Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1607
Peer-review started: November 11, 2022
First decision: December 26, 2022
Revised: January 4, 2023
Accepted: February 10, 2023
Article in press: February 10, 2023
Published online: March 6, 2023
Processing time: 111 Days and 1.5 Hours
Mucosa-associated lymphoid tissue (MALT) lymphoma originates in the marginal zone of lymphoid tissue. lung is one of the most frequent non-gastrointestinal organs involved, here known as bronchus-associated lymphoid tissue (BALT) lymphoma. BALT lymphoma of unknown etiology, and most patients are asymptomatic. The treatment of BALT lymphoma is controversial.
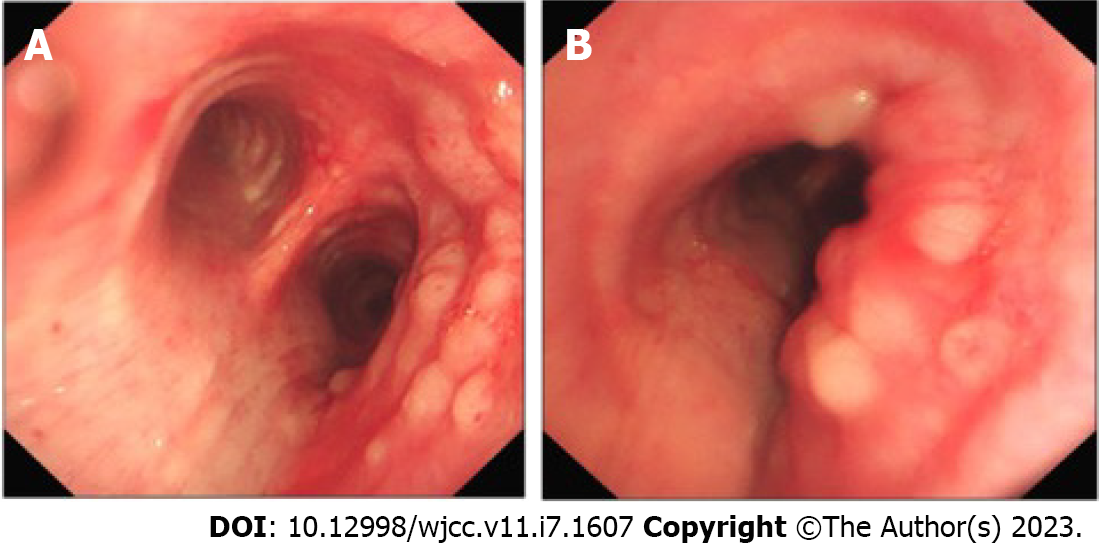
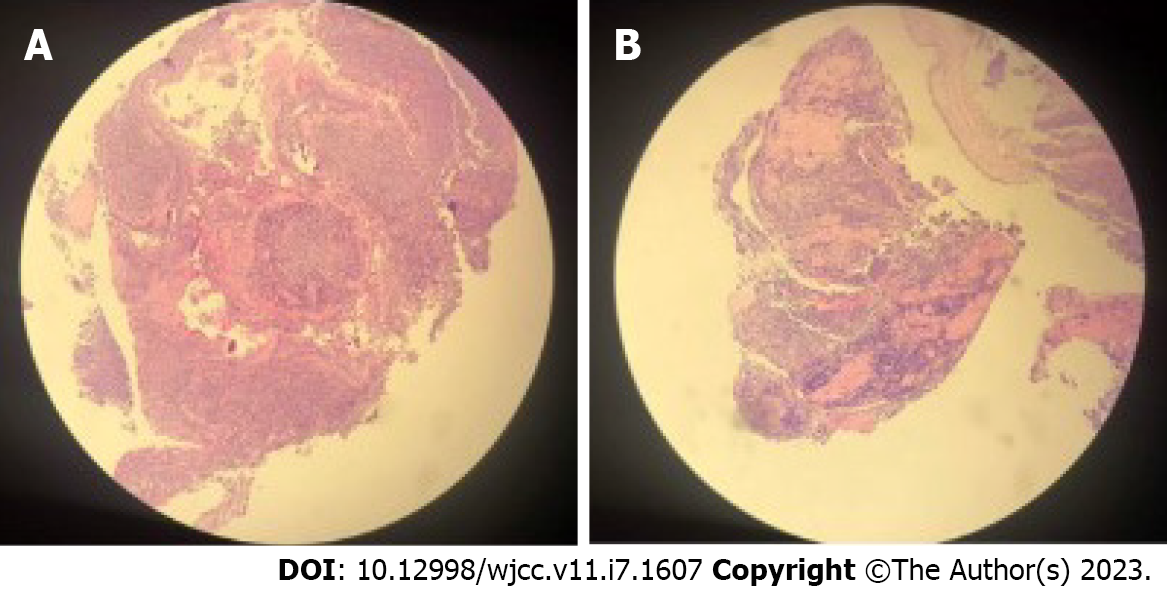
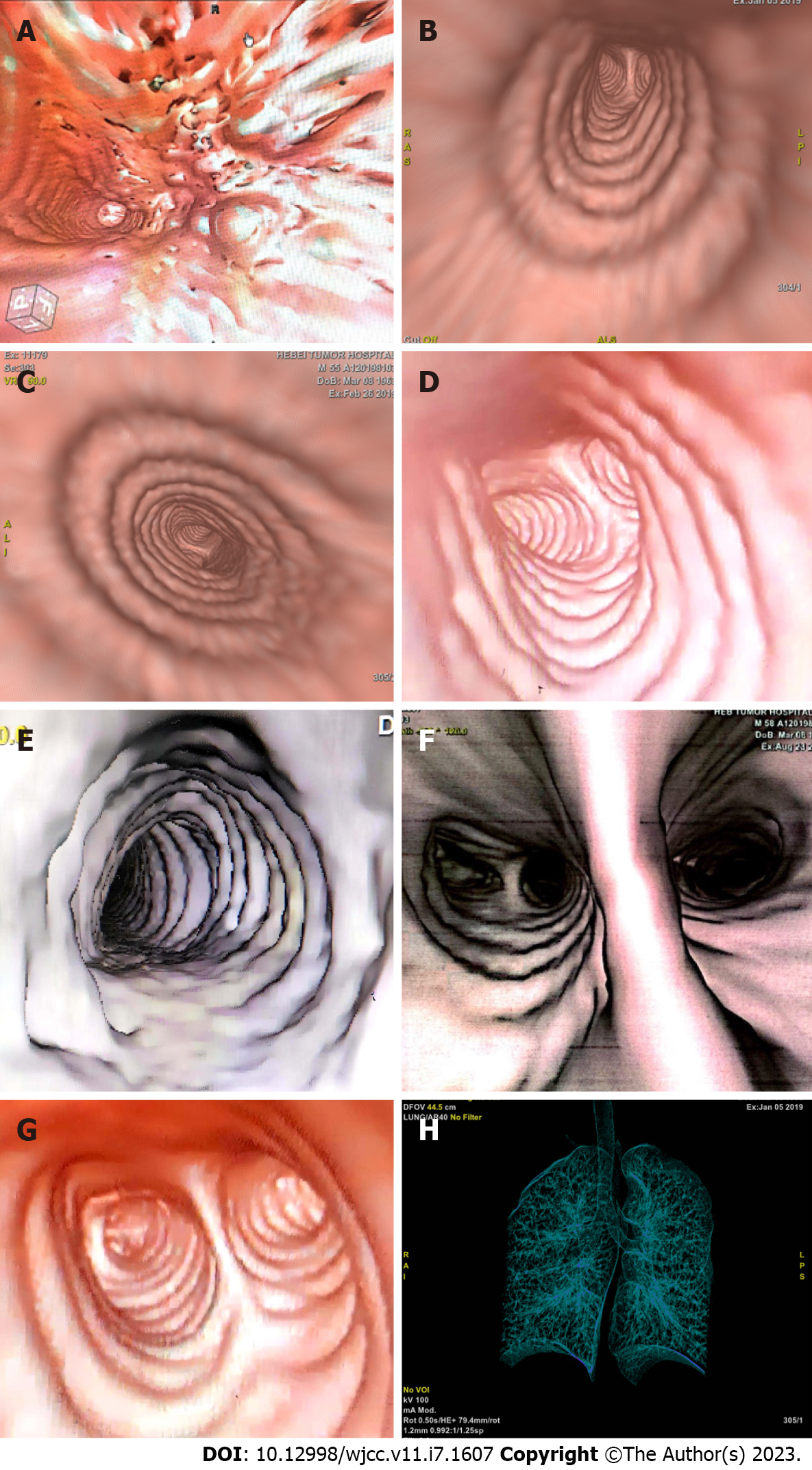
A 55-year-old man admitted to hospital had a three-month history of progressively coughing up yellow sputum, chest stuffiness, and shortness of breath. Fiberoptic bronchoscopy revealed mucosal visible beaded bumps 4 cm from the tracheal carina at 9 o 'clock and 3 o 'clock, the right main bronchus, and the right upper lobe bronchus. Biopsy specimens showed MALT lymphoma. Computed tomography virtual bronchoscopy (CTVB) showed uneven main bronchial wall thickening and multiple nodular protrusion. BALT lymphoma stage IE was diagnosed after a staging examination. We treated the patient with radiotherapy (RT) alone. A total dose of 30.6 Gy/17 f/25 d was given. The patient had no obvious adverse reactions during RT. The CTVB was repeated after RT and showed that the right side of the trachea was slightly thickened. CTVB was repeated 1.5 mo after RT and again showed that the right side of the trachea was slightly thickened. Annual CTVB showed no signs of recurrence. The patient now has no symptoms.
BALT lymphoma is an uncommon disease and shows good prognosis. The treatment of BALT lymphoma is controversial. In recent years, less invasive diagnostic and therapeutic approaches have been emerging. RT was effective and safe in our case. The use of CTVB could provide a noninvasive, repeatable, and accurate method in diagnosis and follow-up.
Core Tip: The treatment of bronchus-associated lymphoid tissue (BALT) lymphoma is controversial. A patient with BALT lymphoma received radiotherapy (RT) alone. A total dose of 30.6 Gy/17 f/25 d was given. The patient had no obvious adverse reactions during RT. The computed tomography virtual bronchoscopy (CTVB) was repeated after RT and showed that the right side of the trachea was slightly thickened. CTVB was repeated 1.5 mo after RT and again showed that the right side of the trachea was slightly thickened. Annual CTVB showed no signs of recurrence.
- Citation: Zhen CJ, Zhang P, Bai WW, Song YZ, Liang JL, Qiao XY, Zhou ZG. Mucosa-associated lymphoid tissue lymphoma of the trachea treated with radiotherapy: A case report. World J Clin Cases 2023; 11(7): 1607-1614
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1607.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1607
Primary pulmonary non-Hodgkin’s lymphomas are uncommon. They represent 3.6% of all extranodal lymphomas and 0.4% of all non-Hodgkin’s lymphomas. Extranodal marginal zone lymphoma of the mucosa-associated lymphoid tissue (MALT) type is the most frequent[1]. MALT lymphoma originates in the marginal zone of lymphoid tissue. The gastrointestinal tract is associated with more than two-thirds of cases, but the lung is one of the most frequent non-gastrointestinal organs involved, here known as bronchus-associated lymphoid tissue (BALT) lymphoma. BALT lymphoma of unknown etiology, and most patients are asymptomatic. The treatment of BALT lymphoma is controversial. A patient with BALT lymphoma received radiotherapy (RT) alone, leading to complete remission of the tumor. A case description as follows.
A 55-year-old man had progressively cough up yellow sputum, chest stuffiness, and shortness of breath.
A 55-year-old man, had a three-month history of progressively coughing up yellow sputum, chest stuffiness, and shortness of breath. He had no chest pain, fever, night sweats, or weight loss.
No lymphadenopathy or chronic lung disease was present.
The patient had no history of smoking or alcohol abuse. Without family history of carcinomas.
Physical examination did not show any signs of superficial lymph node enlargement. No rales, two lungs breathing clearly.
Hematological and biochemical examination results largely normal.
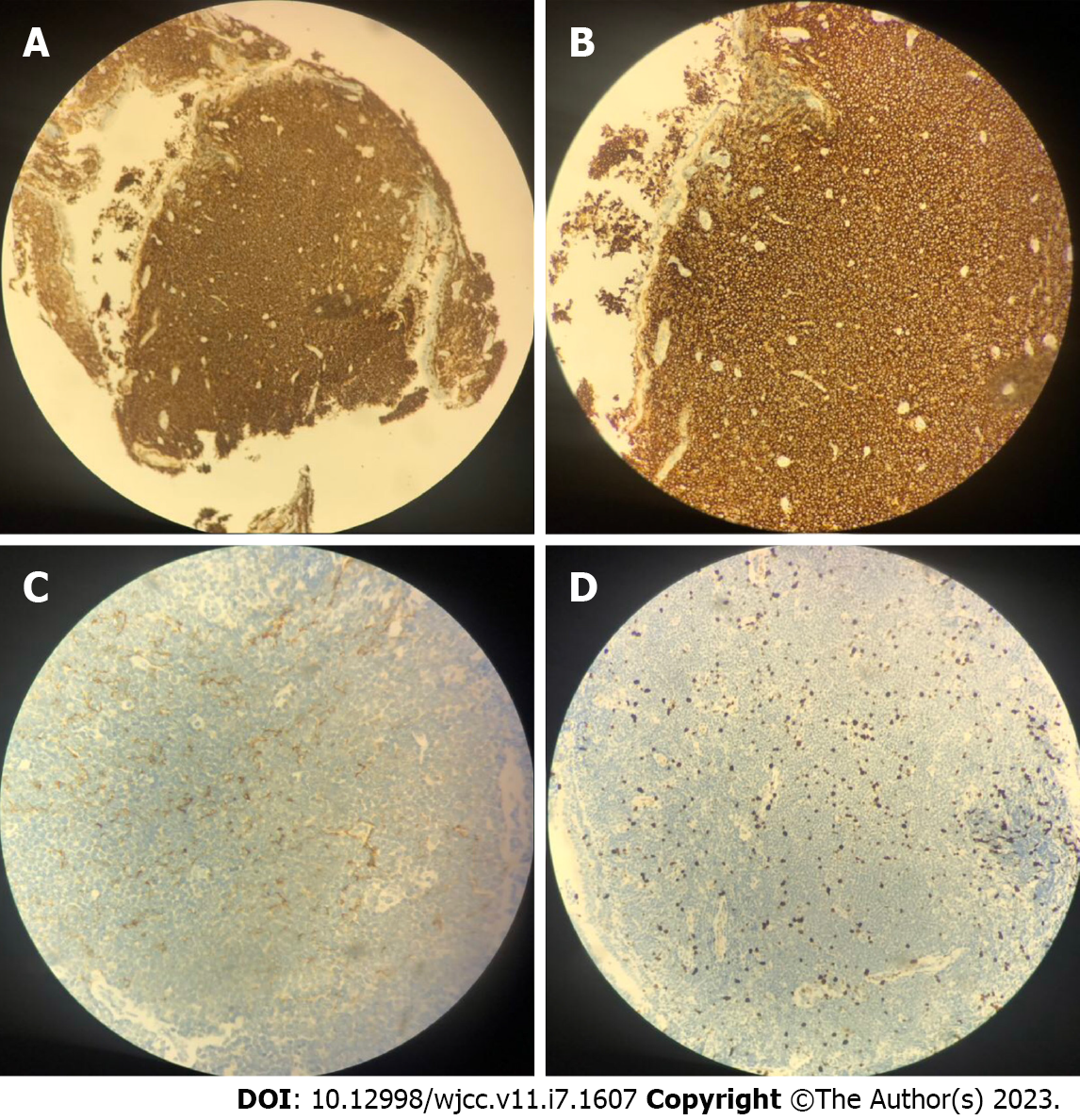
An enhanced CT scan revealed the left upper lobe nodules, considered benign. Two-side pleural multiple heterogeneous hypertrophies with calcification was found. Fiberoptic bronchoscopy showed mucosal visible beaded bumps 4 cm from the tracheal carina at 9 o 'clock and 3 o 'clock, the surface flow was rich, and the bronchus extended to the tracheal carina, the right main bronchus, and the right upper lobe bronchus (Figure 1). Biopsy specimens showed MALT lymphoma (Figure 2). Immunohistochemical staining was performed with Bcl - 6 (-), CD10 (-), CD20 (+), CD21 (remaining FDC net), CD3 (-), CD56
BALT lymphoma stage IE was diagnosed was diagnosed.
We treated the patient with RT alone. Intensity–modulated radiation therapy was performed with Elekta linear accelerator. A total dose of 30.6 Gy/17 f/25 d was given. The process went well.
The patient had no obvious adverse reactions, and all symptoms disappeared. After completing RT, CTVB was repeated and showed the right side of the trachea was slightly thickened (Figure 4B). CTVB was repeated 1.5 mo after RT and showed again that the right side of the trachea was slightly thickened (Figure 4C). The case had been followed up for more than 3.5 years, and annual CTVB showed no signs of recurrence (Figure 4D-G). The patient now shows no symptoms. Long-term efficacy requires further follow-up.
BALT lymphoma is considered to be a consequence of long-term exposure to a variety of antigenic stimuli-including smoking, inflammatory disorders, or autoimmune diseases[2-4]. BALT lymphoma tends to remain localized until late in the natural course. The histological progression from a low-grade BALT lymphoma to a high-grade lymphoma is rare, and they show a good prognosis. Many patients with this disease are asymptomatic. Symptomatic patients evidence some nonspecific pulmonary symptoms, such as cough, dyspnea, and chest pain. It is easy to misdiagnose and miss diagnoses[5,6].
The diagnosis of BALT lymphoma should be based on comprehensive analysis via chest X-ray, computed tomography (CT), magnetic resonance imaging and other imaging, a bone marrow biopsy, bronchoscopy, and positron emission tomography/CT. Imaging is characterized by irregular tracheal or bronchial wall thickening and luminal stenosis, with or without accompanying atelectasis. Microscopic examination of the trachea is performed to diagnose the disease under larger values, mainly for nodules or mucosa hypertrophy.
Because the incidence and prevalence of this disease are rare, it is difficult for large randomized clinical trials to provide an “evidence-based” approach. There is no consensus for the treatment of BALT lymphoma. Surgery, single agent therapy, combination chemotherapy, radiation treatment, and the watchful waiting approach have all been used in single or very small series of cases[7-10]. Some scholars believe that localized disease could be resected, especially for diagnostic and therapeutic purposes. RT may play a role in the case of small localized lesions. If symptomatic, BALT lymphoma should be treated with combination chemotherapy or chemoimmunotherapy. For all other cases (asymp
| Number | Treatment | Dose of radiation | Response | Outcome | Ref. |
| 61 | Surgery (21), Surgery + chemotherapy (16), Surgery + radiation (3), Surgery + chemotherapy + radiation (2), Chemotherapy (16), Observation (3) | Not reported | Not reported | 5-year OS 93.6% | Cordier et al[5] |
| 19 | Chemotherapy (14), Surgery (2), Surgery + chemotherapy (2), Chemotherapy + radiation (1) | Not reported | 79% CR, 21% PR | Not reported | Zinzani et al[17] |
| 41 | Observation (5), Surgery (17), Chemotherapy (12), Surgery + chemotherapy (3), Surgery + radiation (1), Prednisone (2), Unknown (1) | Not reported | Not reported | Lymphoma-specificsurvival was 71.7% at 10 years | Kurtin et al[18] |
| 22 | Observation (2), Chemotherapy alone (2), Rituximab alone (2), Systemic chemotherapy ± Rituximab (12), Chemotherapy with rituximab (8), Surgery (6), Radiotherapy (2) | Not reported | --, 2 PR, 2 PR, 2 CR, 9 PR, 1 SD, 2 CR, 5 PR, 1 SD, 6 CR, 1 CR, 1 PR | 53 mo median PFS | Ahmed et al[19] |
| 18 | Observation (1), Surgery (6), Surgery + chemotherapy (8), Surgery + radiotherapy (1), Surgery + chemotherapy + radiotherapy (2) | Not reported | Not reported | 6 years median time to disease recurrence or death | Graham et al[20] |
| 61 | Surgery alone (17), Surgery + Chemotherapy (3), Surgery + Radiotherapy (2), Chemotherapy (28), Radiotherapy (6), Observation (5) | Not reported | 15 CR, 2 PR, 3 CR, 2 CR, 7 CR, 12 PR, 5 SD, 2 PD, 2 not valuable, 3 CR, 2 PR, 1 SD, 5 not valuable | median time to progression was 5.6 years. 5-year OS 89.7% | Oh et al[21] |
| 10 | Radiotherapy ± surgery ± chemotherapy ± rituximab | 2 Gy × 2 | 6 CR, 4 PR | 87.5% 5-year progression-free survival rate | Girinsky et al[15] |
| 1 | Radiotherapy (1) | 30 Gy | CR | No signs of recurrence are found 4 years after radiotherapy | Hashemi et al[13] |
| 2 | Radiotherapy + rituximab (1), Observation (1) | 50 Gy | CR, -- | Not reported | Kawaguchi et al[14] |
| 1 | Radiotherapy (1) | 30.6 Gy | CR | Survive more than 3.5 years | Our case |
In our case, referring to the biological characteristics of MALT lymphoma and the radiation dose of digestive tract MALT lymphoma, a total dose of 30.6 Gy/17 f was given. There were no obvious symptoms, radioactive lung, or heart injury during and after RT. Until the time of follow-up, there were no signs of recurrence. RT was effective and safe for patients with BALT lymphoma. It also retained the normal physiological structure and improved quality of life. Annual CTVB showed no signs of recurrence. Long-term efficacy still requires further follow-up.
BALT lymphoma can manifest as solitary intraluminal nodules, a diffuse wall thickening, and several tiny nodular protrusions in CT scans. Chest CT alone is not usually sufficient to determine the scope of the lesions. A bronchoscope is important in diagnosis and therapy. In our case, the patient received CTVB before RT, at the end of RT, and 1.5 mo after RT to determine the scope of the lesions and evaluate the effect. Annual CTVB was used for annual reviews. CTVB is a CT-based imaging technique that allows for a noninvasive intraluminal evaluation of the tracheobronchial tree. It can accurately show the lumen and the diameter of the trachea, the left and right main stem bronchi, and the bronchial tree down to the fourth order of bronchial orifices and branches (Figure 4H). The morphology of the carinas can be evaluated accurately, and the images look very similar to those recorded with fiberoptic bronchoscopy (FB)[16]. In contrast to FB, CTVB is noninvasive and repeatable. It can show the outside of the cavity of infringement and indicate its relationship with surrounding structures. It can also show the bronchial lumen across the narrow or blocked bronchial segment and can be used to observe the lumen, which FB cannot achieve. In this case, CTVB was applied for diagnosis, treatment, and follow-up and provided abundant information regarding the above-mentioned characteristics. It can be used in subsequent follow-up and in other cases.
BALT lymphoma is an uncommon disease and shows good prognosis. The treatment of BALT lymphoma is controversial. In recent years, less invasive diagnostic and therapeutic approaches have been emerging. RT was effective and safe in our case. The use of CTVB could provide a noninvasive, repeatable, and accurate method in diagnosis and follow-up.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gursel B, Turkey; Sezer HF, Turkey S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Isaacson PG, Spencer J. Malignant lymphoma of mucosa-associated lymphoid tissue. Histopathology. 1987;11:445-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 490] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 2. | Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am J Respir Cell Mol Biol. 1990;3:131-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 155] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 3. | Richmond I, Pritchard GE, Ashcroft T, Avery A, Corris PA, Walters EH. Bronchus associated lymphoid tissue (BALT) in human lung: its distribution in smokers and non-smokers. Thorax. 1993;48:1130-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Tschernig T, Pabst R. Bronchus-associated lymphoid tissue (BALT) is not present in the normal adult lung but in different diseases. Pathobiology. 2000;68:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 150] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Cordier JF, Chailleux E, Lauque D, Reynaud-Gaubert M, Dietemann-Molard A, Dalphin JC, Blanc-Jouvan F, Loire R. Primary pulmonary lymphomas. A clinical study of 70 cases in nonimmunocompromised patients. Chest. 1993;103:201-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 180] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Li G, Hansmann ML, Zwingers T, Lennert K. Primary lymphomas of the lung: morphological, immunohistochemical and clinical features. Histopathology. 1990;16:519-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 174] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Cadranel J, Wislez M, Antoine M. Primary pulmonary lymphoma. Eur Respir J. 2002;20:750-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Addis BJ, Hyjek E, Isaacson PG. Primary pulmonary lymphoma: a re-appraisal of its histogenesis and its relationship to pseudolymphoma and lymphoid interstitial pneumonia. Histopathology. 1988;13:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 155] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 9. | Wei Z, Li J, Cheng Z, Yuan L, Liu P. A single center experience: rituximab plus cladribine is an effective and safe first-line therapy for unresectable bronchial-associated lymphoid tissue lymphoma. J Thorac Dis. 2017;9:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Isaacson PG, Wotherspoon AC, Diss T, Pan LX. Follicular colonization in B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Surg Pathol. 1991;15:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 193] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Arnaoutakis K, Oo TH. Bronchus-associated lymphoid tissue lymphomas. South Med J. 2009;102:1229-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Sammassimo S, Pruneri G, Andreola G, Montoro J, Steffanoni S, Nowakowski GS, Gandini S, Negri M, Habermann TM, Raderer M, Li ZM, Zinzani PL, Adam P, Zucca E, Martinelli G. A retrospective international study on primary extranodal marginal zone lymphoma of the lung (BALT lymphoma) on behalf of International Extranodal Lymphoma Study Group (IELSG). Hematol Oncol. 2016;34:177-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Hashemi SM, Heitbrink MA, Jiwa M, Boersma WG. A patient with endobronchial BALT lymphoma successfully treated with radiotherapy. Respir Med. 2007;101:2227-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kawaguchi T, Himeji D, Kawano N, Shimao Y, Marutsuka K. Endobronchial Mucosa-associated Lymphoid Tissue Lymphoma: A Report of Two Cases and a Review of the Literature. Intern Med. 2018;57:2233-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Girinsky T, Paumier A, Ferme C, Hanna C, Ribrag V, Leroy-Ladurie F, Ghalibafian M. Low-dose radiation treatment in pulmonary mucosa-associated lymphoid tissue lymphoma: a plausible approach? Int J Radiat Oncol Biol Phys. 2012;83:e385-e389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | De Wever W, Vandecaveye V, Lanciotti S, Verschakelen JA. Multidetector CT-generated virtual bronchoscopy: an illustrated review of the potential clinical indications. Eur Respir J. 2004;23:776-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 55] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Zinzani PL, Magagnoli M, Galieni P, Martelli M, Poletti V, Zaja F, Molica S, Zaccaria A, Cantonetti AM, Gentilini P, Guardigni L, Gherlinzoni F, Ribersani M, Bendandi M, Albertini P, Tura S. Nongastrointestinal low-grade mucosa-associated lymphoid tissue lymphoma: analysis of 75 patients. J Clin Oncol. 1999;17:1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 193] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Kurtin PJ, Myers JL, Adlakha H, Strickler JG, Lohse C, Pankratz VS, Inwards DJ. Pathologic and clinical features of primary pulmonary extranodal marginal zone B-cell lymphoma of MALT type. Am J Surg Pathol. 2001;25:997-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Ahmed S, Kussick SJ, Siddiqui AK, Bhuiya TA, Khan A, Sarewitz S, Steinberg H, Sison CP, Rai KR. Bronchial-associated lymphoid tissue lymphoma: a clinical study of a rare disease. Eur J Cancer. 2004;40:1320-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Graham BB, Mathisen DJ, Mark EJ, Takvorian RW. Primary pulmonary lymphoma. Ann Thorac Surg. 2005;80:1248-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Oh SY, Kim WS, Kim JS, Kim SJ, Kwon HC, Lee DH, Won JH, Hwang IG, Kim MK, Lee SI, Chae YS, Yang DH, Lee GW, Choi CW, Park J, Suh C, Kim HJ. Pulmonary marginal zone B-cell lymphoma of MALT type--what is a prognostic factor and which is the optimal treatment, operation, or chemotherapy? Ann Hematol. 2010;89:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |