataxia is relatively common in CD and can be found in patients without gastrointestinal symptoms. It occurs in both children and adults; the disease is characterized by a cerebellar type of ataxia and sometimes by a sensory type. In gluten ataxia, imaging methods (CT and MRI) reveal cerebellar atrophy, which occurs gradually but can sometimes occur suddenly. Differential diagnosis includes spinocerebellar ataxia and multisystem atrophy cerebellar type (MSA-C). In both diseases, atrophy is present, but typical for MSA-C is the disproportionate atrophy of the cerebellum and the brainstem, as well as the presence of T2 hyperintense lesions (typically in the pontocerebellar tracts, pons and the middle cerebellar peduncles)[52,53].
Nevertheless, we have to acknowledge the limitations of the serological tests and relying to them for making the diagnosis of gluten enteropathy, and gluten ataxia as well. Several studies confirmed that serologic tests, particularly the IgA EMA and the IgA tTGA, have become a relatively sensitive and specific way to initially detect CD. Many studies demonstrate a specificity of IgA tTGA greater than 95% and a sensitivity in the range of 90% to 96% and EMA has a slightly lower and variable sensitivity but an excellent specificity (99.6%). However, many individuals without CD may express AGA IgG antibody (sensitivity of AGA IgA among adults ranges between 0.65 and 1.0 and the specificity between 0.71 and 0.97). In line with this, AGA IgG is similar in sensitivity to the AGA IgA, but the specificity is much lower, approximately 0.5. Because of the variable and generally inferior accuracy of the AGA, the use of AGA IgA and AGA IgG tests is no longer recommended for identifying individuals with CD[54-57]. Nevertheless, false positive antigliadin antibody tests have been recorded in individuals with a variety of other gastrointestinal disorders, including esophagitis, gastritis, gastroenteritis, inflammatory bowel disease, cystic fibrosis and cow's milk protein intolerance. Moreover, we have also bear in mind that serologic tests may have false positive results (usually low antibody titers) in patients with other immune or inflammatory conditions such as many neurological disorders. For this reason and others, AGA Institute recommended testing for CD in persons with peripheral neuropathy, CA, and recurrent migraine, but confirmation of the diagnosis of CD requires an intestinal biopsy in all cases[56].
Based on the data accumulated so far, we can conclude that many autoantibodies related to gluten enteropathy and other conditions could be assessed in patients with ataxia or other undiagnosed neurological disorders, revealing the involvement of immunological mechanisms associated with CD. Some of these antibodies are AGA, antibodies to different isoforms of tissue TG (anti-TG2, 3, and especially anti-TG6 antibodies), anti-GlyR-Abs, anti-GAD antibodies, and anti-DGP antibodies. Some of these autoantibodies are pathogenic, others-epiphenomena, and for others-there is not enough data to conclude. The opposite is also valid-it is recommended to manage strictly and follow-up patients with CD for the development of neurological dysfunction and other extraintestinal complications.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Pavlovic M, Serbia S-Editor: Zheng XM L-Editor: A P-Editor: Guo X
| 1. | Mitoma H, Manto M, Hadjivassiliou M. Immune-Mediated Cerebellar Ataxias: Clinical Diagnosis and Treatment Based on Immunological and Physiological Mechanisms. J Mov Disord. 2021;14:10-28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (1)] |
| 2. | Fasano A, Catassi C. Current approaches to diagnosis and treatment of celiac disease: an evolving spectrum. Gastroenterology. 2001;120:636-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 804] [Cited by in RCA: 737] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 3. | Hadjivassiliou M, Grünewald R, Sharrack B, Sanders D, Lobo A, Williamson C, Woodroofe N, Wood N, Davies-Jones A. Gluten ataxia in perspective: epidemiology, genetic susceptibility and clinical characteristics. Brain. 2003;126:685-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Cooke WT, Smith WT. Neurological disorders associated with adult coeliac disease. Brain. 1966;89:683-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 231] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hadjivassiliou M, Gibson A, Davies-Jones GA, Lobo AJ, Stephenson TJ, Milford-Ward A. Does cryptic gluten sensitivity play a part in neurological illness? Lancet. 1996;347:369-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 266] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Hadjivassiliou M, Grünewald RA, Chattopadhyay AK, Davies-Jones GA, Gibson A, Jarratt JA, Kandler RH, Lobo A, Powell T, Smith CM. Clinical, radiological, neurophysiological, and neuropathological characteristics of gluten ataxia. Lancet. 1998;352:1582-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 244] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 7. | Hadjivassiliou M, Grünewald RA, Davies-Jones GA. Gluten sensitivity: a many headed hydra. BMJ. 1999;318:1710-1711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 8. | Bushara KO. Neurologic presentation of celiac disease. Gastroenterology. 2005;128:S92-S97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 156] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 9. | Chin RL, Latov N, Green PH, Brannagan TH 3rd, Alaedini A, Sander HW. Neurologic complications of celiac disease. J Clin Neuromuscul Dis. 2004;5:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Caio G, De Giorgio R, Venturi A, Giancola F, Latorre R, Boschetti E, Serra M, Ruggeri E, Volta U. Clinical and immunological relevance of anti-neuronal antibodies in celiac disease with neurological manifestations. Gastroenterol Hepatol Bed Bench. 2015;8:146-152. [PubMed] |
| 11. | Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373:1480-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 461] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 12. | Cervio E, Volta U, Verri M, Boschi F, Pastoris O, Granito A, Barbara G, Parisi C, Felicani C, Tonini M, De Giorgio R. Sera of patients with celiac disease and neurologic disorders evoke a mitochondrial-dependent apoptosis in vitro. Gastroenterology. 2007;133:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Volta U, De Giorgio R, Petrolini N, Stangbellini V, Barbara G, Granito A, De Ponti F, Corinaldesi R, Bianchi FB. Clinical findings and anti-neuronal antibodies in coeliac disease with neurological disorders. Scand J Gastroenterol. 2002;37:1276-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Mitoma H, Adhikari K, Aeschlimann D, Chattopadhyay P, Hadjivassiliou M, Hampe CS, Honnorat J, Joubert B, Kakei S, Lee J, Manto M, Matsunaga A, Mizusawa H, Nanri K, Shanmugarajah P, Yoneda M, Yuki N. Consensus Paper: Neuroimmune Mechanisms of Cerebellar Ataxias. Cerebellum. 2016;15:213-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 116] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Manto M, Hadjivassiliou M, Baizabal-Carvallo JF, Hampe CS, Honnorat J, Joubert B, Mitoma H, Muñiz-Castrillo S, Shaikh AG, Vogrig A. Consensus Paper: Latent Autoimmune Cerebellar Ataxia (LACA). Cerebellum. 2024;23:838-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Hadjivassiliou M, Graus F, Honnorat J, Jarius S, Titulaer M, Manto M, Hoggard N, Sarrigiannis P, Mitoma H. Diagnostic Criteria for Primary Autoimmune Cerebellar Ataxia-Guidelines from an International Task Force on Immune-Mediated Cerebellar Ataxias. Cerebellum. 2020;19:605-610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 17. | Huseby ES, Huseby PG, Shah S, Smith R, Stadinski BD. Pathogenic CD8 T cells in multiple sclerosis and its experimental models. Front Immunol. 2012;3:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Zaborowski MP, Michalak S. Cell-mediated immune responses in paraneoplastic neurological syndromes. Clin Dev Immunol. 2013;2013:630602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Zhang W, Ren H, Ren X, Fang F, Guan H. Autoimmune cerebellar ataxia associated with anti-Purkinje cells antibodies: the next frontier of neuroimmunology. Ann Transl Med. 2023;11:285. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Clark HB. The Neuropathology of Autoimmune Ataxias. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Hadjivassiliou M, Sanders DS, Woodroofe N, Williamson C, Grünewald RA. Gluten ataxia. Cerebellum. 2008;7:494-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Catassi C, Bai JC, Bonaz B, Bouma G, Calabrò A, Carroccio A, Castillejo G, Ciacci C, Cristofori F, Dolinsek J, Francavilla R, Elli L, Green P, Holtmeier W, Koehler P, Koletzko S, Meinhold C, Sanders D, Schumann M, Schuppan D, Ullrich R, Vécsei A, Volta U, Zevallos V, Sapone A, Fasano A. Non-Celiac Gluten sensitivity: the new frontier of gluten related disorders. Nutrients. 2013;5:3839-3853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 23. | Caio G, Volta U, Tovoli F, De Giorgio R. Effect of gluten free diet on immune response to gliadin in patients with non-celiac gluten sensitivity. BMC Gastroenterol. 2014;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Hadjivassiliou M, Aeschlimann P, Sanders DS, Mäki M, Kaukinen K, Grünewald RA, Bandmann O, Woodroofe N, Haddock G, Aeschlimann DP. Transglutaminase 6 antibodies in the diagnosis of gluten ataxia. Neurology. 2013;80:1740-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 25. | Caputo I, Barone MV, Lepretti M, Martucciello S, Nista I, Troncone R, Auricchio S, Sblattero D, Esposito C. Celiac anti-tissue transglutaminase antibodies interfere with the uptake of alpha gliadin peptide 31-43 but not of peptide 57-68 by epithelial cells. Biochim Biophys Acta. 2010;1802:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Sarrigiannis PG, Hoggard N, Aeschlimann D, Sanders DS, Grünewald RA, Unwin ZC, Hadjivassiliou M. Myoclonus ataxia and refractory coeliac disease. Cerebellum Ataxias. 2014;1:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Ferlazzo E, Polidoro S, Gobbi G, Gasparini S, Sueri C, Cianci V, Sofia V, Giuliano L, Giallonardo AT, Di Bonaventura C, Casciato S, Messana T, Coppola A, Striano S, Bilo L, Monoriti M, Genovese G, Sarica P, Arcudi L, Aguglia U. Epilepsy, cerebral calcifications, and gluten-related disorders: Are anti-transglutaminase 6 antibodies the missing link? Seizure. 2019;73:17-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Stenberg R, Dahle C, Lindberg E, Schollin J. Increased prevalence of anti-gliadin antibodies and anti-tissue transglutaminase antibodies in children with cerebral palsy. J Pediatr Gastroenterol Nutr. 2009;49:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 29. | Stenberg R, Dahle C, Magnuson A, Hellberg D, Tysk C. Increased prevalence of antibodies against dietary proteins in children and young adults with cerebral palsy. J Pediatr Gastroenterol Nutr. 2013;56:233-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Stenberg R, Hadjivassiliou M, Aeschlimann P, Hoggard N, Aeschlimann D. Anti-transglutaminase 6 antibodies in children and young adults with cerebral palsy. Autoimmune Dis. 2014;2014:237107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Stamnaes J, Dorum S, Fleckenstein B, Aeschlimann D, Sollid LM. Gluten T cell epitope targeting by TG3 and TG6; implications for dermatitis herpetiformis and gluten ataxia. Amino Acids. 2010;39:1183-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 32. | Thomas H, Beck K, Adamczyk M, Aeschlimann P, Langley M, Oita RC, Thiebach L, Hils M, Aeschlimann D. Transglutaminase 6: a protein associated with central nervous system development and motor function. Amino Acids. 2013;44:161-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Boscolo S, Lorenzon A, Sblattero D, Florian F, Stebel M, Marzari R, Not T, Aeschlimann D, Ventura A, Hadjivassiliou M, Tongiorgi E. Anti transglutaminase antibodies cause ataxia in mice. PLoS One. 2010;5:e9698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Wang JL, Yang X, Xia K, Hu ZM, Weng L, Jin X, Jiang H, Zhang P, Shen L, Guo JF, Li N, Li YR, Lei LF, Zhou J, Du J, Zhou YF, Pan Q, Wang J, Li RQ, Tang BS. TGM6 identified as a novel causative gene of spinocerebellar ataxias using exome sequencing. Brain. 2010;133:3510-3518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 189] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 35. | Li M, Pang SY, Song Y, Kung MH, Ho SL, Sham PC. Whole exome sequencing identifies a novel mutation in the transglutaminase 6 gene for spinocerebellar ataxia in a Chinese family. Clin Genet. 2013;83:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 36. | Gadoth A, Nefussy B, Bleiberg M, Klein T, Artman I, Drory VE. Transglutaminase 6 Antibodies in the Serum of Patients With Amyotrophic Lateral Sclerosis. JAMA Neurol. 2015;72:676-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Sato K, Nanri K. [Gluten Ataxia: Anti-Transglutaminase-6 Antibody as a New Biomarker]. Brain Nerve. 2017;69:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 38. | Kass-Iliyya L, Sarrigiannis PG, Sanders DS, Hadjivassiliou M. Glycine receptor antibodies and coeliac disease-related neurological dysfunction. Cerebellum Ataxias. 2021;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (1)] |
| 39. | Hadjivassiliou M, Aeschlimann D, Grünewald RA, Sanders DS, Sharrack B, Woodroofe N. GAD antibody-associated neurological illness and its relationship to gluten sensitivity. Acta Neurol Scand. 2011;123:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Baizabal-Carvallo JF, Jankovic J. Stiff-person syndrome: insights into a complex autoimmune disorder. J Neurol Neurosurg Psychiatry. 2015;86:840-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 41. | Hadjivassiliou M, Sarrigiannis PG, Shanmugarajah PD, Sanders DS, Grünewald RA, Zis P, Hoggard N. Clinical Characteristics and Management of 50 Patients with Anti-GAD Ataxia: Gluten-Free Diet Has a Major Impact. Cerebellum. 2021;20:179-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Manto MU, Laute MA, Aguera M, Rogemond V, Pandolfo M, Honnorat J. Effects of anti-glutamic acid decarboxylase antibodies associated with neurological diseases. Ann Neurol. 2007;61:544-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 43. | Crisp SJ, Dixon CL, Jacobson L, Chabrol E, Irani SR, Leite MI, Leschziner G, Slaght SJ, Vincent A, Kullmann DM. Glycine receptor autoantibodies disrupt inhibitory neurotransmission. Brain. 2019;142:3398-3410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 44. | Carvajal-González A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain. 2014;137:2178-2192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 45. | Ashizawa T, Xia G. Ataxia. Continuum (Minneap Minn). 2016;22:1208-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 46. | Tomandl BF, Klotz E, Handschu R, Stemper B, Reinhardt F, Huk WJ, Eberhardt KE, Fateh-Moghadam S. Comprehensive imaging of ischemic stroke with multisection CT. Radiographics. 2003;23:565-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 109] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 47. | Mariotti C, Fancellu R, Di Donato S. An overview of the patient with ataxia. J Neurol. 2005;252:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Eichler L, Bellenberg B, Hahn HK, Köster O, Schöls L, Lukas C. Quantitative assessment of brain stem and cerebellar atrophy in spinocerebellar ataxia types 3 and 6: impact on clinical status. AJNR Am J Neuroradiol. 2011;32:890-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 49. | Frismand S, Salem H, Panouilleres M, Pélisson D, Jacobs S, Vighetto A, Cotton F, Tilikete C. MRI findings in AOA2: Cerebellar atrophy and abnormal iron detection in dentate nucleus. Neuroimage Clin. 2013;2:542-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Pagani E, Ginestroni A, Della Nave R, Agosta F, Salvi F, De Michele G, Piacentini S, Filippi M, Mascalchi M. Assessment of brain white matter fiber bundle atrophy in patients with Friedreich ataxia. Radiology. 2010;255:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Cook A, Giunti P. Friedreich's ataxia: clinical features, pathogenesis and management. Br Med Bull. 2017;124:19-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 52. | Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal Manifestations of Celiac Disease: Early Detection for Better Long-Term Outcomes. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 53. | Zis P, Hadjivassiliou M. Treatment of Neurological Manifestations of Gluten Sensitivity and Coeliac Disease. Curr Treat Options Neurol. 2019;21:10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | ESPGHAN. New Guidelines for the Diagnosis of Paediatric Coeliac Disease. Available from: https://www.espghan.org/dam/jcr:a82023ac-c7e6-45f9-8864-fe5ee5c37058/2020_New_Guidelines_for_the_Diagnosis_of_Paediatric_Coeliac_Disease._ESPGHAN_Advice_Guide.pdf. |
| 55. | Hill ID, Fasano A, Guandalini S, Hoffenberg E, Levy J, Reilly N, Verma R. NASPGHAN Clinical Report on the Diagnosis and Treatment of Gluten-related Disorders. J Pediatr Gastroenterol Nutr. 2016;63:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 56. | Husby S, Murray JA, Katzka DA. AGA Clinical Practice Update on Diagnosis and Monitoring of Celiac Disease-Changing Utility of Serology and Histologic Measures: Expert Review. Gastroenterology. 2019;156:885-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 158] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 57. | National Institute of Diabetes and Digestive and Kidney Diseas. Celiac Disease Tests. Available from: https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/digestive-diseases/celiac-disease-health-care-professionals. |