Published online Mar 16, 2023. doi: 10.12998/wjcc.v11.i8.1878
Peer-review started: January 8, 2023
First decision: January 30, 2023
Revised: February 6, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 16, 2023
Processing time: 58 Days and 2 Hours
Sclerosing odontogenic carcinoma is a rare primary intraosseous neoplasm that was featured recently as a single entity in the World Health Organization classification of Head and Neck Tumors 2017, with only 14 cases published to date. The biological characteristics of sclerosing odontogenic carcinoma remain indistinct because of its rarity; however, it appears to be locally aggressive, with no regional or distant metastasis reported to date.
We reported a case of sclerosing odontogenic carcinoma of the maxilla in a 62-year-old woman, who presented with an indolent right palatal swelling, which progressively increased in size over 7 years. Right subtotal maxillectomy with surgical margins of approximately 1.5 cm was performed. The patient remained disease free for 4 years following the ablation surgery. Diagnostic workups, treatment, and therapeutic outcomes were discussed.
More cases are needed to further characterize this entity, understand its biological behavior, and justify the treatment protocols. Resection with wide margins of approximately 1.0 to 1.5 cm is proposed, while neck dissection, post-operative radiotherapy, or chemotherapy are deemed unnecessary.
Core Tip: Sclerosing odontogenic carcinoma is a rare disease entity with only 14 cases published to date, this case report will further substantiate the understanding to this disease and the management protocols.
- Citation: Soh HY, Zhang WB, Yu Y, Zhang R, Chen Y, Gao Y, Peng X. Sclerosing odontogenic carcinoma of maxilla: A case report. World J Clin Cases 2023; 11(8): 1878-1887
- URL: https://www.wjgnet.com/2307-8960/full/v11/i8/1878.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i8.1878
Sclerosing odontogenic carcinoma (SOC) is an unusual primary intraosseous neoplasm that was added to the 4th edition World Health Organization (WHO) classification of Head and Neck Tumors in 2017[1]. This disease entity was first described by Landwehr and Allen[2] in 1996 and subsequently reported by Koutlas et al[3] in 2008. Nevertheless, this distinct entity remains poorly understood, with only 14 cases published to date[2-6], despite its recent inclusion in the latest WHO classification of Head and Neck Tumors. Clinically, it is characterized by locally aggressive, non-metastasizing properties, while histopathologically, it is typically illustrated by infiltrative thin cords and small nests of epithelial cells in the diffused sclerotic stroma[3]. However, there is no specific or distinctive immunohistochemical staining for SOC. Radiologically, it mostly presents as an osteolytic lesion, with or without bony perforation[4]. The histological resemblance of SOC to other disease entities poses a challenge to the accurate diagnosis of this neoplasm, while the paucity of literature makes standardizing treatment protocols more difficult. Herein, we describe a case of SOC of the maxilla, including diagnostic workups, treatment, and therapeutic outcomes.
A 62-year-old woman presented to her local hospital in December 2017 with a 7-year history of right palatal swelling. The patient first noticed a small, indolent swelling at the right anterior palate 7 years previously, which gradually increased in size over the past 2 years, associated with intermittent toothache and occasional facial swelling.
Initial clinical examination revealed a firm mass over the anterior palate, without apparent buccal and lingual expansion. The initial dental panoramic tomogram (DPT) revealed radiolucency with a welldefined sclerotic border of the right maxilla extending into the right maxillary sinus and significant root resorption of the upper central, lateral incisors, and upper right first molar. Excisional biopsy of the anterior palate was performed under local anesthesia via an intraoral approach, with extraction of the upper right central and lateral incisors at the local hospital. The histopathology examination showed SOC. Hematoxylin and eosin (H&E) sections showed small epithelial tumor cell cords in a densely collagenized stroma. No obvious dysplastic features were observed in the given specimen. The swelling resolved; however, the patient noticed the swelling of the palate again in April 2018, for which she was eventually referred to our institution for management of the right maxillary tumor (Supplementary material).
Except for long-standing diabetes mellitus and hypertension, her past medical history was unremar
Unremarkable.
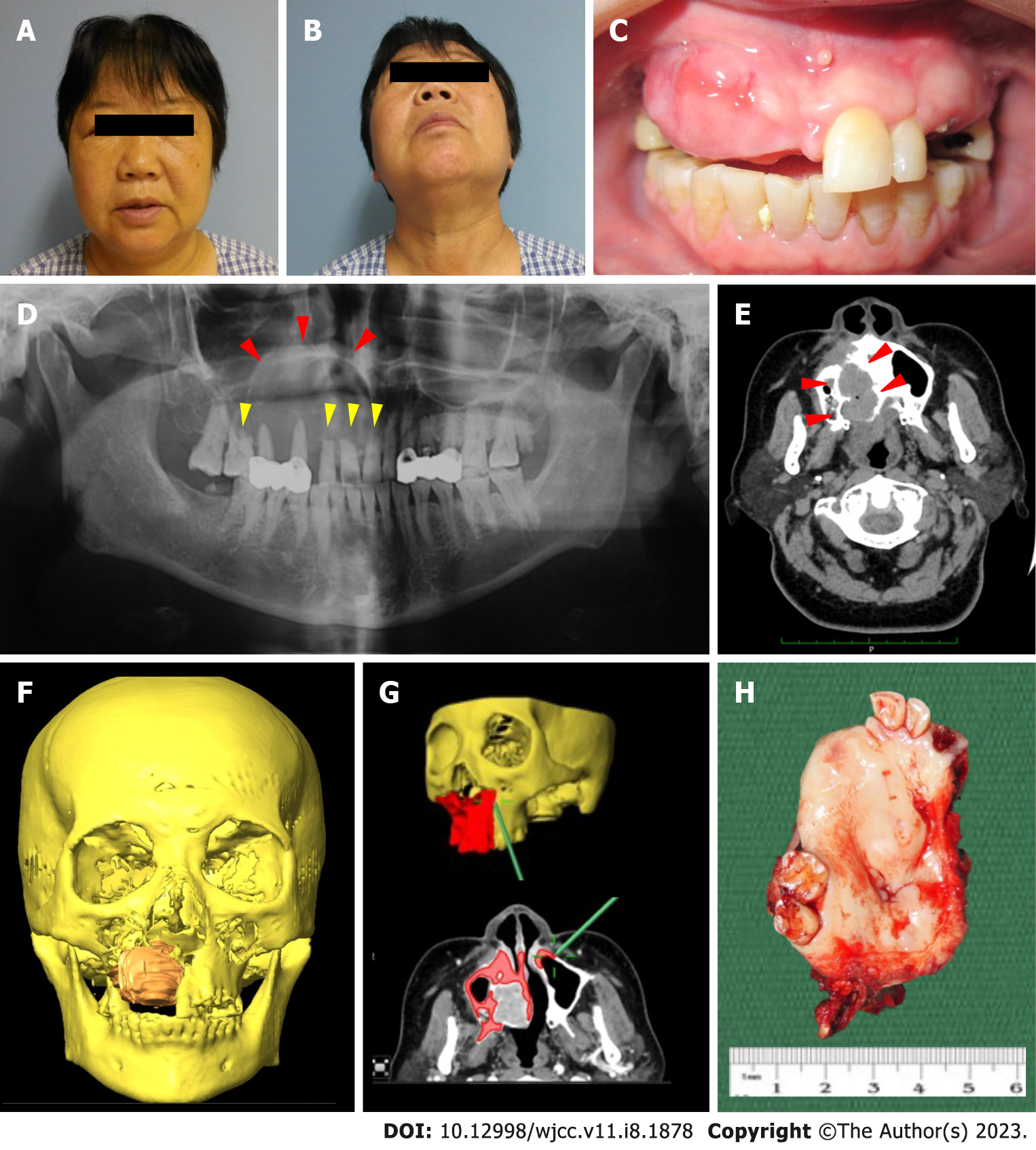
Clinically, the patient presented with diffuse right facial swelling without overlying skin changes. The swelling was diffuse and firm, causing obliteration of the right nasolabial fold. Mouth opening was not restricted and there was no palpable cervical lymphadenopathy. There were no neurosensory changes to the right infraorbital region (Figure 1A and B). Intra-oral examination showed an irregular mass at the anterior maxilla, extending from the tooth 11 to 15 region, but without obliteration of the buccal sulcus. The swelling was firm and non-tender upon palpation, with non-ulcerated overlying mucosa. The adjacent teeth showed no marked increase in mobility and there was no fluid discharge noted upon palpation (Figure 1C).
The histopathology examination of the excisional biopsy that was performed at the local hospital showed SOC. H&E sections showed small epithelial tumor cell cords in a densely collagenized stroma. No obvious dysplastic features were observed in the given specimen.
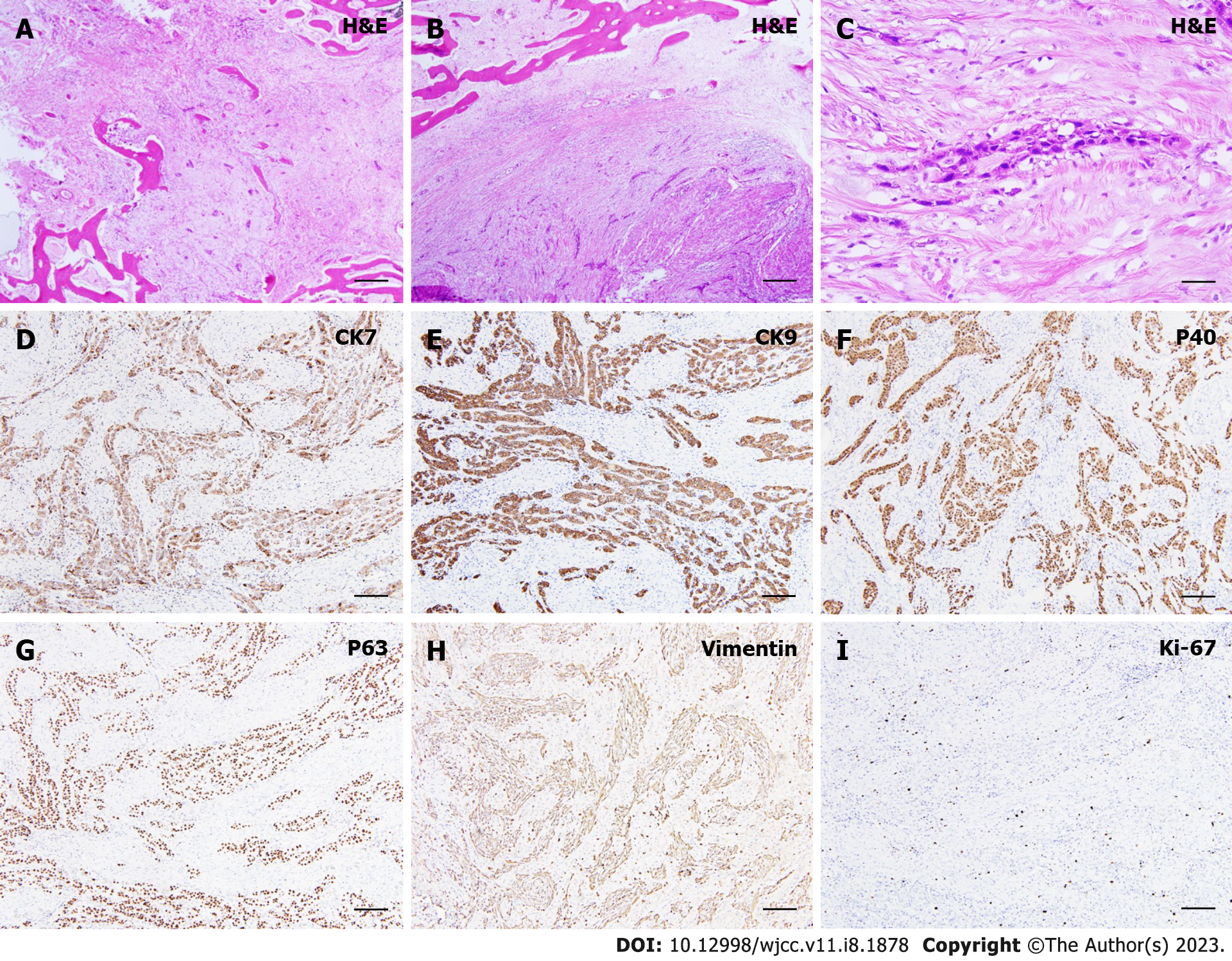
A gross pathological examination revealed a firm, expansile mass involving the right palate and right maxillary sinus. However, the overlying mucosa appeared intact and not ulcerated (Figure 1H). The resected specimens were fixed in 10% formalin, processed, and embedded in paraffin blocks for histopathological examination. H&E sections showed small nests or cords of small neoplastic epithelial cells, immersed within a sclerotic stroma, with perivascular infiltration. Under low power magnification, the tumor cells demonstrated an infiltrating nature towards mature lamellar bone fragments and generally, the tumor appeared to be non-encapsulated. The epithelial cells appeared to be faintly hyperchromatic, while focal areas of tumor islands exhibited round hyperchromatic nuclei with clear cytoplasm. Pleomorphism was uncommon and mitotic figures were scarce, with no significant cellular atypia present (Figure 2A-C). Immunohistochemically, strong positive staining was observed for cytokeratin 7 (CK7) (Figure 2D) and CK19 (Figure 2E). Tumor cell showed positive expression of p40 (Figure 2F) and p63 (Figure 2G). The tumor cells stained negative for vimentin (Figure 2H) and the proliferative activity was approximately 5%-10% according to the Ki-67 staining results, suggesting a low-grade malignancy (Figure 2I). The sections also stained negative for S-100.
The initial DPT revealed radiolucency with a welldefined sclerotic border of the right maxilla extending into the right maxillary sinus and significant root resorption of the upper central, lateral incisors, and upper right first molar. DPT was repeated and revealed an ill-defined radiolucency at the right maxilla, with a slight increase in size, as compared with the initial DPT (Figure 1D). The computed tomography (CT) scan showed an expansile enhancing osteolytic mass at the right maxilla, with marked buccal and palatal bone perforation (Figure 1E). The lesion extended into the right inferior turbinate and breached the nasal septum. No prominent radiological evidence of lymphatic spread to the cervical region was seen.
A diagnosis of SOC was reached based on the clinical features and the radiological and histopathological findings.
Right subtotal maxillectomy and reconstruction with a vascularized free fibula flap were performed under the guidance of an intraoperative navigation system (BrainLAB, AG, Feldkirchen, Germany). Approximately 1.5 cm surgical margins were resected, guided by the intraoperative navigation system, to ensure clear surgical margins (Figure 1F and G). The ipsilateral free vascularized fibular flap was harvested simultaneously with the tumor resection. Neck dissection was not performed because of negative clinical and radiological findings of the cervical region. Intraoperative frozen section biopsy from the surgical margins showed that all margins were tumor-free.
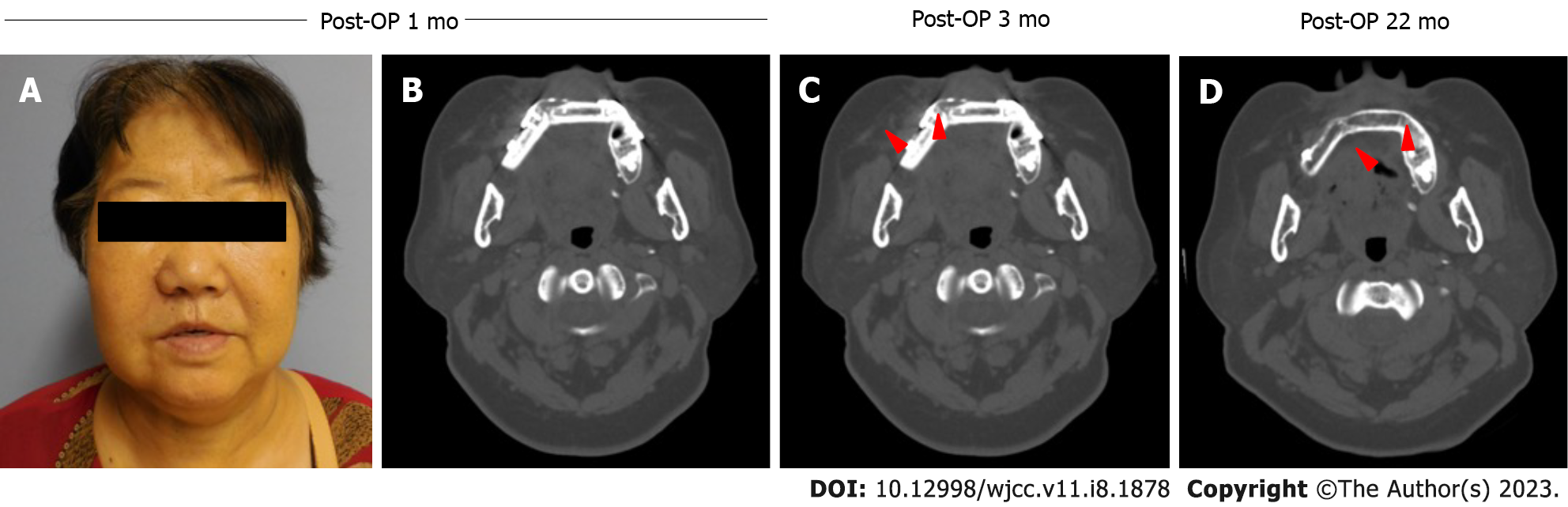
Post-operative recovery was uneventful, and the patient was subjected to standardized oral oncology follow-up. At the post-operative one and six month-reviews, the patient was pleased with her post-operative appearance and functions (Figure 3A). Upon clinical examination, the vascularized free fibula flap provided good oroantral seal and support to the facial profile. The CT scan revealed excellent bony consolidation of the graft and there was no obvious recurrence or metastasis noted radiographically or clinically (Figure 3B-D). The patient remains diseasefree 22 mo after the surgery.
SOC is a relatively rare and disputable entity that was recently featured in the 2017 WHO classification of Head and Neck Tumours[1]. Despite its recent addition to the WHO classification and its locally aggressive nature, the characteristics and treatment protocol for SOC are inadequately described because of its scarcity. The current literature review yielded 14 cases with comparable characteristics, as summarized in Table 1[2-7,9-13,15-18].
| Ref. | Gender, age (yr) | Site, symptoms | Duration | Radiological features | Treatment | Histopathological features | Outcome | ||
| H&E | IMC | ||||||||
| Positive staining | Negative staining | ||||||||
| Landwehr and Allen[2], 1996 (Koutlas et al[3], 2008) | F, 46 | Pain in the right mandible | Not mentioned | Poorly defined osteolytic lesion with perforation of buccal plate and thinning of lingual plate | Wide resection with 1 cm margin | Islands of moderately pleomorphic neoplastic epithelial cells interspersed with dense fibrous connective tissue | CK5/6, CK19 | Not mentioned | No recurrence after 12 yr |
| Koutlas and Warnock[8], 2005 (Koutlas et al[3], 2008) | M, 72 | Left mandibular mass (33-35) protruding into vestibule with mental nerve paraesthesia | “Long” duration | Radiolucency affecting the lower left canine and premolar | Wide resection with ipsilateral neck dissection | Thin cords and small nests of epithelium in densely collagenized stroma with invasion of striated muscle and perineural infiltration | CK5/6, CK19, CK7 (focal), p63, E-cadherin | CK8/18, CK20, S-100, SMA, CEA, desmin | No recurrence after 5 yr |
| Chaisuparat et al[6], 2006 (Koutlas and Warnock[8], 2005; Koutlas et al[3], 2008) | F, 73 | Enlargement of right maxilla | Not mentioned | Diffuse radiolucency involving alveolar ridge and extended into maxillary sinus | Wide resection with post-operative radiotherapy | Small nests and slender cords of epithelial cells in densely collagenized stroma with muscle and perineural infiltration | p63 | Not mentioned | No recurrence after 3.5 yr |
| Ide et al[13], 2009 | F, 47 | 2 cm mass on lower left lingual gingiva | 2 yr | Unilocular radiolucency with sclerotic inferior border surrounding roots of mandibular left second premolar and first molar | Resection with neck dissection | Small islands of tumour cells reminiscing epithelial cell rests of Malassez infiltrating the cancellous bone | Not mentioned | No recurrence after 6 yr | |
| Irié et al[12], 2010 | M, 67 | Paraesthesia in left mental region | Not mentioned | Focally expansile lesion with thinning of buccal cortical bone with admixed of radiolucent and radiopaque areas | First surgery: curettage; Second surgery: Segmental mandibulectomy with chemotherapy | Foci of thin cords and small nests of epithelial cells in fibrous stroma with epithelial cells invading into the mandibular canal | p63, CK6, CK19, CK7 (focal), AE1/AE3 | S100, CEA, calretinin, CD34, vimentin, CK8, CK20, SMA, amelogenin, MIB-1 < 3% | Recurrence 8 mo after the first surgery.No recurrence after 15 mo of the second surgery |
| Hussain et al[11], 2013 | M, 54 | Sensitivity of upper right canine with a firm lump | Not mentioned | Well defined radiolucency associated with the upper right lateral incisor and canine teeth with loss of the lamina dura and irregular resorption of the canine root was seen | Resection with close follow-up | Small infiltrative islands in densely fibrous stroma with perineural infiltration | AE1-3, CK5/6, CK19 | Not mentioned | No recurrence after 19 mo |
| Saxena et al[9], 2013 | M, 42 | Firm swelling at left mandibular lateral incisor to second premolars | 11 mo | Well-defined unilocular lytic lesion and perforation of both buccal and lingual cortices | First surgery: excision; Second surgery: Hemimandibulectomy with radical neck dissection and radiotherapy | Cords and nests of tumour cells in dense fibrous sclerosing stroma with vascular invasion | CK5/6, P63 | S100, SMA, Desmin | No recurrence after 10 mo |
| Tan et al[10], 2014 | F, 31 | 1 cm hard swelling at lower right first molar region | Lower right first molar was extracted 10 yr ago | Well-circumscribed round radiolucent lesion with scattered specks of radiopacities with a distinct sclerotic peripheral margin | Enucleation | Small clusters neoplastic cells in diffusely sclerotic stroma | CK7, CK5/6, CK19, CK8/18, CAM 5.2, p63, p16 (weak), p53E-cadherin | Vimentin, CEA, EMA, CK20, SMA, S-100, CD1a, ER, PR, FISH EWSR 1, calretinin, CD34, desmin, Ki-67 < 2% | No recurrence after 1 yr |
| Wood et al[7], 2016 (Gordon et al[18], 2015) | F, 43 | Asymptomatic firm lump at right anterior hard palate | Not mentioned | Enhancing soft tissue mass arising from the right hard palate with no bone destruction | Maxillectomy with wide margins and reconstruction with obturator | Small groups and prominent cords of bland hyperchromatic cells with minimal nuclear pleomorphism and eosinophilic cytoplasm | CK14, CK19, E-cadherin, weak nuclear staining to p63 | S100, PR, FISH EWSR 1 | Disease free after 17 mo |
| Hanisch et al[4], 2017 | M, 60 | Swelling at left premolar/molar region | Not mentioned | Ill-defined osteolytic changes with expansion, erosion, and perforation | Left hemimandibulectomy with ipsilateral radical neck dissectionSecondary reconstruction with CAD/CAM endoprosthesis (replacement of TMJ) and reconstruction with fibula flap | Small epithelial tumour cells and cords infiltrating lamellar bone | CK5/6, p40, p63, and MNF116 | Not mentioned | Disease free after 22 mo |
| Todorovic et al[5], 2019 | M, 62 | Progressive left maxillary swelling with recurrent sinus infections and mobility of teeth | 6 mo | Ground glass appearance with loss of trabeculations of left maxilla | Left maxillectomy and removal of skull base involving the infratemporal fossaUnderwent high-dose radiotherapy (66Gy in 33 fractions) for recurrence | Non-encapsulated tumour with mixed epithelial and mesenchymal components. Epithelial component consisted of highly infiltrative nests and cords of small polygonal and cuboidal cells with eosinophilic cytoplasm and mild-moderate nuclear atypia, usually associated with a dense background stroma. Significant intratumoral variability was observed | CK5/6, CK14, p63 | CK7, CK19, CK20, EBER ISH, ER, PAX8, CDX2, FISH EWSR 1, Ki-67 10% | Recurrence at 5 mo after surgery;No recurrence 19 mo following radiotherapy |
| Seyiti et al[15], 2020 | F, 54 | Discomfort at left posterior mandibular region, associated with numbness of lower lip | 3 mo | CBCT/SCT: irregular extensive osteolytic lesion with poorly defined borders and patchy calcifications were noted in the lesion. Slight resorption of cementum in apical region was seen. Obvious thickening of bilateral mandibular body was seen | Extensive resection and reconstruction with free fibula flap | Strands of epithelial tumor cells with clear cytoplasm infiltrating the fibrous stroma, osseous trabeculae and perineural invasion was observed | CK5/6, p63, | SMA, S-100, desmin, Ki-67 approx. 10%, EWSR1 | Not mentioned |
| O’Connor et al[16], 2019 | F, 43 | Asymptomatic, incidental finding of radiolucency of right anterior maxilla | 16 yr | Well-defined radiolucency with resorption of tooth roots and cortical thinning and erosion | First surgery: Biopsy; Second surgery: Conservative enucleation; Third surgery: Resection with a margin of 5 mm | Islands of epithelium within fibrous connective tissue that are mostly collagenous and sclerosed. Evidence of perineural invasion was seen | AE1/3, CK5, CK14, CK19 | CK7, Ki-67 < 1%, FISH EWSR1 | No recurrence 12 mo post-op |
| Kataoka et al[17], 2018 | F, 68 | Rapid, painless swelling of anterior mandibular region, with ulcerated overlying gingiva | 3 mo | CT: Radiolucency around the root of central incisor, with resorption of labial cortex; no root resorption; MRI: Well-defined internal heterogenous and extraosseous mass | En-bloc resection of 4 incisors and alveolar bone preserving lingual periosteum | Eosinophilic polyhedral tumor cells scattered under epithelium. Dispersed tumor nests with circular patterns and pressed by sclerosing fibrous stroma. No perineural and vascular infiltration, or invasion of skeletal muscle | AE1/AE3, EMA, p63, CK19 | CK5/6, Ki-67 approx. 2%, CK7 | No recurrence or metastasis more than 5 yr after surgery |
| Present case | F, 62 | Small indolent swelling at anterior palate, associated with intermittent toothache | 7 yr | Well-defined sclerotic border of the right maxilla extending into the right maxillary sinus with significant root resorption was seen on upper right central and lateral incisors and upper right first molar | First surgery: Excisional biopsy; Second surgery: Right subtotal maxillectomy and reconstruction with free fibular flap | Small nests or cords of small epithelial cells, and occasionally clear cells, immersed in a diffuse sclerotic and collagenous stroma. The epithelial cells appeared to be faintly hyperchromatic and mitotic figures were uncommon. Perivascular and perineural infiltration were observed | CK7, CK19, p40, p63 | Vimentin, Ki-67 5%-10% | Disease free after 22 mo |
SOC seems to have peak incidence in the fourth to seventh decades of life, with a female predilection[9]. The tumor appears to have a greater propensity to affect the anterior mandible[9], with only 4 out of 11 cases involving the maxilla[2,6,7,11]. To date, including the case presented herein, there are only five reported cases involving the maxilla. Patients frequently complain of long-standing swelling[2-4,7-12], paraesthesia[3,12], pain[2,3], and tooth sensitivity[11]. Similar to our case, the patient complained of long-standing swelling, with occasional pain in the area affected. The wide spectrum of clinical presentations make determining the nature of the lesion, whether benign or malignant, difficult[14].
Radiologically, this tumor could present as well-circumscribed or poorly-defined lytic radiolucency with cortical bone perforation[2-4,9,12,13]. Our case demonstrated similar radiographical features, with both well- and ill-defined sclerotic lesions and notable cortical bone perforation. Root resorption was only described in one of the published cases[11], despite the locally aggressive nature of the tumor. The infiltrative and locally aggressive nature was demonstrated in this case, as indicated by the marked buccal and palatal bone perforation and distinct tooth root resorption on both plain radiographs and CT scans.
Histologically, the tumor is typically characterized by infiltrative thin cords or small nests of epithelial cells in the densely sclerotic stroma. Perineural, intraneural, or vascular invasion, which is another distinguishing feature, were also described in seven cases[3,8,9,11-13], similar to our case, which displayed perivascular infiltration in the H&E section. Although there is no distinctive immunohistochemical marker for SOC, consistent cytokeratin immunoreactivity was seen, with positive staining for CK5/6, p40, and p63 in most reported cases. Only one case demonstrated weak nuclear staining for p63[7] and two cases displayed positivity for CK14[5,7]. Most cases reported negative staining for CK7, whereas our case demonstrated diffuse CK7 expression, which is similar to that reported by Tan et al[10], while Koutlas et al[3] and Irié et al[12] showed focal expression of CK7. Most cases reported negative results for vimentin and S-100, which is similar to the present case; only one case reported unexpected negative staining for CK19[5]. Ki-67 was used in most cases to assess the proliferative index of the tumor, which appeared to be insignificant in the reported cases, which is similar to the current case.
Our case demonstrated substantial demographical, histopathological, and radiographical similarities with previous cases. In this case, the tumor was neglected, possibly because of the vague signs and symptoms. Our immunohistochemistry results were also comparable to those of other published cases, with positive staining of the tumor cells for CK19, p40, and p63. The infiltrative growth pattern with vascular invasion was also similar to the case reported by Saxena et al[9], while eight published cases showed evidence of perineural invasion[3,4,7,9,11,12,15,16]. In contrast, four cases reported a lack of perineural or vascular infiltration[5,10,13,17]. Although perineural invasion was often associated with poorer locoregional control and prognosis in squamous cell carcinoma, this appears to be a distinctive histopathological feature in most of the reported cases reported. However, more cases are required to further validate this specific feature as a prognostic factor or tumor grading for SOC. Distant or regional metastasis is yet to be reported in SOC, based on the available published data.
Exclusion from the differential diagnosis can be difficult because of the histopathological resemblance of SOC to other histological differential diagnoses, such as central odontogenic fibroma and desmoplastic ameloblastoma. Central odontogenic fibroma (particularly the epithelial-rich type) is clinically less aggressive than that SOC. The stroma is variably cellular, with fibroblastic connective tissue, and unlike in SOC, the stroma appears to be densely fibrous and sclerotic. Although desmoplastic ameloblastoma demonstrated dense fibrous stroma similar to SOC, it should present with focal ameloblastic columnar cells, even if the presentation is scant. A metastatic tumor was ruled out in this case, given the strong positive expression of p63, which confirmed the basal characteristics of the epithelial cells.
Currently, we lack a standardized treatment protocol because of the rarity of the tumor. SOC demonstrated permeative and locally aggressive characteristics, thus it should warrant more radical resection to prevent local recurrence. Recurrence was reported in two patients following curettage; however, there was no recurrence noted after the subsequent ablative surgery and high-dose radiotherapy, respectively[5,12]. Our case also experienced a recurrence of the tumor at one year following the excisional biopsy; therefore, conservative management of enucleation or curettage might not be adequate. Hussain et al[11] suggested that conservative tumor-free margins of 5 mm should be used. By contrast, Landwehr and Allen[2] reported close margins despite having 1.0 cm resection margins. The invasive properties of SOC and its close margins following 1.0 cm surgical margins, as mentioned in the previous reports, prompted us to propose that the surgical margins should be extended to 1.5 cm for both hard and soft tissues to ensure tumor-negative margins. However, more cases are required to better appreciate the true origin, morphological features, and biological behavior to definitively ascertain the tumor resection margins.
To date, based on the currently available data, there is still no evidence of cervical lymphatic spread or distant metastasis reported. The tumor appears to have no evident metastatic capability, despite a typical long-standing history, which is again demonstrated in our case. Nevertheless, two patients were subjected to radiotherapy[5,6,9], two patients underwent neck dissection in addition to tumor resection[3,8,9], one patient underwent chemotherapy following tumor resection[12], and one patient received high-dose radiotherapy following disease recurrence[5]. However, prophylactic neck dissection or adjunct therapy, such as chemotherapy and radiotherapy, were deemed unnecessary because the tumor has yet to show metastasis potential[9,11]. As the treatment approach and its efficacy for SOC remains ambiguous, we suggest that standard oral oncology follow-up should be carried out for at least 5 years in patients diagnosed with SOC because of the locally aggressive and infiltrative nature of this tumor. More cases are required to further illustrate this entity and guide clinical diagnosis and treatment.
In summary, the biological behaviors, and characteristics of SOC remain ambiguous owing to its rarity, with limited case reports published to date. More cases are needed to further characterize this entity, understand its biological behavior, and justify the treatment protocols. To date, surgical resection with adequate surgical margins remains the mainstay treatment, with no disease recurrence in most cases, while neck dissection and postoperative radiotherapy or chemotherapy were not deemed necessary. We hope that this case report will facilitate the validation of this disease entity and contribute to the establishment of treatment protocols.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Dentistry, oral surgery and medicine
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arumugam EAP, India; Hasan S, India; Sekhar P, India S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Speight PM, Takata T. New tumour entities in the 4th edition of the World Health Organization Classification of Head and Neck tumours: odontogenic and maxillofacial bone tumours. Virchows Arch. 2018;472:331-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 184] [Article Influence: 23.0] [Reference Citation Analysis (6)] |
| 2. | Landwehr D, Allen C. Aggressive odontogenic epithelial neoplasm mimicking metastatic brreast carcinoma:sclerosing odontogenic carcinoma? Oral Surg Oral Med Oral Pathol Oral Radiol. 1996;82:206. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (3)] |
| 3. | Koutlas IG, Allen CM, Warnock GR, Manivel JC. Sclerosing odontogenic carcinoma: a previously unreported variant of a locally aggressive odontogenic neoplasm without apparent metastatic potential. Am J Surg Pathol. 2008;32:1613-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Hanisch M, Baumhoer D, Elges S, Fröhlich LF, Kleinheinz J, Jung S. Sclerosing odontogenic carcinoma: current diagnostic and management considerations concerning a most unusual neoplasm. Int J Oral Maxillofac Surg. 2017;46:1641-1649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 5. | Todorovic E, Berthelet E, O'Connor R, Durham JS, Tran E, Martin M, Hayes MM, Ng TL. Sclerosing Odontogenic Carcinoma with Local Recurrence: Case Report and Review of Literature. Head Neck Pathol. 2019;13:371-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Chaisuparat R, Coletti D, Kolokythas A, Ord RA, Nikitakis NG. Primary intraosseous odontogenic carcinoma arising in an odontogenic cyst or de novo: a clinicopathologic study of six new cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Wood A, Young F, Morrison J, Conn BI. Sclerosing odontogenic carcinoma presenting on the hard palate of a 43-year-old female: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e204-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Koutlas IG, Warnock GMJ. “Intraossesous” sclerosing carcinoma of possible odontogenic origin. Report of two cases. Oral Surg Oral Med Oral Pathol Oral Radiol. 2005;100:187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Saxena S, Kumar S, Rawat S, Arun Kumar KV. An indolent swelling of the parasymphyseal area. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:528-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Tan SH, Yeo JF, Kheem Pang BN, Petersson F. An intraosseous sclerosing odontogenic tumor predominantly composed of epithelial cells: relation to (so-called) sclerosing odontogenic carcinoma and epithelial-rich central odontogenic fibroma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;118:e119-e125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Hussain O, Rendon AT, Orr RL, Speight PM. Sclerosing odontogenic carcinoma in the maxilla: a rare primary intraosseous carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e283-e286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Irié T, Ogawa I, Takata T, Toyosawa S, Saito N, Akiba M, Isobe T, Hokazono C, Tachikawa T, Suzuki Y. Sclerosing odontogenic carcinoma with benign fibro-osseous lesion of the mandible: an extremely rare case report. Pathol Int. 2010;60:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Ide F, Mishima K, Saito I, Kusama K. Diagnostically challenging epithelial odontogenic tumors: a selective review of 7 jawbone lesions. Head Neck Pathol. 2009;3:18-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Lim D, Tan CC, Tilakaratne WM, Goh YC. Sclerosing odontogenic carcinoma - review of all published cases: is it a justifiable addition as a malignancy? Braz J Otorhinolaryngol. 2022;88:118-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Seyiti P, Feng Y, Gao A, Lin Z, Huang X, Sun G, Zhang L, Wang T. An extensive sclerosing odontogenic carcinoma in mandible: a case report and literature review. Dentomaxillofac Radiol. 2020;49:20190426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 16. | O’Connor RC, Khurram SA, Singh T, Jones K. Sclerosing odontogenic carcinoma - what we know so far. Oral Surg. 2019;12:133-138. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 17. | Kataoka T, Fukada K, Okamoto T, Nagashima Y, Ando T. Sclerosing odontogenic carcinoma in the mandible. J Oral Maxillofac Surg, Med Pathol. 2018;30:428-433. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Gordon P, Morrison J, Wood A, Conn B. A case report of sclerosing odontogenic carcinoma of the hard palate. Br J Oral Maxillofac Surg. 2015;53:e49. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (1)] |