Published online Apr 16, 2024. doi: 10.12998/wjcc.v12.i11.1918
Peer-review started: November 26, 2023
First decision: January 24, 2024
Revised: February 6, 2024
Accepted: March 20, 2024
Article in press: March 20, 2024
Published online: April 16, 2024
Processing time: 136 Days and 17.7 Hours
Hypertriglyceridemia is the third leading cause of acute pancreatitis (AP), and its incidence is increasing. Due to its relatively insidious etiology, it is easy to be ignored in the early stages. In China, Chaiqin Chengqi Decoction (CQCQD) has long been employed for treating AP.
To evaluate the effectiveness of CQCQD in patients diagnosed with mild/ moderately severe hypertriglyceridemic AP (HTG-AP).
In this study, the clinical data of 39 patients with HTG-AP admitted from January 2019 to November 2022 were collected. The changes of blood lipids, gastro
Twenty patients were treated with the conventional HTG-AP regimen, and 19 patients were additionally treated with CQCQD. After receiving treatment, the triglycerides (TG) level of the CQCQD group was lower than that of the CQCQD group (3.14 ± 0.25 mmol/L vs 4.96 ± 0.47 mmol/L, P < 0.01). After 3 d of treatment, the patients in the CQCQD group had more bowel movements than the control group (2.51 ± 0.25 times vs 1.00 ± 0.17 times, P = 0.01). The gastrointestinal function of most patients returned to normal, and the acute gastrointestinal injury score was significantly lower than that of the control group (0.11 ± 0.07 vs 0.42 ± 0.11, P < 0.01).
In patients with HTG-AP, CQCQD can significantly reduce the TG level, shorten the recovery time of defecation, significantly improve the gastrointestinal function.
Core Tip: Applying Chaiqin Chengqi Decoction (CQCQD) for treating acute pancreatitis (AP) has a long-standing history in China. To validate the efficacy of CQCQD in treating hypertriglyceridemic AP (HTG-AP), we conducted a retrospective analysis of patients with HTG-AP treated at our hospital. We compared and analyzed changes in blood lipid levels, gastrointestinal symptoms, and abdominal pain before and after treatment. Following treatment, the CQCQD group exhibited significantly lower triglyceride levels compared to the control group (3.14 ± 0.25 mmol/L vs 4.96 ± 0.47 mmol/L, P < 0.01). Additionally, shortened defecation recovery time and a notable improvement in gastrointestinal function were observed.
- Citation: Zhang HF, Su ZX, Feng YH, Li SJ, Xie BY. Chaiqin Chengqi Decoction as an adjuvant treatment for mild/moderately severe hypertriglyceridemic acute pancreatitis: A retrospective study. World J Clin Cases 2024; 12(11): 1918-1928
- URL: https://www.wjgnet.com/2307-8960/full/v12/i11/1918.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i11.1918
After alcohol and cholecystolithiasis, hypertriglyceridemia is the third leading cause of acute pancreatitis (AP), accounting for about 10% of all causes, and its prevalence in the Asian population is higher and increasing[1,2]. In addition, the causes of hypertriglyceridemic AP (HTG-AP) are insidious and are commonly ignored in the early stages. Consequently, it is easy to miss a diagnosis, resulting in a disease that progresses to greater severity[3,4].
Excessive serum triglyceride (TG) levels are necessary for developing HTG-AP, and the severity of pancreatitis increases as TG levels increase[5]. High levels of chylomicrons in plasma increase blood viscosity, impair pancreatic microcirculation, and lead to ischemia[6]. Free fatty acids (FFAs) produced by simultaneous lipolysis of excess TG cause capillary damage and intracellular calcium overload in the pancreas[7]. FFAs can also stimulate the production of inflammatory mediators such as tumor necrosis factor α, interleukin (IL)-6, and IL-10, resulting in an inflammatory cascade that damages the pancreas and other organs[8].
In addition to supportive therapy, as with other causes of AP, HTG-AP treatment includes HTG treatment[9]. Hyperlipidemia is treated with lipid-lowering agents and plasma exchange, which significantly increase the risk of infection[10]. Furthermore, because many traditional Chinese medicines inhibit the inflammatory response and lower blood lipids, many people have attempted to combine traditional Chinese medicine to treat HTG-AP[11]. In treating conventional pancreatitis, Chaiqin Chengqi Decoction (CQCQD)[12], Da-cheng-qi Decoction[13] and Chaihu Guizhi Ganjiang Decoction[14] have demonstrated some curative effects. Rhubarb is included in the above-mentioned decoctions, which have the effects of lowering inflammatory mediators, reducing organ damage, and relieving defecation[15].
To confirm the efficacy of CQCQD in treating HTG-AP, we conducted a retrospective analysis of patients with HTG-AP treated in our hospital from January 2019 to November 2022.
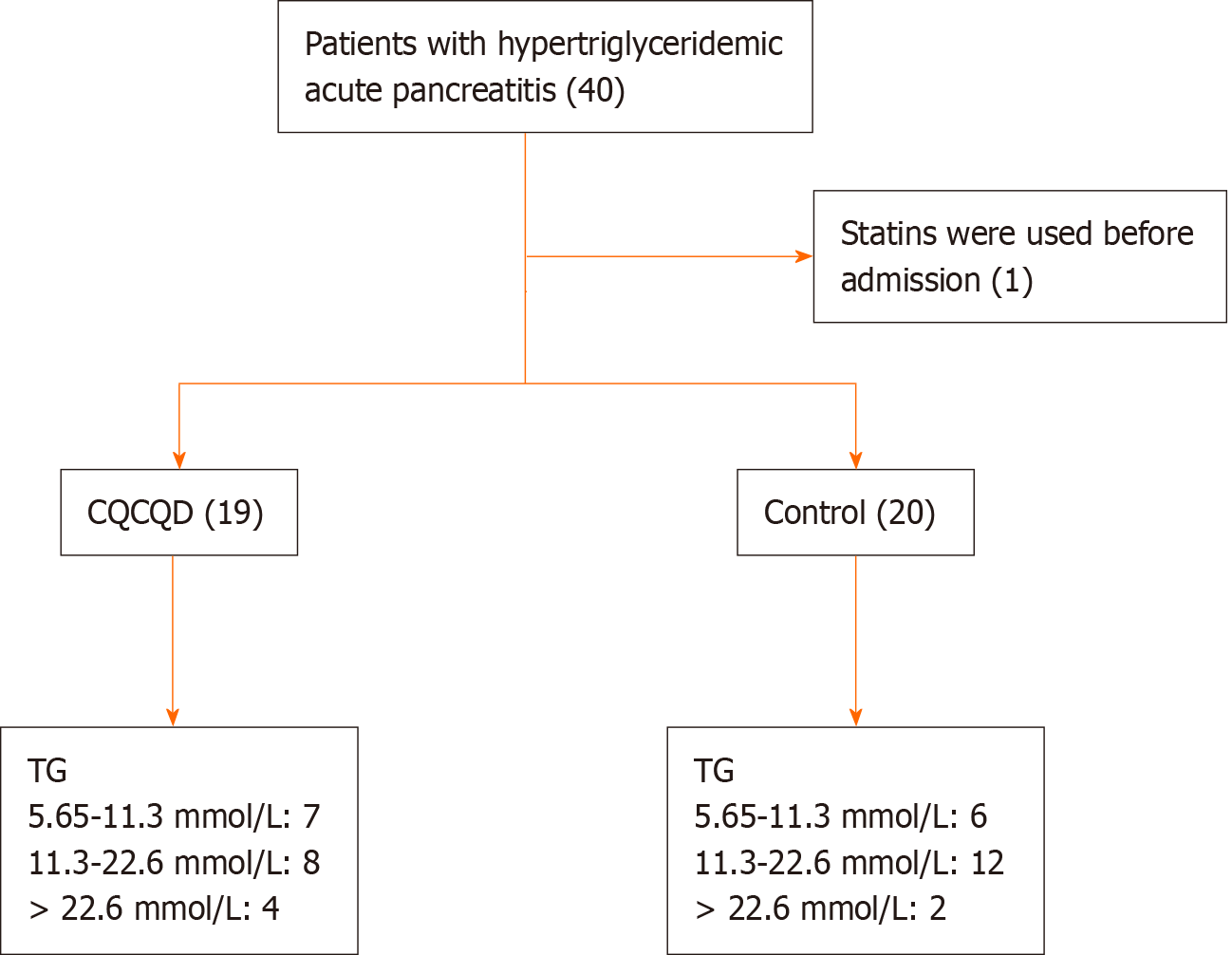
The clinical data of 39 patients with HTG-AP admitted to the First Hospital of Fuyang Hangzhou from January 2019 to November 2022 were retrospectively analyzed. All patients had mild-to-moderate pancreatitis and were previously treated with lipid-lowering therapy if tolerated by the gastrointestinal tract. Among them, 19 patients took oral Chinese medicine in the early stages, whereas the remaining 20 patients did not (Figure 1).
Patients who meet all the following criteria will be included: (1) Satisfied two of three of the following diagnostic criteria for AP: Typical symptoms and signs of abdominal pain (acute, sudden, persistent, and severe epigastric pain radiating to the back), elevated serum amylase and lipase levels of at least three times the upper limit of normal, and imaging findings consistent with AP; (2) The serum TG level > 11.30 mmol/L, or the TG level was between 5.65 and 11.30 mmol/L, but the serum was chylous[16]; (3) Other causes of pancreatitis (e.g., bile duct disease, alcohol consumption, trauma, and tumor) were ruled out; and (4) The severity of pancreatitis ranged from mild to moderate.
Patients who meet all the following criteria will be excluded: (1) Recurrent episodes of chronic pancreatitis; (2) Known history of duodenum, liver, gallbladder, or bile duct neoplasms; (3) Chronic alcohol abuse; (4) Combined cholecystolithiasis and choledocholithiasis were observed; (5) Combined with gastrointestinal bleeding or mechanical ileus; (6) Had comorbid malignancies and had received radiotherapy, chemotherapy, oral targeted agents, or immunotherapy in the previous half-year; (7) Had taken traditional Chinese medicine in the past six months; (8) Had combined familial hypercholesterolemia or were on long-term oral lipid-lowering medication for various arterial stenoses; and (9) Pregnancy or lactation.
Please refer to Table 1 for further details.
| Chinese name | English name | Latin name | Scientific name | Weight (g) |
| Chaihu | Chinese thorowax root | Bupleuri Radix | Bupleurum chinense DC. | 10 |
| Baishao | White paeony root | Paeoniae Radix Alba | Paeonia lactiflora Pall | 10 |
| Huangqin | Baical skullcap root | Scutellariae Radix | Scutellaria baicalensis Georgi | 10 |
| Zhishike | Fructus aurantii immaturus | Aurantii Fructus Immaturus | Citrus aurantium L. | 10 |
| Jianghoupo | Officinal magnolia bark | Cortex Magnoliae Officinalis | Magnolia officinalis Rehd et Wils | 10 |
| Dahuang | Rhubarb root and rhizome | Rhei Radix et Rhizoma | Rheum palmatum L. | 10 |
| Xuanmingfen | Weathered sodium sulfate | Thenardite | Na2SO4 | 10 |
| Jinyinhua | Wild honeysuckle flower | Lonicerae Japonicae Flos | Lonicera japonica Thunb. | 20 |
| Chonglou | Yunnan manyleaf paris rhizome | Paridis Rhizoma | P. polyphylla Smith var. chinensis (Franch.) Hara | 9 |
The presence or absence of gastrointestinal symptoms such as abdominal pain, abdominal distension, nausea and vomiting, and cessation of defecation was used to calculate the acute gastrointestinal injury (AGI) score[17]. The bedside index for severity of AP was used to evaluate the prognosis of patients based on blood urea nitrogen levels > 25 mg/dL, disturbance of consciousness, systemic inflammatory response syndrome, age, and pleural effusion[18]. The Balthazar computed tomography (CT) severity index was used to assess disease severity, and the prognosis was determined by combining the imaging grade of AP and the degree of pancreatic necrosis[19].
All data are presented the mean ± SEM. The IBM SPSS Statistics 23 statistical software was used for statistical analysis. The independent sample t-test was used to compare differences between groups, and statistical significance was set at P < 0.05. All statistical plots were prepared using GraphPad Prism 7 software.
In the present study, 39 patients (Table 2) were included, with an average age of 40 years. Among them, 76.92% were males, and all patients had a body mass index above the upper limit of normal. Twenty patients were treated with a conventional HTG-AP regimen, and 19 were treated with CQCQD for 3 d. Most participants (84.6%) also had fatty liver disease. At the time of admission, 13 patients in the experimental group and 9 in the control group stopped defecating. After gastrointestinal tolerance was achieved, all patients were treated with oral lipid-lowering agents (fenofibrate or atorvastatin). Serologic parameters were commonly rechecked 3-6 d (mean 4.1 d) after treatment. Abdominal CT was performed upon admission for each patient to evaluate their conditions, and most patients (89.7%) underwent CT re-evaluation 3-5 d after treatment.
| Parameters | CQCQD | Control | P value |
| Number of patients | 19 | 20 | |
| Number of male patients | 15 | 15 | 1 |
| Age (yr) | 44.00 ± 13.24 | 41.25 ± 10.19 | 0.15 |
| Hypertension (n) | 2 | 5 | 0.41 |
| Diabetes (n) | 8 | 9 | 1 |
| Fatty liver (n) | 18 | 15 | 0.18 |
| BMI (kg/m2) | 28.87 ± 4.09 | 26.78 ± 4.38 | 0.43 |
| NRS score | 2.26 ± 0.28 | 1.68 ± 0.17 | 0.07 |
| BISAP score | 1.11 ± 0.17 | 0.65 ± 0.17 | 0.06 |
| AGI score | 1.00 ± 0.00 | 0.95 ± 0.05 | 0.32 |
| Balthazar score | 2.63 ± 0.22 | 2.37 ± 0.14 | 0.32 |
| Biochemical data | |||
| WBC (× 109/L) | 13.51 ± 1.16 | 12.21 ± 1.05 | 0.41 |
| HCT | 0.42 ± 0.01 | 0.42 ± 0.01 | 0.96 |
| CRP (mg/L) | 130.55 ± 19.98 | 58.42 ± 13.50 | 0.01a |
| AST (U/L) | 29.37 ± 2.85 | 30.25 ± 5.29 | 0.87 |
| ALT (U/L) | 25.79 ± 4.11 | 34.40 ± ± 4.99 | 0.19 |
| CHO (mmol/L) | 9.76 ± 0.96 | 8.71 ± 0.56 | 0.34 |
| TG (mmol/L) | 14.93 ± 1.87 | 15.71 ± 1.72 | 0.78 |
| APOE (mg/L) | 163.26 ± 23.11 | 169.94 ± 14.52 | 0.81 |
| UA (μmmol/L) | 363.15 ± 31.70 | 383.28 ± 22.85 | 0.61 |
At the time of admission, all patients had higher blood lipid levels [CQCQD group vs control group, TG: 14.93 ± 1.87 mmol/L vs 15.71 ± 1.72 mmol/L, P = 0.78; Cholesterol (CHO): 9.76 ± 0.96 mmol/L vs 8.71 ± 0.56 mmol/L, P = 0.34]. All patients were started on oral lipid-lowering drugs once the gastrointestinal tract could tolerate them in the early stages. The TG levels of the two groups were significantly lower than those at admission to a safe level during the first reexamination of blood lipids. However, the TG level of the CQCQD group was lower than that of the control group (3.14 ± 0.25 mmol/L vs 4.96 ± 0.47 mmol/L, P < 0.01) (Figure 2A-C). Meanwhile, APOA1 Levels were significantly lower in the CQCQD group than in the control group (0.64 ± 0.03 g/L vs 0.82 ± 0.04 g/L, P < 0.01) after treatment (Figure 2D). However, CHO, HDL-C, and APOB levels were not significantly different between the two groups after treatment (Figure 2A).
On admission, 22 patients stopped defecating (13 patients in the CQCQD group), and 35 had varying degrees of upper abdominal pain. Except for one patient in the control group without gastrointestinal symptoms upon admission, all remaining patients had an AGI grade of 1. The time to resume defecation after adding CQCQD was significantly shorter for patients who had stopped defecation at admission than the control group (1.62 ± 0.21 d vs 2.40 ± 0.50 d, P = 0.04) (Figure 3). After 3 d of treatment, the patients in the CQCQD group had more bowel movements than the control group (2.51 ± 0.25 times vs 1.00 ± 0.17 times, P < 0.01) (Figure 4A). Simultaneously, two patients in the CQCQD group exhibited a frequency of defecation that exceeded three times per day.
The gastrointestinal function of most patients returned to normal, and AGI was significantly lower than the control group (0.11 ± 0.07 vs 0.42 ± 0.11, P = 0.02) (Figure 4B). Simultaneously, the defecation recovery time curve revealed that the CQCQD group resumed defecation faster (Hazard ratio: 3.7) (Figure 5). However, in the present study, CQCQD did not prove advantageous in relieving abdominal pain symptoms, which could be attributed to the relatively higher numerical rating scale scores in the experimental group on admission (2.63 ± 1.24 d vs 1.53 ± 0.70 d, P = 0.79) (Figure 3).
Except for hematocrit, the first reexamination of serum biological indicators after treatment revealed no significant difference between CQCQD and control groups (Table 3). The C-reactive protein (CRP) level in the CQCQD group was significantly higher than that in the control group on admission. However, no significant difference was observed in the CRP levels between the two groups in the first review. Furthermore, patients in the CQCQD group had a significant decrease in CRP (86.96 ± 21.62 mg/L vs 29.34 ± 12.63 mg/L, P = 0.03) (Figure 6).
| Parameters | CQCQD | Control | P value |
| Number of patients | 19 | 20 | |
| HCT | 0.38 ± 0.01 | 0.41 ± 0.01 | 0.04 |
| CRP (mg/L) | 43.70 ± 11.06 | 29.09 ± 6.13 | 0.25 |
| ALT (U/L) | 35.11 ± 6.78 | 43.70 ± 11.93 | 0.55 |
| AST (U/L) | 31.78 ± 5.53 | 34.80 ± 7.28 | 0.75 |
| ALB | 36.39 ± 1.11 | 39.33 ± 0.99 | 0.06 |
| UA (μmmol/L) | 258.83 ± 33.34 | 341.65 ± 30.15 | 0.07 |
| CERA | 62.61 ± 3.18 | 68.15 ± 3.26 | 0.23 |
During treatment, three patients (one in the CQCQD group and two in the control group) had transient alanine aminotransferase/aspartate aminotransferase levels higher than three times the upper limit of the normal range, but all returned to normal levels.
The Balthazar score, obtained through imaging, was also used to evaluate pancreatitis progression. There was no statistically significant difference in Balthazar scores between the two groups upon admission (CQCQD group vs control group: 2.63 ± 0.22 vs 2.37 ± 0.14, P = 0.32). Subsequent CT examination after 3-5 d of treatment revealed a slight improvement in Balthazar scores for both groups. However, no significant disparity was observed between the two groups (CQCQD group vs control group: 2.44 ± 0.70 vs 2.29 ± 0.69, P = 0.53) (Figure 7).
Compared with other causes of pancreatitis, HTG-AP has a longer disease course, is more likely to progress to severe disease, and has a worse prognosis[20]. On the one hand, because of the unique pathogenesis of HTG-AP, chylomicrons prevent pancreatic duct obstruction, while FFAs aggravate systemic inflammatory response[21]. On the other hand, a lack of attention to hyperlipidemia results in lower early diagnosis and intervention rates[22]. Consequently, the primary goal of HTG-AP treatment is to reduce TG levels to a safe level at the earliest. Patients with intestinal function tolerance can be treated with oral lipid-lowering drugs, whereas those who cannot tolerate them may require plasma exchange[23].
Oral lipid-lowering agents such as fenofibrate or atorvastatin are commonly used to treat hyperlipidemia. Fenofibrate accelerates chylomicron and TG degradation through the peroxisome proliferator-activated receptor (PPAR) pathway[24]; whereas atorvastatin reduces cholesterol and TG levels by inhibiting HMG-CoA[25]. In addition to hyperlipidemia, patients with HTG-AP frequently have fatty liver, diabetes, and other metabolic diseases. In China, CQCQD has a long history of use for treating AP, and some drugs regulate glucose and lipid metabolism. Bupleurum can improve lipid metabolism by upregulating the FGF21 pathway and increasing the expression of GLUT1 and PGC-1α[26]. Bupleuri radix and paeoniae radix alba synergistically reduce lipid production by activating AMP-activated protein kinase α (AMPK α) and inhibiting PPARγ[27]. Through the MAPK/PI3K/Akt signaling pathway, Scutellariae radix can improve insulin resistance and regulate blood lipid and glucose metabolism[28]. Because CQCQD reduces TG levels through a pathway different from that of statin/fibrate, patients with CQCQD in the present study had significantly lower post-treatment TG levels than those in the control group.
AP is frequently complicated by gastrointestinal dysfunction, which manifests as abdominal pain, distension, ileus, and bowel dilatation and plays an important role in disease progression[29]. Intestinal dysfunction promotes the translocation of opportunistic pathogens in the intestine, which can lead to infection and worsen AP[30]. The findings of the present study revealed that patients in the CQCQD group returned to defecation more quickly, with an average of 2.51 ± 0.25 defecation/d after 3-d treatment. CQCQD contains sodium sulfate, which is commonly used as an ionic laxative, as well as antibacterial and defecation-promoting Chinese herbs such as rhubarb and lonicerae japonicae flos. Rhubarb could improve gastrointestinal symptoms in patients with pancreatitis and significantly reduce the duration of abdominal pain and the time to first defecation[31]. Animal studies have revealed that rhubarb can improve gastrointestinal peristalsis function by increasing motion secretion and inhibiting the activity of Na+-K+-exchanging ATPase in the small intestinal mucosa[32]. Lonicerae japonicae flos has antibacterial activity against Escherichia coli, Candida albicans, and Klebsiella pneumoniae, which can help prevent infection to a certain extent[33].
Meanwhile, we confirmed that CQCQD was beneficial for gastrointestinal function recovery by comparing AGI grading. AGI grading is helpful in determining the severity of gastrointestinal dysfunction in patients with AP and can be used as an important prognostic indicator[34]. After 3-d treatment, the AGI grade in the CQCQD group was significantly lower than that in the control group.
CRP is the most commonly used and least expensive biomarker for pancreatitis, and CRP level 72 h after onset is an excellent indicator of disease severity[35]. Although the CRP levels in the CQCQD group were higher on admission, they declined more quickly. They were no longer significantly different from those in the control group, indicating that they can inhibit the inflammatory response to some extent. CQCQD treatment reduced plasma lipopolysaccharide (LPS), sCd14, and LPS-binding prot levels by inhibiting the upregulation of p-Sre, p-p85a, and c-Fos, and alleviated LPS and cytokine-mediated inflammatory exudation[36]. Saikosaponin can inhibit NLRP3 activation by downregulating the AMPK/mTOR pathway, improving islet function, preventing pancreatitis progression, and inhibiting pancreatic stellate cell activation by preventing fibrosis[37]. In addition, multiple drugs in CQCQD usually exhibit synergistic effects. Network pharmacology analysis revealed that baicalin in CQCQD reduced pancreatic acinar cell damage, and emodin, rhein, and chrysin reduced the inflammatory response by inhibiting activation of the TLR4/NLRP3 pathway[38]. Consequently, adding CQCQD quickly reduced CRP by inhibiting the inflammatory response.
In this retrospective study, no significant difference was observed in the occurrence of abnormal liver function between CQCQD and control groups. Although traditional Chinese medicine has been implicated as a potential cause of drug-induced liver damage, longer clinical observations are warranted to establish conclusive evidence. Notably, even after three consecutive days of CQCQD administration, two cases still experienced more than three episodes of defecation per day, indicating the need for timely dosage adjustment during clinical application.
CQCQD can significantly lower TG and APOA1 Levels, shorten defecation recovery time, improve gastrointestinal function, and inhibit the inflammatory response in patients with HTG-AP.
Hypertriglyceridemia is currently the third leading cause of acute pancreatitis (AP), with its incidence continuing to rise. Moreover, there exists a positive correlation between the severity of pancreatitis and elevated levels of triglycerides (TG). Notably, Chaiqin Chengqi Decoction (CQCQD) has been historically employed in our country for the treatment of AP.
CQCQD has a rich historical background in the management of pancreatitis in China. The lipid-lowering effects of certain Traditional Chinese Medicine components have been observed in previous research. In order to validate its efficacy in treating hypertriglyceridemic AP (HTG-AP) and facilitate its clinical implementation, we conducted a retrospective study.
To assess the impact of CQCQD on blood lipid levels and clinical manifestations in patients with mild, mild/moderately HTG-AP.
The clinical data of 39 patients with HTG-AP admitted to our hospital between January 2019 and November 2020 were retrospectively analyzed. We conducted a comparative analysis of changes in blood lipids, gastrointestinal symptoms, and abdominal computed tomography (CT) findings before and after treatment between the two groups.
Twenty patients were treated with conventional HTG-AP regimen, and 19 patients were additionally treated with CQCQD. After receiving treatment, the TG level of the CQCQD group was lower than that of the CQCQD group (3.14 ± 0.25mmol/L vs 4.96 ± 0.47 mmol/L, P < 0.01). However, there were no significant differences observed in other lipid parameters, including total cholesterol, high-density lipoprotein cholesterol, and apolipoprotein B, between the two groups. After 3 d of treatment, the patients in the CQCQD group had more bowel movements than the control group (2.51 ± 0.25 times vs 1.00 ± 0.17 times, P = 0.01). The gastrointestinal function of most patients returned to normal, and AGI was significantly lower than that of the control group (0.11 ± 0.07 vs 0.42 ± 0.11, P < 0.01). The CT reexamination conducted after 3-5 d of treatment revealed no significant difference in Balthazar score between the two groups (2.44 ± 0.70 vs 2.29 ± 0.69, P = 0.53).
In HTG-AP patients, CQCQD can significantly reduce the TG level, shorten the recovery time of defecation, significantly improve the gastrointestinal function.
More data are required for a more comprehensive analysis in future investigations. Simultaneously, it is imperative to conduct fundamental experiments to elucidate the underlying mechanism of CQCQD.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Matsumoto T, Japan; Nagaya M, Japan S-Editor: Zheng XM L-Editor: A P-Editor: Guo X
| 1. | Zheng Y, Zhou Z, Li H, Li J, Li A, Ma B, Zhang T, Liao Q, Ye Y, Zhang Z, Yang Y, Wang Z, Yang J, Li F. A multicenter study on etiology of acute pancreatitis in Beijing during 5 years. Pancreas. 2015;44:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 2. | de Pretis N, Amodio A, Frulloni L. Hypertriglyceridemic pancreatitis: Epidemiology, pathophysiology and clinical management. United European Gastroenterol J. 2018;6:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Olesen SS, Harakow A, Krogh K, Drewes AM, Handberg A, Christensen PA. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: A population-based cohort study. Pancreatology. 2021;21:334-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 4. | Vipperla K, Somerville C, Furlan A, Koutroumpakis E, Saul M, Chennat J, Rabinovitz M, Whitcomb DC, Slivka A, Papachristou GI, Yadav D. Clinical Profile and Natural Course in a Large Cohort of Patients With Hypertriglyceridemia and Pancreatitis. J Clin Gastroenterol. 2017;51:77-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 5. | Yang AL, McNabb-Baltar J. Hypertriglyceridemia and acute pancreatitis. Pancreatology. 2020;20:795-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 182] [Article Influence: 36.4] [Reference Citation Analysis (0)] |
| 6. | Grisham JM, Tran AH, Ellery K. Hypertriglyceridemia-induced acute pancreatitis in children: A mini-review. Front Pediatr. 2022;10:931336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 7. | Tsuang W, Navaneethan U, Ruiz L, Palascak JB, Gelrud A. Hypertriglyceridemic pancreatitis: presentation and management. Am J Gastroenterol. 2009;104:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 8. | Guo YY, Li HX, Zhang Y, He WH. Hypertriglyceridemia-induced acute pancreatitis: progress on disease mechanisms and treatment modalities. Discov Med. 2019;27:101-109. [PubMed] |
| 9. | Garg R, Rustagi T. Management of Hypertriglyceridemia Induced Acute Pancreatitis. Biomed Res Int. 2018;2018:4721357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 10. | Berberich AJ, Ziada A, Zou GY, Hegele RA. Conservative management in hypertriglyceridemia-associated pancreatitis. J Intern Med. 2019;286:644-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Chi MH, Chao J, Ko CY, Huang SS. An Ethnopharmaceutical Study on the Hypolipidemic Formulae in Taiwan Issued by Traditional Chinese Medicine Pharmacies. Front Pharmacol. 2022;13:900693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 12. | Yang X, Zhang X, Lin Z, Guo J, Yang X, Yao L, Wang H, Xue P, Xia Q. Chaiqin chengqi decoction alleviates severe acute pancreatitis associated acute kidney injury by inhibiting endoplasmic reticulum stress and subsequent apoptosis. Biomed Pharmacother. 2020;125:110024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Zhou Z, Chen Y, Dong W, An R, Liang K, Wang X. Da Cheng Qi Decoction Alleviates Cerulein-Stimulated AR42J Pancreatic Acinar Cell Injury via the JAK2/STAT3 Signaling Pathway. Evid Based Complement Alternat Med. 2021;2021:6657036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Cui L, Li C, Shang Y, Li D, Zhuo Y, Yang L, Cui N, Li Y, Zhang S. Chaihu Guizhi Ganjiang Decoction Ameliorates Pancreatic Fibrosis via JNK/mTOR Signaling Pathway. Front Pharmacol. 2021;12:679557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Hu J, Li P, Zhang T. Rhubarb combined with trypsin inhibitor for severe acute pancreatitis: A systematic review and meta-analysis. Phytother Res. 2018;32:1450-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | An F, Zhan Q, Xia M, Jiang L, Lu G, Huang M, Guo J, Liu S. From moderately severe to severe hypertriglyceridemia induced acute pancreatitis: circulating miRNAs play role as potential biomarkers. PLoS One. 2014;9:e111058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Hu B, Sun R, Wu A, Ni Y, Liu J, Guo F, Ying L, Ge G, Ding A, Shi Y, Liu C, Xu L, Jiang R, Lu J, Lin R, Zhu Y, Wu W, Xie B. Severity of acute gastrointestinal injury grade is a predictor of all-cause mortality in critically ill patients: a multicenter, prospective, observational study. Crit Care. 2017;21:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. Gut. 2008;57:1698-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 516] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 19. | Chatzicostas C, Roussomoustakaki M, Vardas E, Romanos J, Kouroumalis EA. Balthazar computed tomography severity index is superior to Ranson criteria and APACHE II and III scoring systems in predicting acute pancreatitis outcome. J Clin Gastroenterol. 2003;36:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Liu ZY, Tian L, Sun XY, Liu ZS, Hao LJ, Shen WW, Gao YQ, Zhai HH. Development and validation of a risk prediction score for the severity of acute hypertriglyceridemic pancreatitis in Chinese patients. World J Gastroenterol. 2022;28:4846-4860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 21. | Tan HLE, McDonald G, Payne A, Yu W, Ismadi Z, Tran H, Gani J, Wynne K. Incidence and Management of Hypertriglyceridemia-Associated Acute Pancreatitis: A Prospective Case Series in a Single Australian Tertiary Centre. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Hassanloo J, Béland-Bonenfant S, Paquette M, Baass A, Bernard S. Prevalence, severity and management of hypertriglyceridemia-associated pancreatitis; A 7-year retrospective cohort study at Canadian quaternary care hospitals. J Clin Lipidol. 2022;16:455-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Pulipati VP, Amblee A, Yap SET, Shaka H, Tahsin B, Fogelfeld L. Hypertriglyceridemia-associated acute pancreatitis: Response to continuous insulin infusion. PLoS One. 2021;16:e0260495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Oscarsson J, Önnerhag K, Risérus U, Sundén M, Johansson L, Jansson PA, Moris L, Nilsson PM, Eriksson JW, Lind L. Effects of free omega-3 carboxylic acids and fenofibrate on liver fat content in patients with hypertriglyceridemia and non-alcoholic fatty liver disease: A double-blind, randomized, placebo-controlled study. J Clin Lipidol. 2018;12:1390-1403.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah-Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 674] [Article Influence: 112.3] [Reference Citation Analysis (0)] |
| 26. | Wu L, Yan Q, Chen F, Cao C, Wang S. Bupleuri radix extract ameliorates impaired lipid metabolism in high-fat diet-induced obese mice via gut microbia-mediated regulation of FGF21 signaling pathway. Biomed Pharmacother. 2021;135:111187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 27. | Lee J, Park J, Park H, Youn DH, Lee J, Hong S, Um JY. Synergistic Effect of Bupleuri Radix and Scutellariae Radix on Adipogenesis and AMP-Activated Protein Kinase: A Network Pharmacological Approach. Evid Based Complement Alternat Med. 2018;2018:5269731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Cui X, Qian DW, Jiang S, Shang EX, Zhu ZH, Duan JA. Scutellariae Radix and Coptidis Rhizoma Improve Glucose and Lipid Metabolism in T2DM Rats via Regulation of the Metabolic Profiling and MAPK/PI3K/Akt Signaling Pathway. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 168] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 29. | Agarwala R, Rana SS, Sharma R, Kang M, Gorsi U, Gupta R. Gastrointestinal Failure Is a Predictor of Poor Outcome in Patients with Acute Pancreatitis. Dig Dis Sci. 2020;65:2419-2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Hu X, Gong L, Zhou R, Han Z, Ji L, Zhang Y, Zhang S, Wu D. Variations in Gut Microbiome are Associated with Prognosis of Hypertriglyceridemia-Associated Acute Pancreatitis. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Zhou Y, Wang L, Huang X, Li H, Xiong Y. Add-on effect of crude rhubarb to somatostatin for acute pancreatitis: A meta-analysis of randomized controlled trials. J Ethnopharmacol. 2016;194:495-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Shi Y, Xu J, Ding B, Chen G, Jin L, Ke L, Xu X, Wang J, Sun Q. Gastrointestinal Motility and Improvement Efficacy of Shenhuang Plaster Application on Shenque: Identification, Evaluation, and Mechanism. J Immunol Res. 2020;2020:2383970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Zheng S, Liu S, Hou A, Wang S, Na Y, Hu J, Jiang H, Yang L. Systematic review of Lonicerae Japonicae Flos: A significant food and traditional Chinese medicine. Front Pharmacol. 2022;13:1013992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 34. | Ding L, Chen HY, Wang JY, Xiong HF, He WH, Xia L, Lu NH, Zhu Y. Severity of acute gastrointestinal injury grade is a good predictor of mortality in critically ill patients with acute pancreatitis. World J Gastroenterol. 2020;26:514-523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Stirling AD, Moran NR, Kelly ME, Ridgway PF, Conlon KC. The predictive value of C-reactive protein (CRP) in acute pancreatitis - is interval change in CRP an additional indicator of severity? HPB (Oxford). 2017;19:874-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Wu W, Luo R, Lin Z, Xia Q, Xue P. Key Molecular Mechanisms of Chaiqinchengqi Decoction in Alleviating the Pulmonary Albumin Leakage Caused by Endotoxemia in Severe Acute Pancreatitis Rats. Evid Based Complement Alternat Med. 2016;2016:3265368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Cui L, Li C, Zhuo Y, Yang L, Cui N, Li Y, Zhang S. Saikosaponin A inhibits the activation of pancreatic stellate cells by suppressing autophagy and the NLRP3 inflammasome via the AMPK/mTOR pathway. Biomed Pharmacother. 2020;128:110216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Wen Y, Han C, Liu T, Wang R, Cai W, Yang J, Liang G, Yao L, Shi N, Fu X, Deng L, Sutton R, Windsor JA, Hong J, Phillips AR, Du D, Huang W, Xia Q. Chaiqin chengqi decoction alleviates severity of acute pancreatitis via inhibition of TLR4 and NLRP3 inflammasome: Identification of bioactive ingredients via pharmacological sub-network analysis and experimental validation. Phytomedicine. 2020;79:153328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |