Published online Jan 6, 2024. doi: 10.12998/wjcc.v12.i1.130
Peer-review started: September 19, 2023
First decision: December 5, 2023
Revised: December 11, 2023
Accepted: December 19, 2023
Article in press: December 19, 2023
Published online: January 6, 2024
Processing time: 104 Days and 21.7 Hours
Mycosis fungoides is the most common primary cutaneous T-cell lymphoma, whereas generalized erythroderma is rare. In this report, we describe a case of mycosis fungoides with generalized erythroderma using complete clinical data and [18F]fluoroDglucose positron emission tomography/computed tomography (18F-FDG PET/CT) images.
Systemic skin redness with desquamation for three years confirmed mycosis fungoides within one month. The patient underwent left axillary lymphadenectomy biopsy; pathological biopsy suggested abnormal T-cell lesions consistent with mycosis fungoides involving lymph nodes. The patient received metho
The 18F-FDG PET/CT is essential for early diagnosis and timely treatment.
Core Tip: Mycosis fungoides is the most common primary cutaneous T-cell lymphoma, whereas generalized erythroderma is rare. Patients with mycosis fungoides with erythroderma lesions are more severe and require poor treatment. The [18F]fluoroDglucose positron emission tomography/computed tomography is essential for early diagnosis and timely treatment. Understanding the clinical and radiographic features of this rare disease will facilitate a better therapeutic diagnosis in clinical practice.
- Citation: Xu WB, Zhang YP, Zhou SP, Bai HY. Erythrodermic mycosis fungoides: A case report. World J Clin Cases 2024; 12(1): 130-135
- URL: https://www.wjgnet.com/2307-8960/full/v12/i1/130.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v12.i1.130
Mycosis fungoides, the most common type of cutaneous T-cell lymphoma, is a rare disease with an incidence of ten cases per million people worldwide[1]. The cause has not yet been defined, but infectious agents, ultraviolet radiation, or occupational exposure may be the triggers[2]. Most patients with mycosis fungoides are adults and older adults; it is less common in children and adolescents. The main clinical manifestations are chronic skin pruritus and rashes, which can eventually develop into skin ulcers. Its histological features include the infiltration of small-to medium-sized T lym
This paper reports a case of biopsy-confirmed mycosis fungoides with skin lesions and the complete clinical and imaging data.
Systemic skin redness with desquamation for three years confirmed mycosis fungoides within one month.
A 58-year-old man with total skin redness for three years, pruritus, and desquamation was considered to have eczema at various local hospitals, with no improvement after symptomatic treatment. One month prior, due to high fever and skin pain in the left lower limb, he was treated at another hospital and underwent a skin biopsy of the left foot, which suggested mycosis fungoides. The patient was admitted to our hospital for further treatment.
The patient had no history of illness.
The patient denied any family history of malignancy.
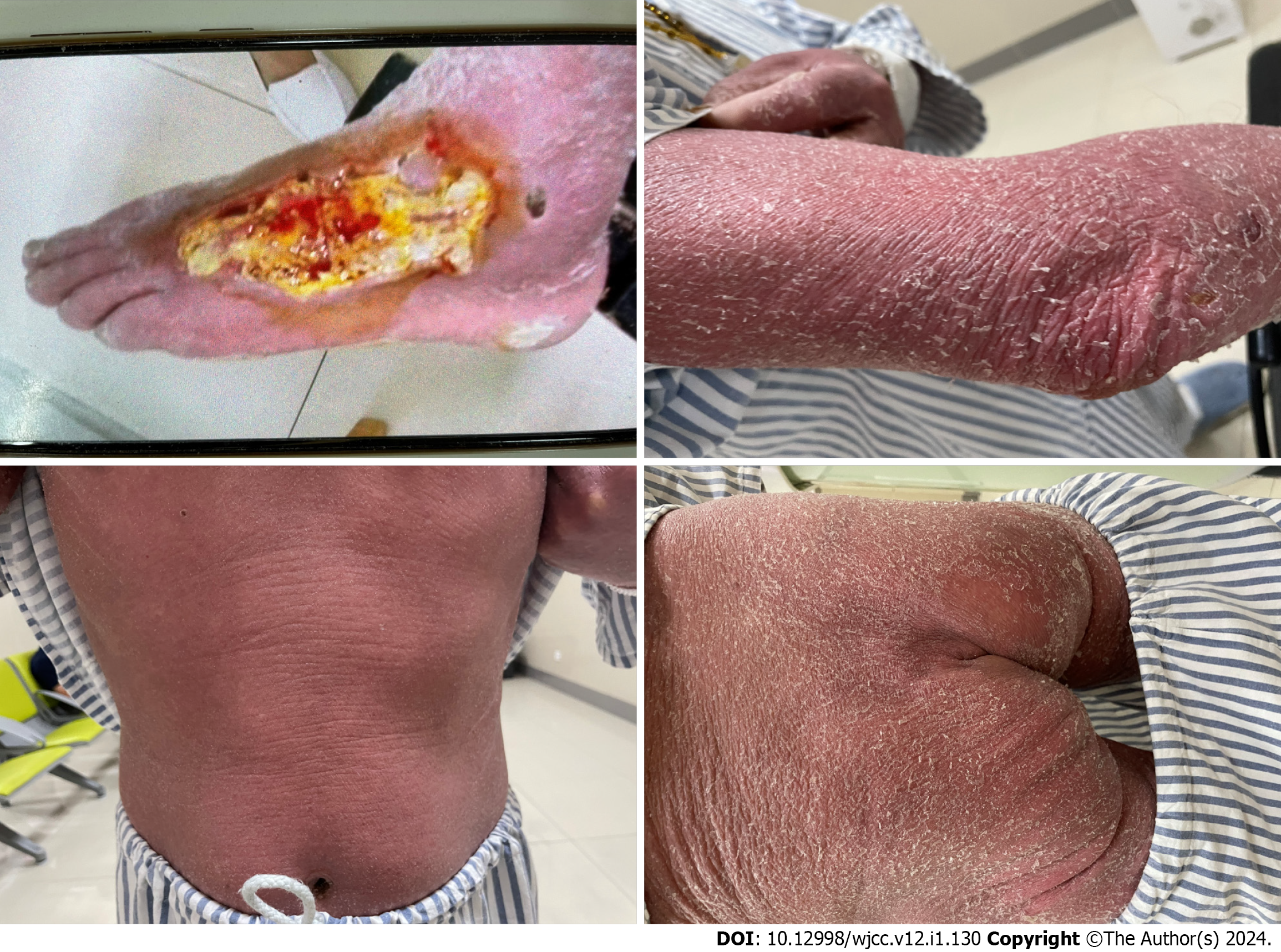
The patient's vital signs were as follows: Body temperature 36.5 ℃, blood pressure 130/97 mmHg, heart rate 107 beats/min, and respiratory rate 20 beats/min. There was total skin redness with desquamation, enlarged lymph nodes palpable in both groins, and a rupture 227 mm outside the left foot (Figure 1).
White blood cell count: 15.61 × 109/L; red blood cell count: 3.25 × 1012/L; hemoglobin count: 107 g/L. Erythrocyte sedimentation rate: 25 mm/h. Detection of T cells infected with total bilirubin: 43.88 pg/mL. Biochemical analysis revealed a C-reactive protein level of 31.36 mg/L. Blood Sezary syndrome (SS) cells (-).
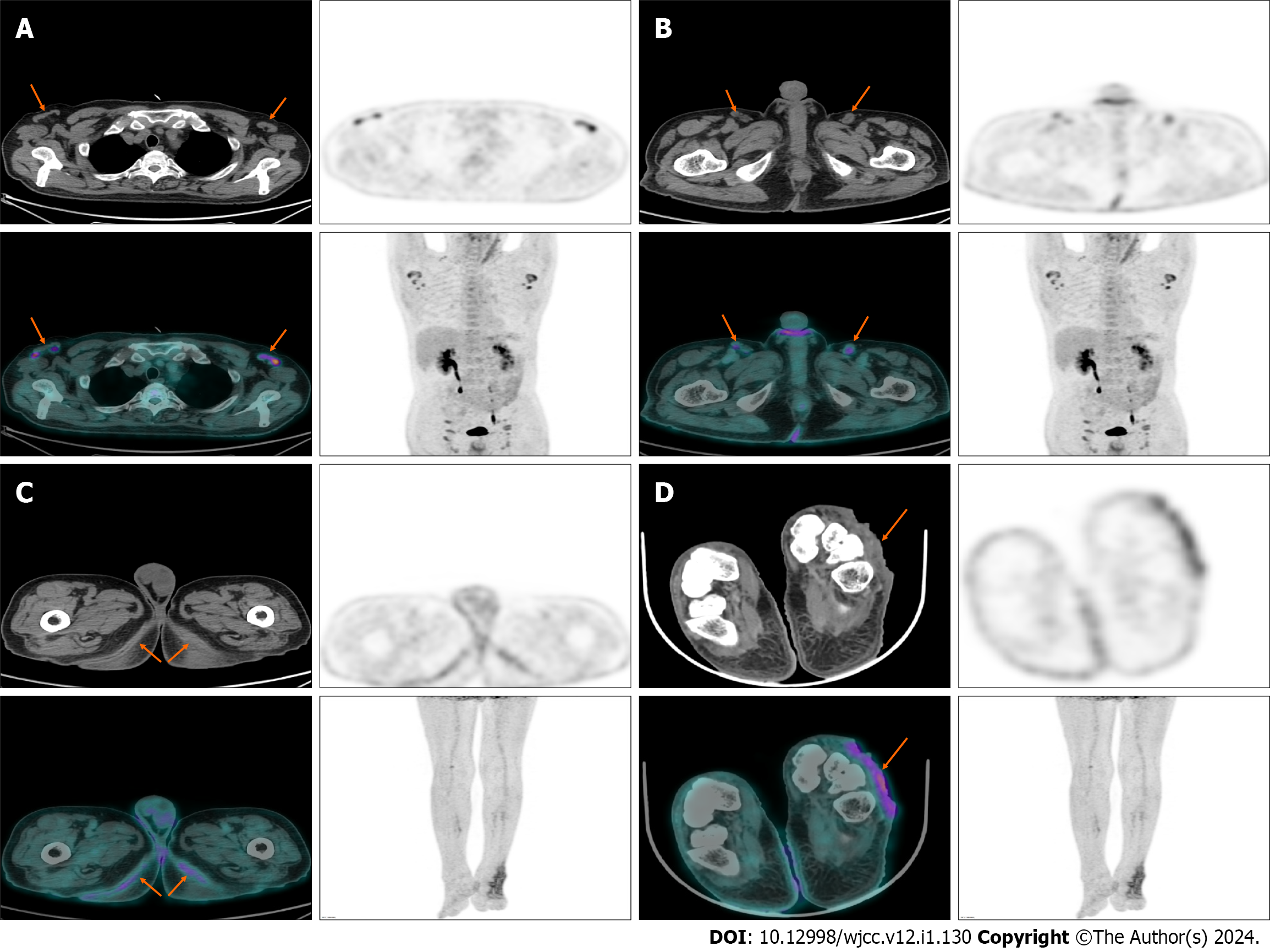
Ultrasonographic examination of the head, neck, and surface masses revealed bilateral lymph nodes in the axillary and inguinal areas. Systemic 18F-FDG PET/CT examination suggested multiple lymph node enlargement of the bilateral neck, axillary, iliac fossa, pelvic wall, and inguinal area with increased fluoroDglucose(FDG) metabolism; maximum standardized uptake value (SUVmax) was 4.92; bilateral symmetrical skin thickening, mild increase of FDG uptake, SUVmax of about 2.78; uneven thickening of soft tissue of the left back foot, visible ulcer formation, and uneven density, with high uptake of sheet FDG, and SUVmax of about 3.95 (Figure 2).
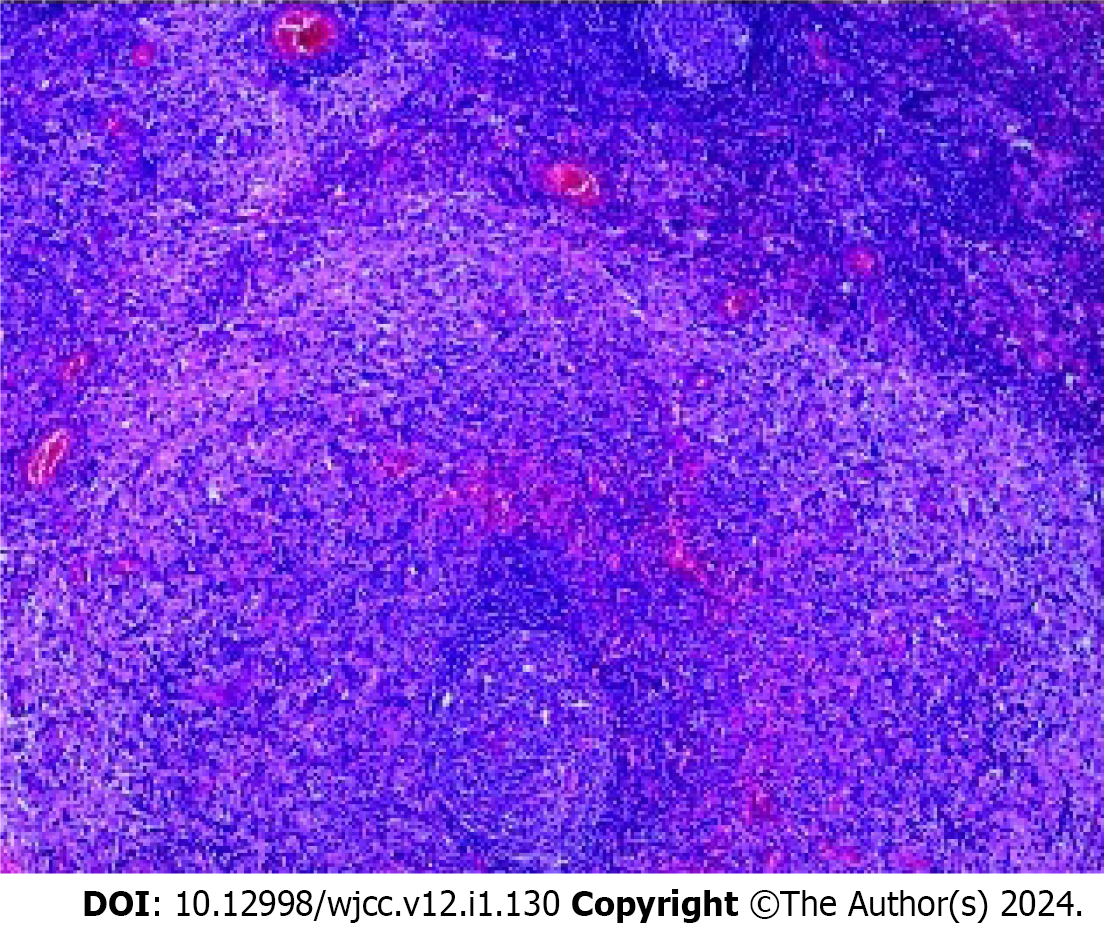
The patient underwent left axillary lymphadenectomy biopsy; pathological biopsy suggested abnormal T-cell lesions consistent with mycosis fungoides involving lymph nodes; and immunohistochemical analysis showed CD2 (+), CD3 (+), CD30 (-), Ki-67 (+, 40%), CD8 (+), CD20 (-), CD5 (+), CD4 (+), TIA-1 (part +), granzyme B (+), CD68 (part +), CD15 (part +), S-100 (+), HMB-45 (-), periodic acid-Schiff staining (-) (Figure 3).
The patient received methotrexate, 5 mg twice weekly, as part of their chemotherapy regimen.
Patients in January half after discharge, no obvious cause of high fever, left axillary lymph nodes with red heat pain, and rupture entered our hospital for treatment. Blood routine prompts white blood cells to significantly increase, biochemical combinations suggest polymerase chain reaction significantly increases, axillary ultrasound suggests left axillary mixed echo mass with surrounding lymph nodes, treatment with imipenem combined with vancomycin, moxifloxacin against infection, and methotrexate treatment of mycosis fungoides. After a series of active treatments, patients with cold fever and other discomfort improved. The condition was stable, systemic skin damage showed no significant improvement, and regular outpatient follow-ups were continued.
Mycosis fungoides is a non-Hodgkin lymphoma derived from T cells that mainly involves the skin[6], Generalized erythroderma damage is a rare clinical manifestation of mycosis fungoides; patients present with generalized skin redness, often with pruritus; some patients progress through mycosis fungoides, show limited skin damage at initial presentation, and later develop erythroderma[7]. Patients with mycosis fungoides with erythroderma lesions are more severe and require poor treatment, according to the tumor-node-metastasis classification of mycosis fungoides proposed by the International Cutaneous Lymphoma Association and the Cutaneous Lymphoma Working Group of the European Organization for Research and Treatment of Cancer[8]. Patients with mycosis fungoides with erythrocosis were classified as mycosis fungoides stage III or IV, stage A/B based on the presence of tumor cells in the blood, and stage A/B based on the presence of lymphatic and visceral involvement. There are no specific drugs for the treatment of erythroderma mycosis fungoides. For most patients with generalized erythroderma, skin-oriented therapies such as topical steroids, retinoids and phototherapy, and systemic therapies such as chemotherapy and systemic biological therapy (methotrexate, interferon)[9,10]. In this case, the patient had multiple systemic lymph node involvements, but no visceral involvement, stage A disease, a poor prognosis, no significant improvement in skin symptoms after methotrexate treatment, and a severe infection that recurred after discharge. Accurate diagnosis and staging of erythroderma mycosis fungoides are difficult[11]. First of all, due to the lack of typical clinical and tissue physiological manifestations, the clinical diagnosis is easy to be confused with specific dermatitis, eczema, SS, and other skin manifestations of erythroderma diseases[12]. In this case, when the pathological biopsy was not performed in the initial stage, eczema was considered in many local hospitals. It is worth mentioning that mycosis fungoides with erythroderma damage and SS are different independent entities, but they are erythroderma skin T-cell lymphoma, and histopathology is not specific. Mycosis fungoides with erythroderma damage is defined as the histopathological characteristics of mycosis fungoides with no typical SS blood involvement, according to whether peripheral blood contains SS cells, to distinguish mycosis fungoides with erythroderma lesions and SS. Second, despite clear histological pathology, the appropriate biopsy site is difficult to define. Finally, the routine computed tomography (CT) and magnetic resonance (MR) examination of small lymph nodes and visceral involvement is often difficult to identify, which causes trouble with the accurate staging of patients. 18F-FDG PET/CT has been used to evaluate the stage and prognosis of various lymphomas. Mycosis fungoides with erythroderma damage lesions absorb more FDG than normal tissue, and 18F-FDG PET/CT can provide metabolic activity within specific lesions and guide the localization of lesions requiring biopsy, which is important for accurate diagnosis of lesions[6]. In addition, PET/CT has higher sensitivity and specificity than CT alone and MR imaging in identifying the affected lymph nodes and detecting the affected sites of lymphoma, which can provide more accurate mycosis fungoides with erythroderma lesions staging and prognostic information, making the staging more accurate and helping patients with mycosis fungoides obtain more accurate medical treatment[13].
Herein, we report a case of mycosis fungoides with generalized erythroderma. The 18F-FDG PET/CT is essential for early diagnosis and timely treatment. Understanding the clinical and radiographic features of this rare disease will facilitate a better therapeutic diagnosis in clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Keppeke GD, Chile S-Editor: Li L L-Editor: A P-Editor: Zhao S
| 1. | Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica. 2010;102:83-87. [PubMed] |
| 2. | Damsky WE, Choi J. Genetics of Cutaneous T Cell Lymphoma: From Bench to Bedside. Curr Treat Options Oncol. 2016;17:33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Willemze R, Jaffe ES, Burg G, Cerroni L, Berti E, Swerdlow SH, Ralfkiaer E, Chimenti S, Diaz-Perez JL, Duncan LM, Grange F, Harris NL, Kempf W, Kerl H, Kurrer M, Knobler R, Pimpinelli N, Sander C, Santucci M, Sterry W, Vermeer MH, Wechsler J, Whittaker S, Meijer CJ. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2714] [Cited by in RCA: 2577] [Article Influence: 128.9] [Reference Citation Analysis (2)] |
| 4. | Domínguez-Gómez MA, Reyes-Salcedo CA, Morales-Sánchez MA, Jurado-Santa CF. Clinical variants of mycosis fungoides in a cohort. Gac Med Mex. 2021;157:41-46. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 5. | Xu L, Pang H, Zhu J, Chen X, Guan L, Wang J, Chen J, Liu Y. Mycosis fungoides staged by 18F-flurodeoxyglucose positron emission tomography/computed tomography: Case report and review of literature. Medicine (Baltimore). 2016;95:e5044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | D'Souza MM, D'Souza P, Sharma R, Jaimini A, Mondal A. Mycosis fungoides: Positron emission tomography/computed tomography in staging and monitoring the effect of therapy. Indian J Nucl Med. 2015;30:165-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Kamijo H, Sugaya M. Two distinct variants of mycosis fungoides (MF): Folliculotropic MF and erythrodermic MF. J Dermatol. 2019;46:1136-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Miyagaki T, Sugaya M. Erythrodermic cutaneous T-cell lymphoma: how to differentiate this rare disease from atopic dermatitis. J Dermatol Sci. 2011;64:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, Robson A, Calonje E, Stefanato CM, Wain EM, Wilkins B, Fields PA, Dean A, Webb K, Scarisbrick J, Morris S, Whittaker SJ. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730-4739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 597] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 10. | Suzuki SY, Ito K, Ito M, Kawai K. Prognosis of 100 Japanese patients with mycosis fungoides and Sézary syndrome. J Dermatol Sci. 2010;57:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Vandergriff T, Nezafati KA, Susa J, Karai L, Sanguinetti A, Hynan LS, Ambruzs JM, Oliver DH, Pandya AG. Defining early mycosis fungoides: validation of a diagnostic algorithm proposed by the International Society for Cutaneous Lymphomas. J Cutan Pathol. 2015;42:318-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hodak E, Amitay-Laish I. Mycosis fungoides: A great imitator. Clin Dermatol. 2019;37:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 13. | Alanteri E, Usmani S, Marafi F, Esmail A, Ali A, Elhagracy RS, Alshemmari S. The role of fluorine-18 fluorodeoxyglucose positron emission tomography in patients with mycosis fungoides. Indian J Nucl Med. 2015;30:199-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |