Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1586
Peer-review started: October 27, 2022
First decision: January 9, 2023
Revised: January 14, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 6, 2023
Processing time: 126 Days and 0.2 Hours
It is not uncommon to develop autoimmune encephalitis and paraneoplastic neurological syndromes (PNS). 4 kinds of antibody-positive autoimmune paraneoplastic limbic encephalitis (PLE) have not been reported.
PNS are distant effects of cancer on the nervous system, rather than syndromes in which cancer directly invades and metastasizes to the nerves and/or muscle tissues. If the limbic lobe system of the brain is involved, this will result in PLE. The detection of patients with PNS is challenging since tumors that cause paraneoplastic neurologic disorders are often asymptomatic, obscure, and thus easily misdiagnosed or missed. Currently, single- or double-antibody-positive paraneoplastic marginal encephalitis has been reported. However, no cases of three or more-antibody-positive cases have been reported. Here, we report a case of PLE that is anti-collapsing response-mediator protein-5, anti-neuronal nuclear antibody-type 1, anti-aminobutyric acid B receptor, and anti-glutamate deglutase positive, and address relevant literature to improve our understanding of the disease.
This article reports on the management of a case of PLE with four positive antibodies, a review of the literature, in order to raise awareness among clinicians.
Core Tip: This patient mainly had persistent epilepsy as the main clinical manifestation, and a lumbar puncture examination showed anti-collapsing response mediator protein-5; anti-neuronal nuclear antibody-type 1; anti-aminobutyric acid B receptor; anti-glutamate deglutase positive, and further improvement of chest computed tomography and medical examination showed lung small cell carcinoma.
- Citation: Huang P, Xu M. Four kinds of antibody positive paraneoplastic limbic encephalitis: A rare case report. World J Clin Cases 2023; 11(7): 1586-1592
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1586.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1586
Autoimmune encephalitis (AE) refers to a class of encephalitis mediated by the autoimmune mechanism. With the continuous development of examination technology in recent years, a variety of AE-related antibodies have been found, among which anti-aminobutyric acid B receptor (GABA-BR) and anti-glutamate deglutase (GAD) antibodies are most common. Rather than direct lyses and transference to nerve, muscle, or neuromuscular joints, paraneoplastic neurological syndromes (PNS) refer to the long-term effects of tumors on the nervous system. In recent years, a variety of antibodies have been found in serum or cerebrospinal fluid of PNS patients, which contribute to the diagnosis of PNS.
When AE combined with tumor, it is called parathymoma-associated AE, and if it conforms to the limbic system, it is called Paraneoplastic limbic encephalitis (PLE).
A 50-year-old male was hospitalized mainly because of “repeated loss of consciousness, convulsions of limbs, and abnormal mental behavior for 10 d”.
Ten days before his admission into the hospital, the patient suddenly fell onto the ground while working. After his fall, people tried to speak to him, but he did not reply, his teeth were clenched and his eyes turned upwards. About 2-3 min later, his convulsions stopped and he gradually recovered consciousness. Then he complained that he felt pain and discomfort throughout his entire body, but no incontinence was present. His limb movement was disorderly and his pronunciation was unclear. He was admitted to the local hospital for treatment and his disease was considered to be “epilepsy”, and thus sodium valproate was used to treat him. On the night of admission, the patient began to exhibit nonsensical behavior: he played with his fingers, kept making the beds, and was biting the quilts. At night, he was in high spirits and walked around. After treatment in the local hospital, the symptoms of loss of consciousness and limb convulsions still repeated. After performing chest computed tomography (CT) and considering lung cancer, the lung puncture examination indicated that the lower leaf of the right lung was on base section: small cell lung cancer, low-differentiated adenocarcinoma, salivating adenocarcinoma, immunohistochemistry which signaled small cell lung cancer. After the patient underwent convulsion again and did not recover consciousness, he was then urgently transferred to our hospital. The emergency department diagnosed him with “epileptic continuity”. He was also diagnosed with lung cancer and admitted to our hospital.
He had no special medical history.
He had been smoking for 30 years with 20 cigarettes per day. He had no alcohol habits.
Admission check: body temperature 38.6 °C, wet rales can be heard at the base of both lungs. There were no obvious cardiac abnormalities. Nervous system check: sleepy, inability to cooperate with cognitive function tests. Double pupils were circular and their diameters were about 2.5 mm, but they appeared dull to light reflection. No obvious facial tongue palsy and the post-group cranial nerve was not willing to cooperate. There was no atrophy or involuntary movement of the limbs, and the muscle tone of the limbs decreased. During pain stimulation, flexion movements of the limbs were visible. Sensory system and working movement were not willing to cooperate. No movement in the limbs and weak tendon reflexes. Negative pathological signs and signs of meningeal irritation.
The number of blood leukocytes was 20.69 × 109/L (3.5-9.5 × 109/L), of which neutral cells accounted for 94% (40%-75%). CRP 0.499 mg/L (0.5-10 mg/L), blood sink, serum tumor markers, clotting, acute infection-related items, liver and kidney function, electrolytes, blood lipids, and a functional, autoimmune antibody spectrum were negative.
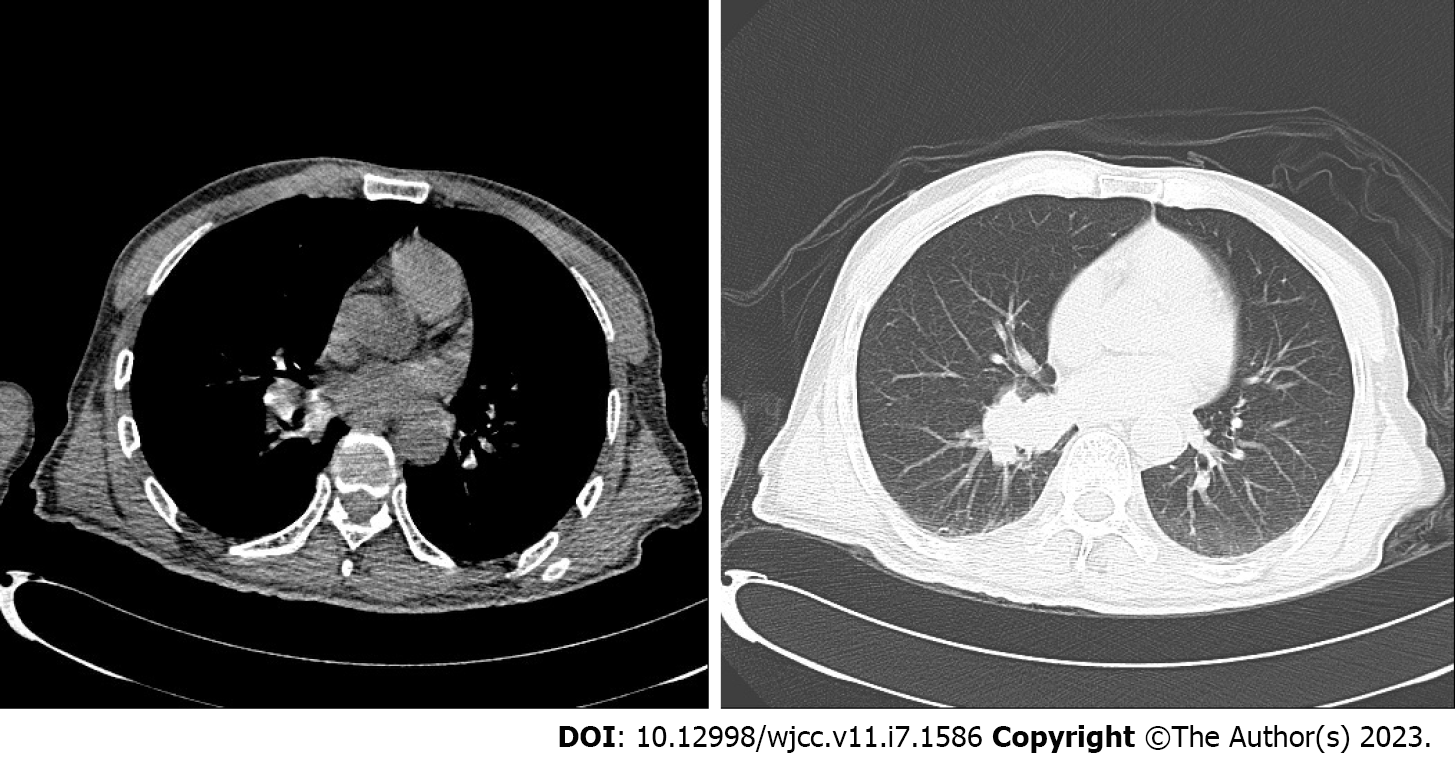
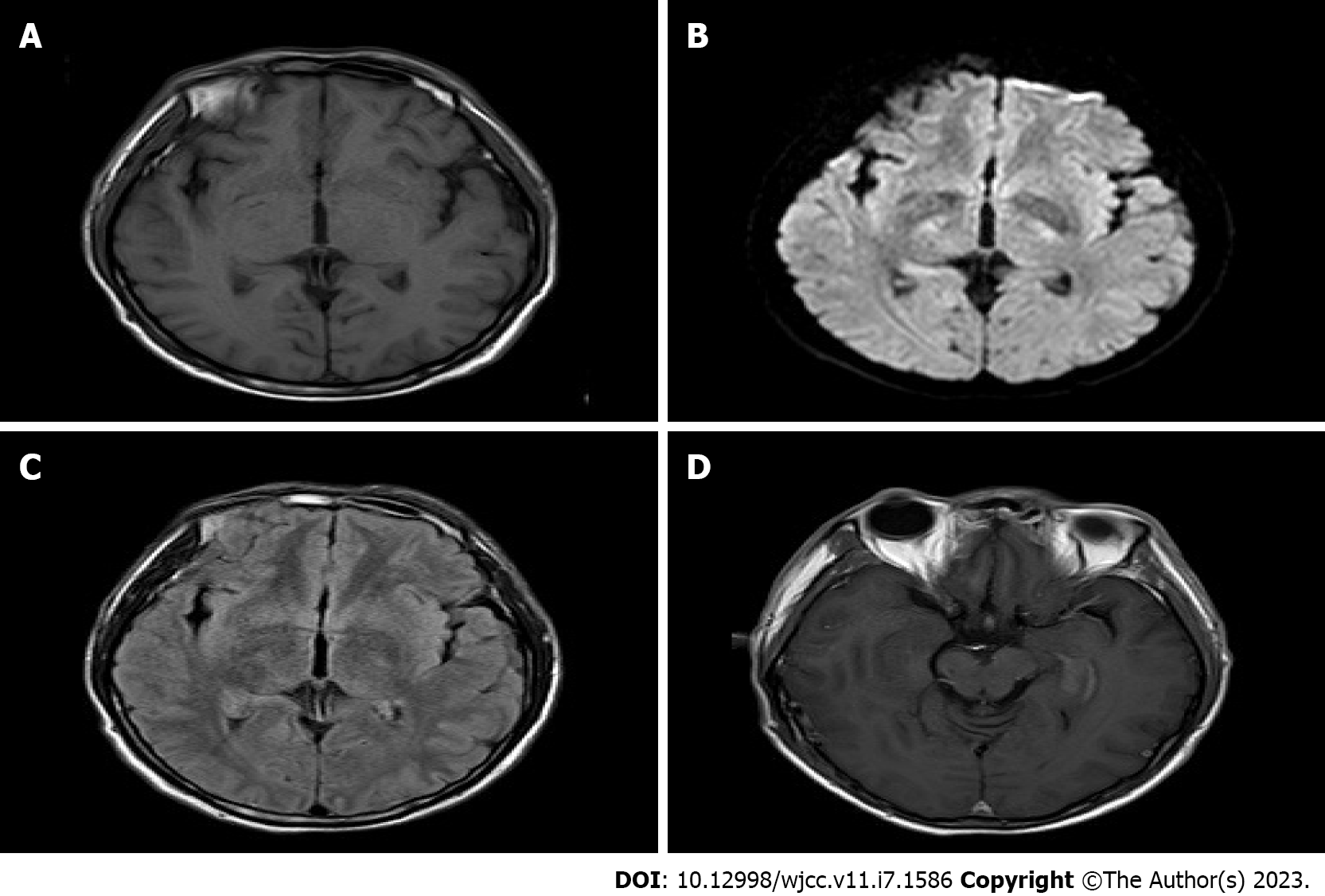
CT revealed no abnormality in his brain essence. After scanning and strengthening the chest CT, an increase of soft tissue cluster shadows near the lower left of the right lung was visible (Figure 1A and B). The local bronchial was slightly blocked and there was a strip of increasing density shadow in the distant lung. Considering the possibility of central lung cancer, we recommended him to undergo further fiber optic examination. Scanning and strengthening the head magnetic resonance imaging (MRI) revealed that the left hippocampus was thickened, and there was a signal that fluid attenuated inversion recovery (FLAIR) was slightly higher. After the thickening of the left hippocampus, there was a strip of high signal shadow near the left hippocampus and temporal lobe. There was no dispersion restriction near diffusion-weighted imaging (Figure 2A-D).
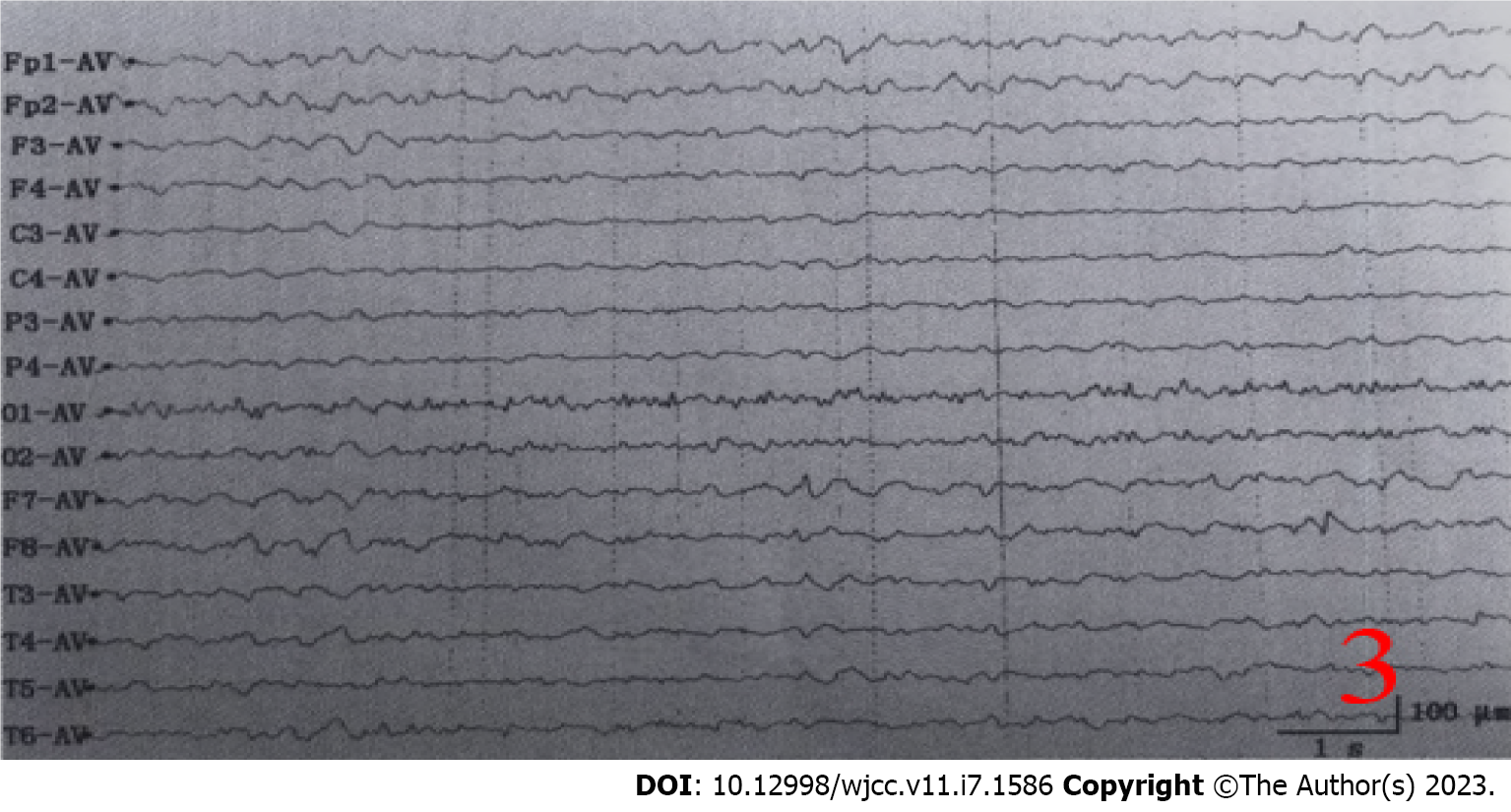
Serum paratumor-related series anti-collapsing response mediator protein-5 (CRMP5/CV2) and anti-neuronal nuclear antibody-type 1 (ANNA1/Hu) antibody were positive. After perfecting lumbar puncture, the results indicated that the cerebrospinal fluid was colorless and transparent, pressure 260 mmH2O (1 mmH2O = 0.009 kPa), protein quantification 0.69 g/L (0-0.43 g/L), cerebrospinal fluid nuclear cell 0.008 × 109/L (0-0.015 × 109/L), adenosine deaminase: 5 U/L (0-4 U/L). Cerebrospinal fluid AE examination showed GABA-BR antibody IgG (++1:10), anti-GAD antibody IgG (+1:10). Cerebral spinal fluid nervous system paratumor syndrome series, herpes simplex virus DNA testing, TB DNA, ink staining, antacid staining were all negative. Video electroencephalography showed that before the illness, the patient’s electroencephalography was in sleeping I waveform. The left forehead to the top was most significant. There were many fast waves between medium and high wave amplitude and then there was a collection-like trend spreading to the whole guide wave range and exhibiting high waves. It was mixed with ratchet rhythm and myoelectric promiscuity phase and the patient exhibited synchronized strong straight state. When the patient underwent numerous convulsions, the brain wave fully showed very high wave amplitudes until 2-3 Hz, after which a gradual slowdown appeared. This decrease to restore the background brain wave indicated that the background brain waves were mainly low, medium-wave slow wave activities. There was no obvious α wave in the top area. As for the sleep brain wave, there was no obvious K-composite wave (Figure 3). During the sleeping period, there was slow wave and rapid eye rotating.
The diagnoses of the patient were: (1) Paraneoplastic limbic encephalitis with positive anti-CV2, anti-Hu, anti-GABA-BR, and anti-GAD; (2) secondary status epilepticus (Generalized tonic-clonic seizure); (3) right lung cancer; and (4) lung infection.
The patient was given methylprednisolone and gamma-globulin shock treatment, epilepsy control, antiviral and anti-infection treatments, nutritional support, and multidisciplinary collaboration treatment. After 17 d, his limb convulsions, nonsensical behavior, and fever symptoms were gradually controlled.
After the patient’s admission to our hospital, our hospital provided methylprednisolone and gamma-globulin shock treatment, epilepsy control, antiviral and anti-infection treatments, nutritional support, and multidisciplinary collaboration treatment. After 17 d, his limb convulsions, nonsensical behavior, and fever symptoms were gradually controlled. When the patient was ready to be discharged from the hospital, he exhibited a slightly slow response, poor memory, and poor self-care performance. Two months later, through telephone consultation, our hospital was informed that he could eat independently and maintain brief conversations. The patient signed an informed consent document and expressed his knowledge of the study.
AE refers to a class of encephalitis mediated by the autoimmune mechanism. It was first reported by Corsellis that AE represented approximately 10%-20% of encephalitis cases[1]. AE often affects the hippocampus, temporal lobe, and dentate gyrus, and exhibits clinical manifestations such as acute or sub-acute epilepsy-like seizures[2], nonsensical and abnormal behavior, and memory loss. With the continuous development of examination technology in recent years, a variety of AE-related antibodies have been found, among which anti-GABA-BR and anti-GAD antibodies are most common. GABA-BR is widely expressed in the hippocampus, cortex, and cerebellum, and belongs to the inhibitory receptors of the central nervous system[3]. When GABA-BR are knocked down in mice, convulsive behavior results, which is associated with the removal of GABA-BR inhibition in the central nervous system. GAD is a catalytic aminobutyric acid that is catalyzed in cells. As a speed limit enzyme, the GABA complex plays a role in central nervous system inhibitory dystonia and can catalyze the transformation of glutamate into the inhibitory neurotransmitter GABA. Currently, it is believed that anti-GAD represents the connection between positive encephalitis patients and their non-tumor autoimmune diseases[4]. Clinical manifestations mainly consist of affected temporal lobes after which epilepsy-like symptoms appear with poor prognosis.
Rather than direct lyses and transference to nerve, muscle, or neuromuscular joints, PNS refers to the long-term effects of tumors on the nervous system. In recent years, a variety of antibodies have been found in serum or cerebrospinal fluid of PNS patients, which contribute to the diagnosis of PNS. The Hu antibody, also known as ANNA1, is a multi-cloned IgG antibody. The target antigen of Hu is found in the central and peripheral nervous system of all neurons as well as in tumor cells. The anti-Hu antibody in tumor patients can attack the tumor, but will also attack the central or peripheral nervous system at the same time, which causes symptoms to appear[5]. Studies have shown that anti-Hu antibodies exhibit a high specificity (95%-100%) and sensitivity (> 80%) to PNS diagnosis[6]. CRMP-5 is widely expressed in the hippocampus and cortex and promotes synaptic proliferation[7]. Furthermore, CRMP-5 can also be highly expressed in small cell lung cancer and thymus tumor and promotes tumor proliferation. CV2 antibody is a small cell lung cancer antibody[8], that can specifically bind with CRMP-5 to form a CV2/CRMP-5 antibody complex. This complex is also called a paratumor antibody and its presence indicates potential tumors. Compared with anti-Hu antibodies, the average survival time of anti-CV2 antibodies in positive patients is significantly longer. The prognosis is good and not affected by tumor types[9].
AE-merging tumors, known as paratumor-dependent AE, is associated with the limbic system and is classified as PLE. In head MRI examinations of such patients, there is a 66% possibility to find that FLAIR shows high signals in the temporal lobe and hippocampus, indicating that the limbic system is affected[10]. To date, only one or two cases of antibody-positive paratumor AE have been reported. Approximately 50% of patients developing anti-GABA-BR antibody-positive AE are easy to merge tumors, mainly small cell lung cancer, melanoma, or thymus tumor. For such patients, AE symptoms often occur before the tumor is diagnosed. However, at present, there is no report that all the above four antibodies are positive at the same time. Compared with the two antibodies, the positive diagnosis of three or more antibodies was difficult. Combining clinical performance of the patient in this case, imaging examination, history of lung cell carcinoma and related antibody results, according to the relevant diagnostic criteria[10], it is clear that the patient is diagnosed with anti-CV2, anti-Hu, anti-GABA-BR, anti-GAD antibody-positive paratumor autoimmune limbenitis. However, in this case, the patient was also considered (due to his fever) to possibly have viral encephalitis in the early stages, before the results of autoimmune antibodies and paratumor-related antibodies were known. Moreover, a recent study reported that viral encephalitis can be a secondary trigger of AE[11].
Immunotherapy, including intravenous glucocorticoids and intravenous immunoglobulin administration, is currently indicated for all patients with first-episode AE, with intravenous glucocorticoids as the preferred regimen[12-15]. In general, treatment with a combination of glucocorticoids and immunoglobulins should be administered. If there is no significant improvement after 2 wk on both first-line immunotherapy, intravenous rituximab should be initiated. There are no clear guidelines for the treatment of paraneoplastic AE, but because of the similarity of many pathogenic mechanisms and antibodies, treatment of paraneoplastic AE is similar to that of AE associated with anti-neuronal cell surface or synaptic protein antibodies, but it should be noted that in T cell-mediated paraneoplastic AE (e.g., anti-Hu antibody-associated encephalitis), irreversible neuronal damage can occur early and rapidly and therefore may respond poorly to immunotherapy[16-18].
In summary, clinical patients who are suspected to have PLE should first undergo related antibody testing and imaging examination to search for evidence of extracranial tumors as early as possible. Early treatment can often achieve better results. However, it should be noted that some clinical cancer patients may appear to have the first secondary tumor-related antibodies or exhibit negative results for AE antibodies. For such patients, we need to follow them closely and review timely in order to reduce missed diagnosis. For patients who are positive for the antibodies but have no related imaging evidence of tumors, the screening of specific systemic tumors can be carried out according to the positive antibody results.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Clinical neurology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mehdipour P, Iran; Samizadeh B; Spataru A, Canada S-Editor: Chang KL L-Editor: A P-Editor: Chang KL
| 1. | Jain A, Balice-Gordon R. Cellular, synaptic, and circuit effects of antibodies in autoimmune CNS synaptopathies. Handb Clin Neurol. 2016;133:77-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalá C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 395] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 3. | Dalmau J, Graus F. Antibody-Mediated Encephalitis. N Engl J Med. 2018;378:840-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 620] [Cited by in RCA: 785] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 4. | Gresa-Arribas N, Ariño H, Martínez-Hernández E, Petit-Pedrol M, Sabater L, Saiz A, Dalmau J, Graus F. Antibodies to inhibitory synaptic proteins in neurological syndromes associated with glutamic acid decarboxylase autoimmunity. PLoS One. 2015;10:e0121364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 117] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Senties-Madrid H, Vega-Boada F. Paraneoplastic syndromes associated with anti-Hu antibodies. Isr Med Assoc J. 2001;3:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 89] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti-Hu antibodies: A beneficial antitumor immune response going awry. Oncoimmunology. 2013;2:e27384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 7. | Dale RC, Brilot F, Duffy LV, Twilt M, Waldman AT, Narula S, Muscal E, Deiva K, Andersen E, Eyre MR, Eleftheriou D, Brogan PA, Kneen R, Alper G, Anlar B, Wassmer E, Heineman K, Hemingway C, Riney CJ, Kornberg A, Tardieu M, Stocco A, Banwell B, Gorman MP, Benseler SM, Lim M. Utility and safety of rituximab in pediatric autoimmune and inflammatory CNS disease. Neurology. 2014;83:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Gozzard P, Maddison P. Which antibody and which cancer in which paraneoplastic syndromes? Pract Neurol. 2010;10:260-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Flanagan EP, McKeon A, Lennon VA, Kearns J, Weinshenker BG, Krecke KN, Matiello M, Keegan BM, Mokri B, Aksamit AJ, Pittock SJ. Paraneoplastic isolated myelopathy: clinical course and neuroimaging clues. Neurology. 2011;76:2089-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 10. | Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Höftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Prüss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostásy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2328] [Cited by in RCA: 2641] [Article Influence: 293.4] [Reference Citation Analysis (0)] |
| 11. | Thomas L, Mailles A, Desestret V, Ducray F, Mathias E, Rogemond V, Didelot A, Marignier S, Stahl JP, Honnorat J; Steering Committee and Investigators Group. Autoimmune N-methyl-D-aspartate receptor encephalitis is a differential diagnosis of infectious encephalitis. J Infect. 2014;68:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2139] [Cited by in RCA: 2126] [Article Influence: 177.2] [Reference Citation Analysis (0)] |
| 13. | Nosadini M, Eyre M, Molteni E, Thomas T, Irani SR, Dalmau J, Dale RC, Lim M; International NMDAR Antibody Encephalitis Consensus Group, Anlar B, Armangue T, Benseler S, Cellucci T, Deiva K, Gallentine W, Gombolay G, Gorman MP, Hacohen Y, Jiang Y, Lim BC, Muscal E, Ndondo A, Neuteboom R, Rostásy K, Sakuma H, Sartori S, Sharma S, Tenembaum SN, Van Mater HA, Wells E, Wickstrom R, Yeshokumar AK. Use and Safety of Immunotherapeutic Management of N-Methyl-d-Aspartate Receptor Antibody Encephalitis: A Meta-analysis. JAMA Neurol. 2021;78:1333-1344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 14. | Dubey D, Britton J, McKeon A, Gadoth A, Zekeridou A, Lopez Chiriboga SA, Devine M, Cerhan JH, Dunlay K, Sagen J, Ramberger M, Waters P, Irani SR, Pittock SJ. Randomized Placebo-Controlled Trial of Intravenous Immunoglobulin in Autoimmune LGI1/CASPR2 Epilepsy. Ann Neurol. 2020;87:313-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 111] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Huang HJ, Chen WB, Liu G, Liu F, Su YY. Clinical efficacy of plasma exchange in patients with autoimmune encephalitis. Ann Clin Transl Neurol. 2021;8:763-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Devine MF, Kothapalli N, Elkhooly M, Dubey D. Paraneoplastic neurological syndromes: clinical presentations and management. Ther Adv Neurol Disord. 2021;14:1756286420985323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 17. | Rosenfeld MR, Dalmau J. Diagnosis and management of paraneoplastic neurologic disorders. Curr Treat Options Oncol. 2013;14:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Tanaka K, Tanaka M, Inuzuka T, Nakano R, Tsuji S. Cytotoxic T lymphocyte-mediated cell death in paraneoplastic sensory neuronopathy with anti-Hu antibody. J Neurol Sci. 1999;163:159-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |