Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1634
Peer-review started: December 17, 2022
First decision: January 17, 2023
Revised: January 28, 2023
Accepted: February 13, 2023
Article in press: February 13, 2023
Published online: March 6, 2023
Processing time: 75 Days and 5.3 Hours
Pulmonary nocardiosis is difficult to diagnose by culture and other conventional testing, and is often associated with lethal disseminated infections. This difficulty poses a great challenge to the timeliness and accuracy of clinical detection, especially in susceptible immunosuppressed individuals. Metagenomic next-generation sequencing (mNGS) has transformed the conventional diagnosis pattern by providing a rapid and precise method to assess all microorganisms in a sample.
A 45-year-old male was hospitalized for cough, chest tightness and fatigue for 3 consecutive days. He had received a kidney transplant 42 d prior to admission. No pathogens were detected at admission. Chest computed tomography showed nodules, streak shadows and fiber lesions in both lung lobes as well as right pleural effusion. Pulmonary tuberculosis with pleural effusion was highly suspected based on the symptoms, imaging and residence in a high tuberculosis-burden area. However, anti-tuberculosis treatment was ineffective, showing no improvement in computed tomography imaging. Pleural effusion and blood samples were subsequently sent for mNGS. The results indicated Nocardia farcinica as the major pathogen. After switching to sulphamethoxazole combined with minocycline for anti-nocardiosis treatment, the patient gradually improved and was finally discharged.
A case of pulmonary nocardiosis with an accompanying bloodstream infection was diagnosed and promptly treated before the dissemination of the infection. This report emphasizes the value of mNGS in the diagnosis of nocardiosis. mNGS may be an effective method for facilitating early diagnosis and prompt treatment in infectious diseases, which overcomes the shortcomings of conventional testing.
Core Tip: Nocardiosis is challenging to diagnosis due to its infrequency in clinical practice, non-specificity in clinical presentation and imaging, and the limitations of detection methods. This report highlighted the value of metagenomic next-generation sequencing (mNGS) in the rapid and precise diagnosis of nocardiosis and underscores the limitations of conventional testing. mNGS may be an effective method for early diagnosis and prompt treatment of infectious diseases.
- Citation: Deng ZF, Tang YJ, Yan CY, Qin ZQ, Yu N, Zhong XB. Pulmonary nocardiosis with bloodstream infection diagnosed by metagenomic next-generation sequencing in a kidney transplant recipient: A case report. World J Clin Cases 2023; 11(7): 1634-1641
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1634.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1634
Nocardia is an opportunistic pathogen that most commonly causes infections in immunocompromised hosts. The largest group of patients with nocardiosis is solid organ transplant recipients, with the highest morbidity in heart and lung recipients and patients with a particularly high level of immunosuppression[1-5]. Pulmonary nocardiosis is the most common type of Nocardia infections, which tends to develop into a disseminated infection[6]. However, nocardiosis is rare in clinical practice, and the current methods of conventional testing for diagnosis have many limitations. Nocardia culture requires at least 2 wk to form colonies, making it difficult to achieve early identification and prompt treatment for nocardiosis[1]. Here, we applied metagenomic next-generation sequencing (mNGS) to identify Nocardia farcinica (N. farcinica) infection involving the pleura and blood in an allogeneic kidney transplant recipient. The patient was prevented from a possible disseminated infection by timely and appropriate treatment.
A 45-year-old male patient presented with cough, chest tightness and fatigue that had persisted for 3 consecutive days.
The patient received an allogeneic kidney transplant in the Department of Urology Surgery (Transplantation ward) at the Ruikang Hospital Affiliated to Guangxi University of Chinese Medicine 42 d prior to admission. After surgery, he was treated with oral tacrolimus (alternating 2 mg and 1.5 mg bid), mycophenolate mofetil (1 g qd) and methylprednisolone (8 mg qd) for immunosuppression.
The patient had a 5-year history of uremia and was on regular hemodialysis 3 times a week. Meanwhile, he also had a 5-year history of hypertension, which was treated with valsartan and terazosin irregularly.
The patient denied other specific diseases and family history.
The patient’s vital signs were normal, including body temperature of 36.5 °C, pulse of 90 beats/min, respiratory rate of 20 breaths/min, and blood pressure of 113/84 mmHg. Breathing sound was slightly decreased in the right lung, and neurological examination was normal.
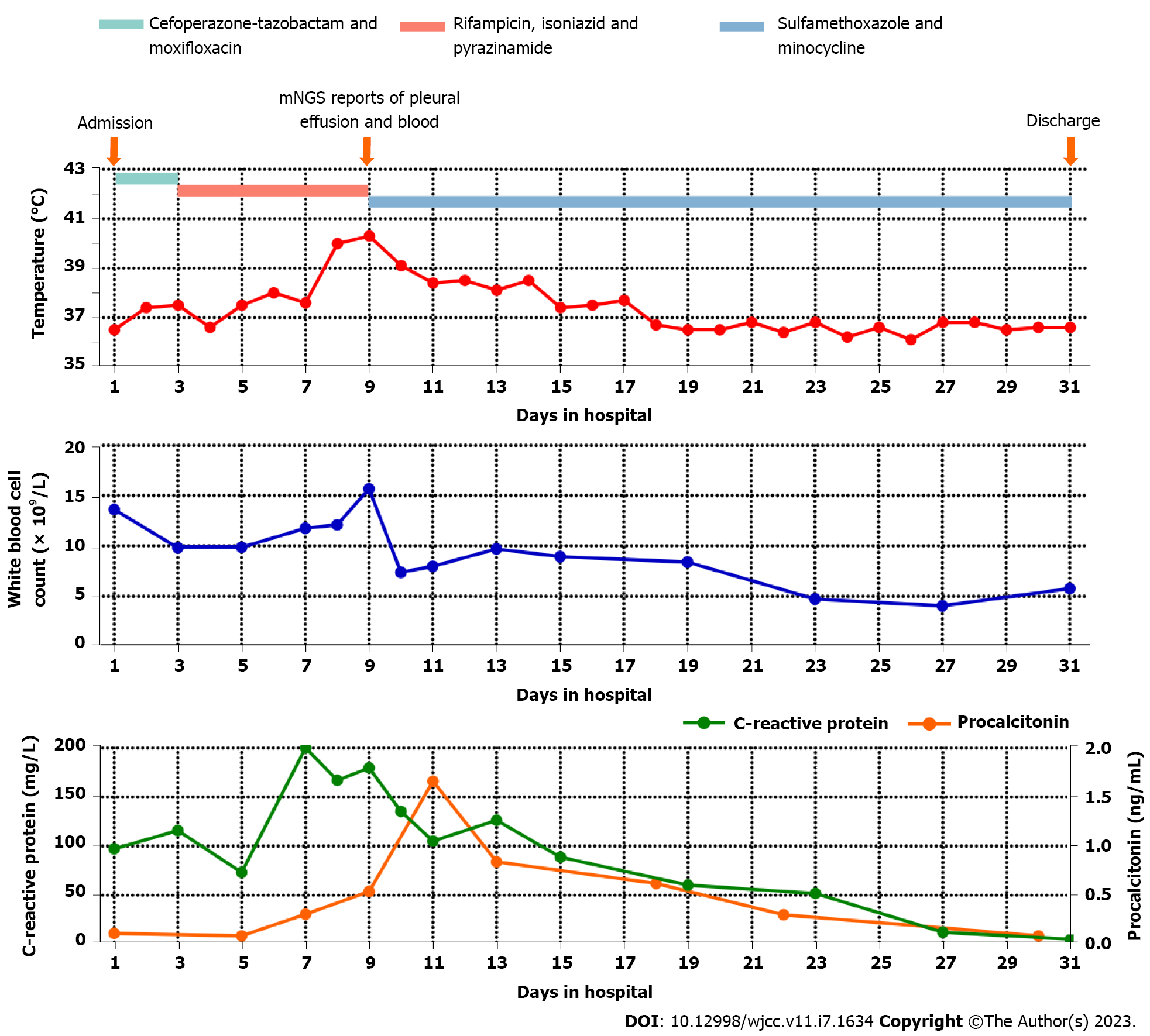
Results of laboratory examinations included increased white blood cell count (13.61 × 109/L; normal range: 3.5-9.5 × 109/L) with elevated neutrophil percentage (92.6%; normal range: 40%-75%), increased C-reactive protein level (97.0 mg/L; normal range: 0.5-3.0 mg/L), normal procalcitonin level (0.11 ng/mL), and normal serum creatinine concentration (120.00 μmol/L). Serum fungal β-(1,3)-D-glucan and galactomannan were within the normal ranges. Peripheral blood was drawn for microbial culture, T cells spot test of tuberculosis infection and antibody detection of Mycobacterium tuberculosis, which were all negative.
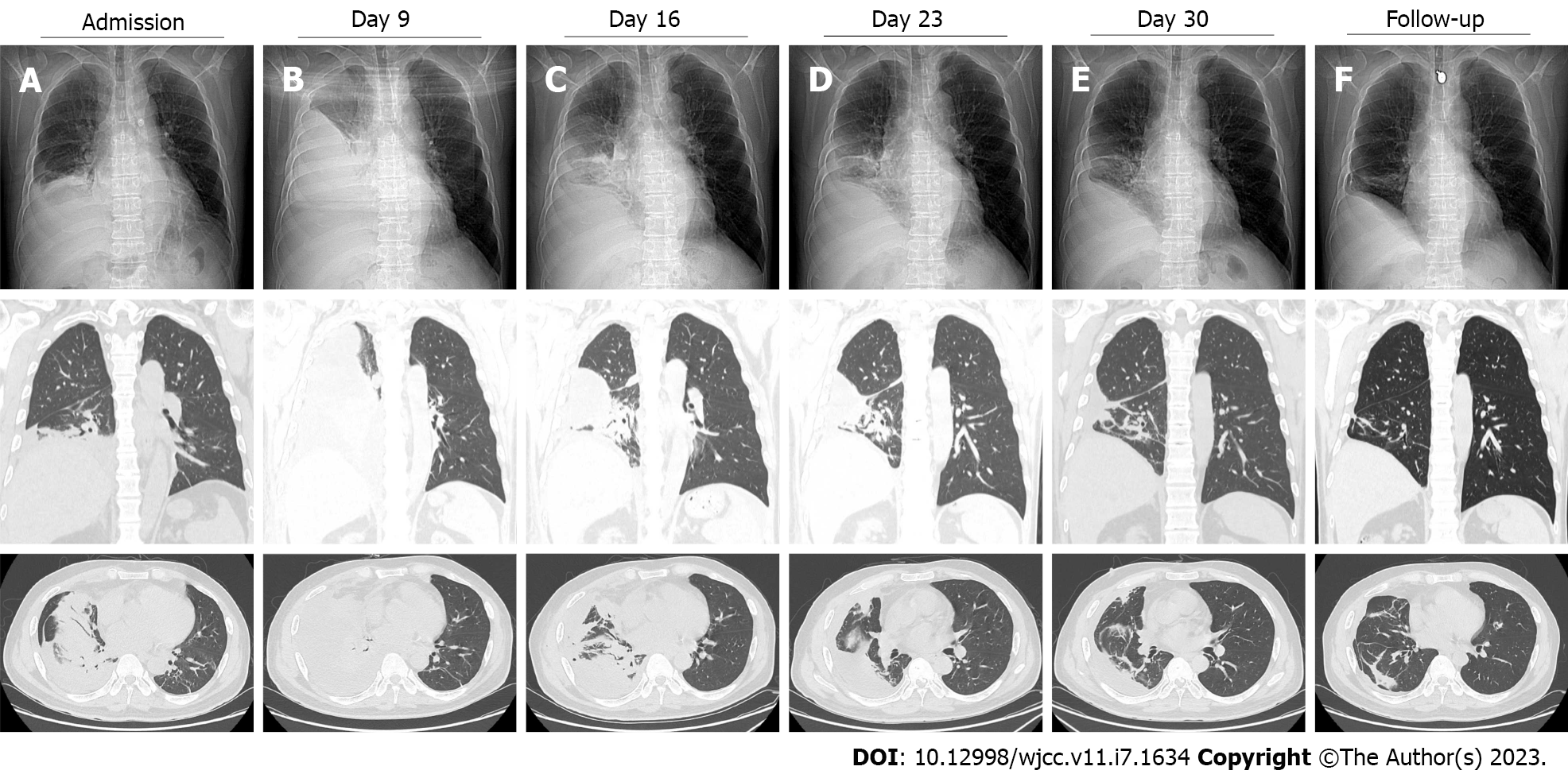
The chest computed tomography (CT) showed right pleural effusion, nodules and streak shadows in the right cardiophrenic angle region. Nodules surrounded by fibrosis and calcification were observed in the left upper lobe (Figure 1A).
On day 3 after admission, the patient experienced recurrent fevers, and an upward trend was seen in his body temperature (Figure 2). Although Mycobacterium tuberculosis was not detected, the patient was presumptively diagnosed with pulmonary tuberculosis due to recurring low fevers, tuberculosis-like imaging findings and a high prevalence of tuberculosis in the patient’s residential area. Anti-tuberculosis treatment with isoniazid, rifampicin and pyrazinamide was started empirically. On day 9, the patient’s body temperature increased again, reaching a maximum of 40.3 °C. Re-examination CT showed a significant increase in pleural effusion and no reduction in the lung lesions (Figure 1B).
Subsequently, ultrasound-guided thoracentesis drainage was performed to investigate the etiology of the pulmonary inflammation. The results of bacterial and fungal culture of the pleural effusion were negative. Antibody detection and PCR for Mycobacterium tuberculosis were also negative. The biochemical examination revealed that the protein level was elevated (41 g/L; normal range: < 25 g/L). Lactate dehydrogenase was also elevated (1445 U/L; normal range: < 200 U/L), while adenosine deaminase was normal (24 U/L; normal range for non-tuberculous pleuritis: < 40 U/L).
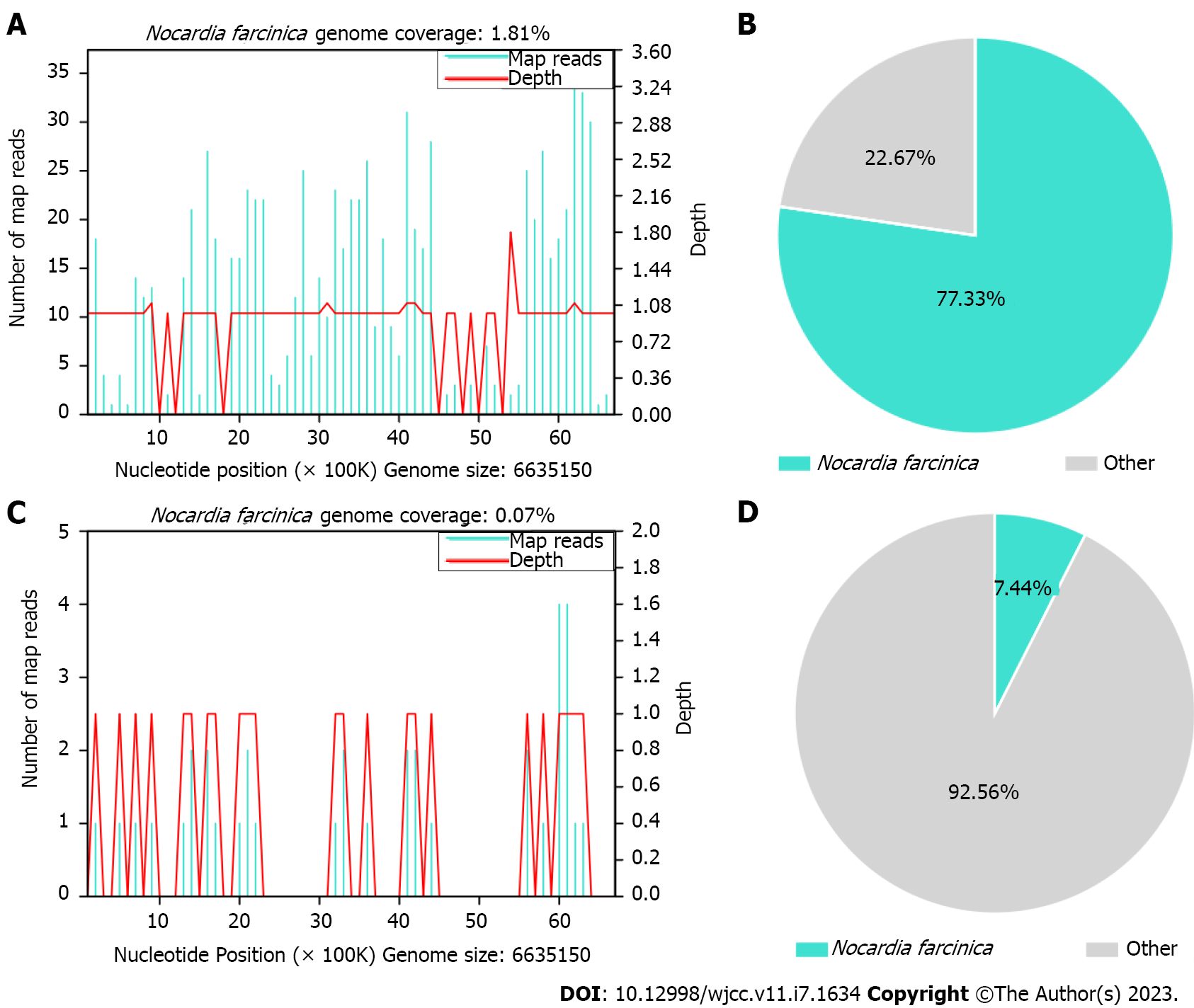
Meanwhile, the pleural effusion and a blood sample were collected for mNGS. The methods of mNGS were described in Supplementary material. The mNGS results on day 10 indicated that N. farcinica was present in both the pleural effusion and blood. There were 464 reads detected for N. farcinica in the pleural effusion with genome coverage of 1.81% and species relative abundance of 96.58%, making up 77.33% of the total DNA reads (Figure 3A and B). Simultaneously, 18 reads for N. farcinica were reported in the blood with genome coverage of 0.07% and species relative abundance of 10.39%, making up 7.44% of the total DNA reads (Figure 3C and D).
Pulmonary nocardiosis with bloodstream infection.
Antibacterial treatment with cefoperazone-tazobactam (1 g q8h) and moxifloxacin (0.4 g qd) was started empirically when the patient was admitted. Subsequently, anti-tuberculosis treatment (isoniazid 0.3 g, rifampicin 0.45 g and pyrazinamide 1.25 g qd) was started on day 3 when the patient was presum
After ongoing treatment of nocardiosis, the patient’s general condition and laboratory examinations improved significantly (Figure 2). The chest CT on days 16, 23 and 30 revealed that the pulmonary infection gradually improved, and the pleural effusion, nodules and streak shadows were reduced (Figure 1C-E). Finally, the patient was discharged on day 31 and continued oral TMP-SMZ and minocycline treatment. The patient returned to the hospital for follow-up 28 d after discharge. He recovered well without any clinical symptoms and complications. The laboratory examination revealed normalized white blood cell count (8.96 × 109/L) with a normalized neutrophil percentage (58.4%) and a nearly normal C-reactive protein level (< 5.00 mg/L). CT showed significant absorption of the right pleural effusion (Figure 1F).
N. farcinica is an aerobic, partially acid-fast, Gram-positive bacillus that commonly exists in soil, decaying vegetation and ventilation equipment[7]. The genus Nocardia is recognized as an opportunistic pathogen, of which N. farcinica is a relatively infrequent species of clinical significance[8]. N. farcinica can invade through the respiratory tract or skin and primarily infects the immunocompromised. Pneumonia is the most common disease caused by N. farcinica[9]. A review reported that 59.7% of patients with N. farcinica had pulmonary or pleural involvement[10]. Moreover, N. farcinica is prone to disseminated infections causing brain abscesses[11], skin infections[12], keratitis[13], etc. Disseminated infections were reportedly found in more than half of patients with pulmonary nocardiosis[14]. The mortality rate of N. farcinica infections can be as high as 14%-40% without early diagnosis and prompt treatment and can reach 100% in cases of disseminated infections[15].
At present, the clinical diagnosis of nocardiosis depends on microbial culture. However, it takes about 2 wk for the colony formation due to the slow growth of Nocardia. Then, the formation of colonies with characteristic morphology requires at least 4 wk[1,7]. In addition, the clinical manifestations of pulmonary nocardiosis lack specificity. Common symptoms include fever, cough, expectoration, fatigue and general malaise, and imaging examination is characterized by invasive lesions, consolidation, nodules, and cavities[16]. Hence, Nocardia infection is usually missed, diagnosis is delayed, or it is misdiagnosed as tuberculosis, Aspergillus infection or lung cancer.
mNGS is an emerging and culture-independent high-throughput sequencing technology that can quickly and objectively detect all pathogenic microorganisms including viruses, bacteria, fungi, and parasites in clinical samples without specific amplification[17-19]. An advantage of mNGS in diagnosing infectious disease is the detection of pathogens that are difficult to detect by conventional testing. In addition, mNGS can also rule out infections when testing results are negative if the sequencing coverage is high enough to ensure the existence of pathogenic microorganisms in samples[20]. Results from mNGS are available between 6 h to 7 d, with an average of 48 h. Completing data analysis depends on sequencing technologies and bioinformatics analysis methods available[21,22]. Thus, mNGS has attracted clinical attention and has recently been applied to the etiological diagnosis of acute, complicated and rare infections.
In this case, the results of routine blood work, inflammatory markers and imaging examination all indicated the presence of a pulmonary infection. However, no pathogens were detected. The patient’s clinical presentation and imaging manifestations were also non-specific. Meanwhile, empirical anti-bacterial and anti-tuberculosis treatment were ineffective. mNGS provided another approach to detect pathogens, and N. farcinica was detected in the patient’s pleural effusion and blood samples. Isolation of Nocardia from the respiratory tract or blood indicates colonization, contamination or infection. Detection of Nocardia in immunosuppressive patients (such as our patient) with obvious infectious symptoms is generally considered an infection because such patients are at high risk[23]. Thus, the detection by mNGS and the high relative abundance of N. farcinica indicated that our patient should be diagnosed with nocardiosis.
SMZ has traditionally been the preferred choice for nocardiosis treatment. SMZ-based combination treatment elicits a favorable response[24,25]. As such, we treated the patient with TMP-SMZ and minocycline. This treatment regimen had high efficacy in our patient, which supported the diagnosis of pulmonary nocardiosis. The patient improved significantly and was discharged from the hospital. We hypothesize that the timely diagnosis and treatment of N. farcinica invading the bloodstream prevented the patient from a disseminated infection, avoiding a worse outcome.
There are several limitations in this case report. First, due to the poor physical condition of the patient, no bronchoalveolar lavage fluid was collected for mNGS assessment. Second, the absence of the pathogen was not confirmed after treatment due to financial reasons. Finally, no other methods, such as PCR or Sanger sequencing, were used to verify the presence of N. farcinica.
This case highlights the challenges of diagnosing pulmonary nocardiosis, which can cause disseminated infections particularly in immunosuppressed patients. When the pathogen is unknown and all cultures are negative, then mNGS can provide a faster and more accurate diagnosis of acute, complicated, and rare infections. Timely treatment with TMP-SMZ and minocycline prevented a disseminated infection in this immunosuppressed patient.
The authors would like to thank Chunhong Li, Xiaocui Liang, Weiying Jiang, Fang Ren, Meng Wu, Chunhua Huang, Zixing Liu, Dingli Wei, Na Peng and Lu Liu for their help in metagenomic next-generation sequencing report interpretation and experimental procedure.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Micsik T, Hungary; Moyses Neto M, Brazil S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Margalit I, Lebeaux D, Tishler O, Goldberg E, Bishara J, Yahav D, Coussement J. How do I manage nocardiosis? Clin Microbiol Infect. 2021;27:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Goodlet KJ, Tokman S, Nasar A, Cherrier L, Walia R, Nailor MD. Nocardia prophylaxis, treatment, and outcomes of infection in lung transplant recipients: A matched case-control study. Transpl Infect Dis. 2021;23:e13478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 3. | Coussement J, Lebeaux D, van Delden C, Guillot H, Freund R, Marbus S, Melica G, Van Wijngaerden E, Douvry B, Van Laecke S, Vuotto F, Tricot L, Fernández-Ruiz M, Dantal J, Hirzel C, Jais JP, Rodriguez-Nava V, Lortholary O, Jacobs F; European Study Group for Nocardia in Solid Organ Transplantation. Nocardia Infection in Solid Organ Transplant Recipients: A Multicenter European Case-control Study. Clin Infect Dis. 2016;63:338-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Haussaire D, Fournier PE, Djiguiba K, Moal V, Legris T, Purgus R, Bismuth J, Elharrar X, Reynaud-Gaubert M, Vacher-Coponat H. Nocardiosis in the south of France over a 10-years period, 2004-2014. Int J Infect Dis. 2017;57:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Averbuch D, De Greef J, Duréault A, Wendel L, Tridello G, Lebeaux D, Mikulska M, Gil L, Knelange N, Zuckerman T, Roussel X, Robin C, Xhaard A, Aljurf M, Beguin Y, Le Bourgeois A, Botella-Garcia C, Khanna N, Van Praet J, Kröger N, Blijlevens N, Ducastelle Leprêtre S, Ho A, Roos-Weil D, Yeshurun M, Lortholary O, Fontanet A, de la Camara R, Coussement J, Maertens J, Styczynski J; European Study Group for Nocardia in Hematopoietic Cell Transplantation. Nocardia Infections in Hematopoietic Cell Transplant Recipients: A Multicenter International Retrospective Study of the Infectious Diseases Working Party of the European Society for Blood and Marrow Transplantation. Clin Infect Dis. 2022;75:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 6. | Soueges S, Bouiller K, Botelho-Nevers E, Gagneux-Brunon A, Chirouze C, Rodriguez-Nava V, Dumitrescu O, Triffault-Fillit C, Conrad A, Lebeaux D, Hodille E, Valour F, Ader F. Prognosis and factors associated with disseminated nocardiosis: a ten-year multicenter study. J Infect. 2022;85:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 7. | Brown-Elliott BA, Brown JM, Conville PS, Wallace RJ Jr. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 692] [Cited by in RCA: 787] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 8. | Huang L, Chen X, Xu H, Sun L, Li C, Guo W, Xiang L, Luo G, Cui Y, Lu B. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Galar A, Martín-Rabadán P, Marín M, Cercenado E, Sánchez-Carrillo C, Valerio M, Bouza E, Muñoz P. Revisiting nocardiosis at a tertiary care institution: Any change in recent years? Int J Infect Dis. 2021;102:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Budzik JM, Hosseini M, Mackinnon AC Jr, Taxy JB. Disseminated Nocardia farcinica: literature review and fatal outcome in an immunocompetent patient. Surg Infect (Larchmt). 2012;13:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Corsini Campioli C, Castillo Almeida NE, O'Horo JC, Challener D, Go JR, DeSimone DC, Sohail MR. Clinical Presentation, Management, and Outcomes of Patients With Brain Abscess due to Nocardia Species. Open Forum Infect Dis. 2021;8:ofab067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Ramos-E-Silva M, Lopes RS, Trope BM. Cutaneous nocardiosis: A great imitator. Clin Dermatol. 2020;38:152-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | DeCroos FC, Garg P, Reddy AK, Sharma A, Krishnaiah S, Mungale M, Mruthyunjaya P; Hyderabad Endophthalmitis Research Group. Optimizing diagnosis and management of nocardia keratitis, scleritis, and endophthalmitis: 11-year microbial and clinical overview. Ophthalmology. 2011;118:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 14. | Lafont E, Conan PL, Rodriguez-Nava V, Lebeaux D. Invasive Nocardiosis: Disease Presentation, Diagnosis and Treatment - Old Questions, New Answers? Infect Drug Resist. 2020;13:4601-4613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 15. | Martínez R, Reyes S, Menéndez R. Pulmonary nocardiosis: risk factors, clinical features, diagnosis and prognosis. Curr Opin Pulm Med. 2008;14:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Bargehr J, Flors L, Leiva-Salinas C, Flohr TR, Sawyer R, Bonatti H, Hagspiel KD. Nocardiosis in solid-organ transplant recipients: spectrum of imaging findings. Clin Radiol. 2013;68:e266-e271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14:319-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 389] [Cited by in RCA: 828] [Article Influence: 118.3] [Reference Citation Analysis (0)] |
| 18. | Han D, Diao Z, Lai H, Han Y, Xie J, Zhang R, Li J. Multilaboratory assessment of metagenomic next-generation sequencing for unbiased microbe detection. J Adv Res. 2022;38:213-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Gu W, Deng X, Lee M, Sucu YD, Arevalo S, Stryke D, Federman S, Gopez A, Reyes K, Zorn K, Sample H, Yu G, Ishpuniani G, Briggs B, Chow ED, Berger A, Wilson MR, Wang C, Hsu E, Miller S, DeRisi JL, Chiu CY. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids. Nat Med. 2021;27:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 20. | Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20:341-355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 901] [Article Influence: 150.2] [Reference Citation Analysis (0)] |
| 21. | Simner PJ, Miller S, Carroll KC. Understanding the Promises and Hurdles of Metagenomic Next-Generation Sequencing as a Diagnostic Tool for Infectious Diseases. Clin Infect Dis. 2018;66:778-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 502] [Article Influence: 83.7] [Reference Citation Analysis (0)] |
| 22. | Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76:225-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 23. | Steinbrink J, Leavens J, Kauffman CA, Miceli MH. Manifestations and outcomes of nocardia infections: Comparison of immunocompromised and nonimmunocompromised adult patients. Medicine (Baltimore). 2018;97:e12436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 24. | Lebeaux D, Freund R, van Delden C, Guillot H, Marbus SD, Matignon M, Van Wijngaerden E, Douvry B, De Greef J, Vuotto F, Tricot L, Fernández-Ruiz M, Dantal J, Hirzel C, Jais JP, Rodriguez-Nava V, Jacobs F, Lortholary O, Coussement J; European Study Group for Nocardia in Solid Organ Transplantation; European Study Group for Nocardia in Solid Organ Transplantation. Outcome and Treatment of Nocardiosis After Solid Organ Transplantation: New Insights From a European Study. Clin Infect Dis. 2017;64:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Lebeaux D, Bergeron E, Berthet J, Djadi-Prat J, Mouniée D, Boiron P, Lortholary O, Rodriguez-Nava V. Antibiotic susceptibility testing and species identification of Nocardia isolates: a retrospective analysis of data from a French expert laboratory, 2010-2015. Clin Microbiol Infect. 2019;25:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |