Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1576
Peer-review started: October 24, 2022
First decision: November 25, 2022
Revised: December 3, 2022
Accepted: February 16, 2023
Article in press: February 16, 2023
Published online: March 6, 2023
Processing time: 129 Days and 0.2 Hours
Intracranial hemorrhage is extremely rare during the initial stages of glioma. Here, we report a case of glioma with unclassified pathology and intracranial bleeding.
After the second surgery for intracerebral hemorrhage, the patient experienced weakness in the left arm and leg, but could walk unassisted. One month after discharge, the weakness in the left limbs had exacerbated and the patient also suffered from headaches and dizziness. A third surgery was ineffective against the rapidly growing tumor. Intracerebral hemorrhage may be the initial symptom of glioma in some rare cases, and atypical perihematomal edema can be used for diagnosis during an emergency. Certain histological and molecular features seen in our case were similar to that of glioblastoma with a primitive neuronal component, which is termed diffuse glioneuronal tumor with features similar to oligodendroglioma and nuclear clusters (DGONC). The patient underwent three surgeries to remove the tumor. The first tumor resection had been performed when the patient was 14-years-old. Resection of the hemorrhage and bone disc decompression were performed when the patient was 39-years-old. One month after the last discharge, the patient underwent neuronavigation-assisted resection of the right frontotemporal parietal lesion plus extended flap decompression. On the 50th d after the third operation, computed tomography imaging showed rapid tumor growth accompanied by brain hernia. The patient was discharged and died 3 d later.
Glioma can present as bleeding in the initial stage and should be considered in such a setting. We have reported a case of DGONC, which is a rare molecular subtype of glioma with a unique methylation profile.
Core Tip: Intracranial hemorrhage is extremely rare during the initial stages of glioma. We hereby report a case of glioma with an unclassified pathology and two instances of intracranial hemorrhage in the initial stage, and atypical perihematomal edema may help in its diagnosis. Certain histological and molecular features in this case are reminiscent of the newly-defined oligodendroglioma and nuclear clusters, a subtype of glioblastoma with a primitive neuronal component, which is currently not recognized by the World Health Organization.
- Citation: Xu EX, Lu SY, Chen B, Ma XD, Sun EY. Manifestation of the malignant progression of glioma following initial intracerebral hemorrhage: A case report . World J Clin Cases 2023; 11(7): 1576-1585
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1576.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1576
Approximately 6% of intracerebral hemorrhages are associated with brain tumors, and most are the result of cerebral metastases and glioblastoma multiforme (GBM)[1]. The incidence of intracerebral hemorrhage in glioma ranges from 3.7% to 7.2%[2], and the majority of these cases are high-grade gliomas with abnormal vascular alignment and immature growth, resulting in bleeding. Conversely, low-grade gliomas (LGGs) rarely bleed.
Gliomas hemorrhage infrequently during the initial stages[3,4], which may lead to misdiagnosis by computed tomography (CT) imaging since this hemorrhage can completely obscure the tumor radiographically. The hemorrhage, in turn, can cause cerebral edema to expand or to present at an earlier time point[5]. Therefore, follow-up neuroimaging is necessary to ensure that a malignancy is not missed and to determine whether the hemorrhage requires emergency surgery or not. The risk of hemorrhage in pilocytic astrocytoma, a low grade glioma based on World Health Organization (WHO) classification[6], is extremely low, especially at later time points (e.g., decades after tumor onset). In fact, only 1 case of bleeding that occurred 18 years post-onset has been reported[7]. Pilocytic astrocytomas bleed as a result of abnormal vessels, or endothelial proliferation in parts of the tumor that have characteristics of oligodendroglioma, leading to rupture of an encased aneurysm or bleeding from retiform capillaries.
In this report, we present a case of progression of glioma following initial intracerebral hemorrhage.
The patient was admitted to our hospital 3 h after experiencing sudden-onset aphasia and left-sided hemiplegia.
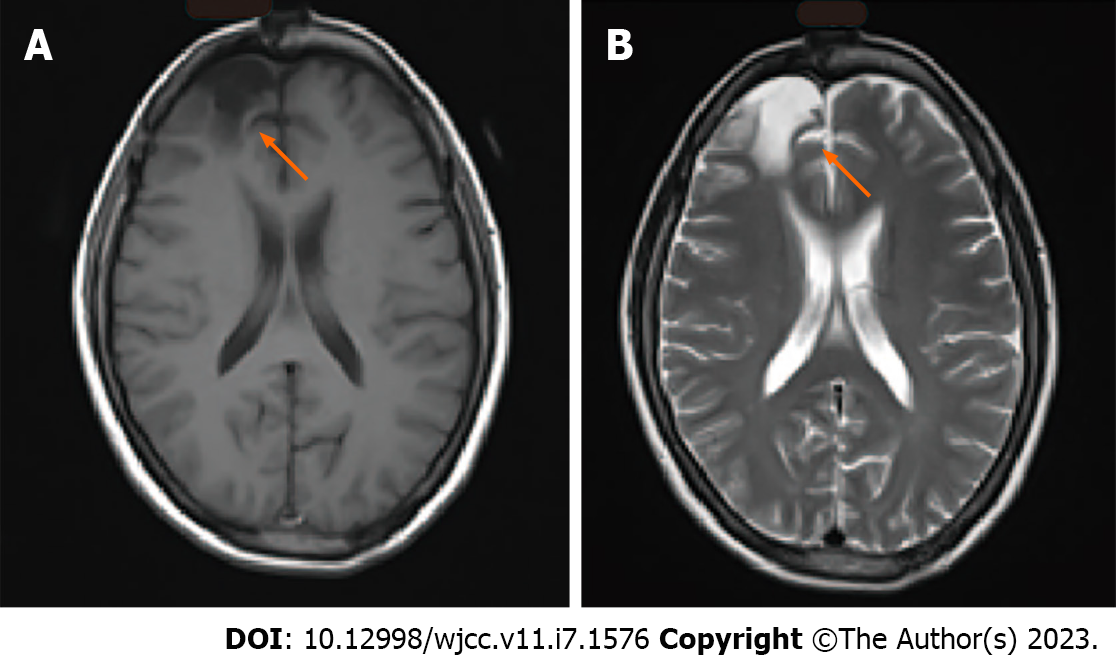
When the patient was 14-years-old, she underwent surgery for the resection of a brain tumor, after which she experienced frequent headaches. At that time, the postoperative pathological results indicated pilocytic astrocytoma (only the discharge summary from that admission is available for our review). The patient was followed annually, and imaging (Figure 1) showed that she had recovered well without the need for radiotherapy or chemotherapy. The patient lived normally, got married, and gave birth to a son.
None of relevance.
None of relevance.
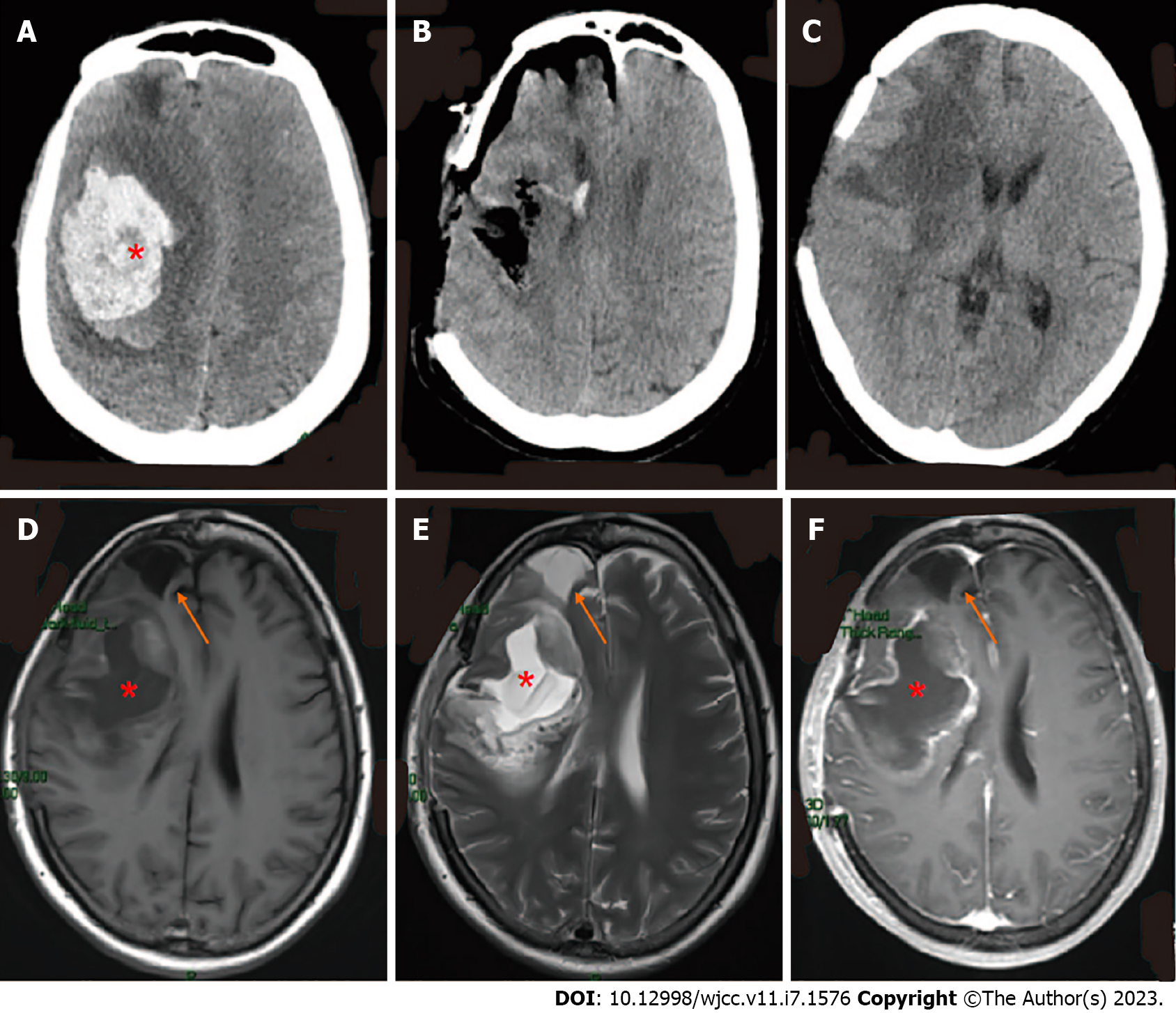
Initial CT showed right basal ganglia hemorrhage and cerebral hernia formation (Figure 2A).
The final diagnosis of this hemorrhage is glioblastoma (WHO IV) with extensive necrosis and hemorrhage.
Initial surgery was performed to remove the hematoma and perform bone disc decompression (Figure 2B).
CT also showed the softening of the frontal lobe and basal ganglia (Figure 2C), while cranial magnetic resonance imaging (MRI) showed a right frontal lobe and basal ganglia residual cavity with marginal enhancement (Figure 2D-F) 11 d following the surgery. The patient was discharged from the hospital on the 13th postoperative day. At the time of discharge, she was alert and could walk unassisted. In addition, the surgical incision had healed well, the bone window tension was not high, and the patient’s left-sided muscle strength was at level IV upon physical examination.
One month after being discharged, the patient suffered headaches and dizziness, and exhibited exacerbation of left-sided weakness.
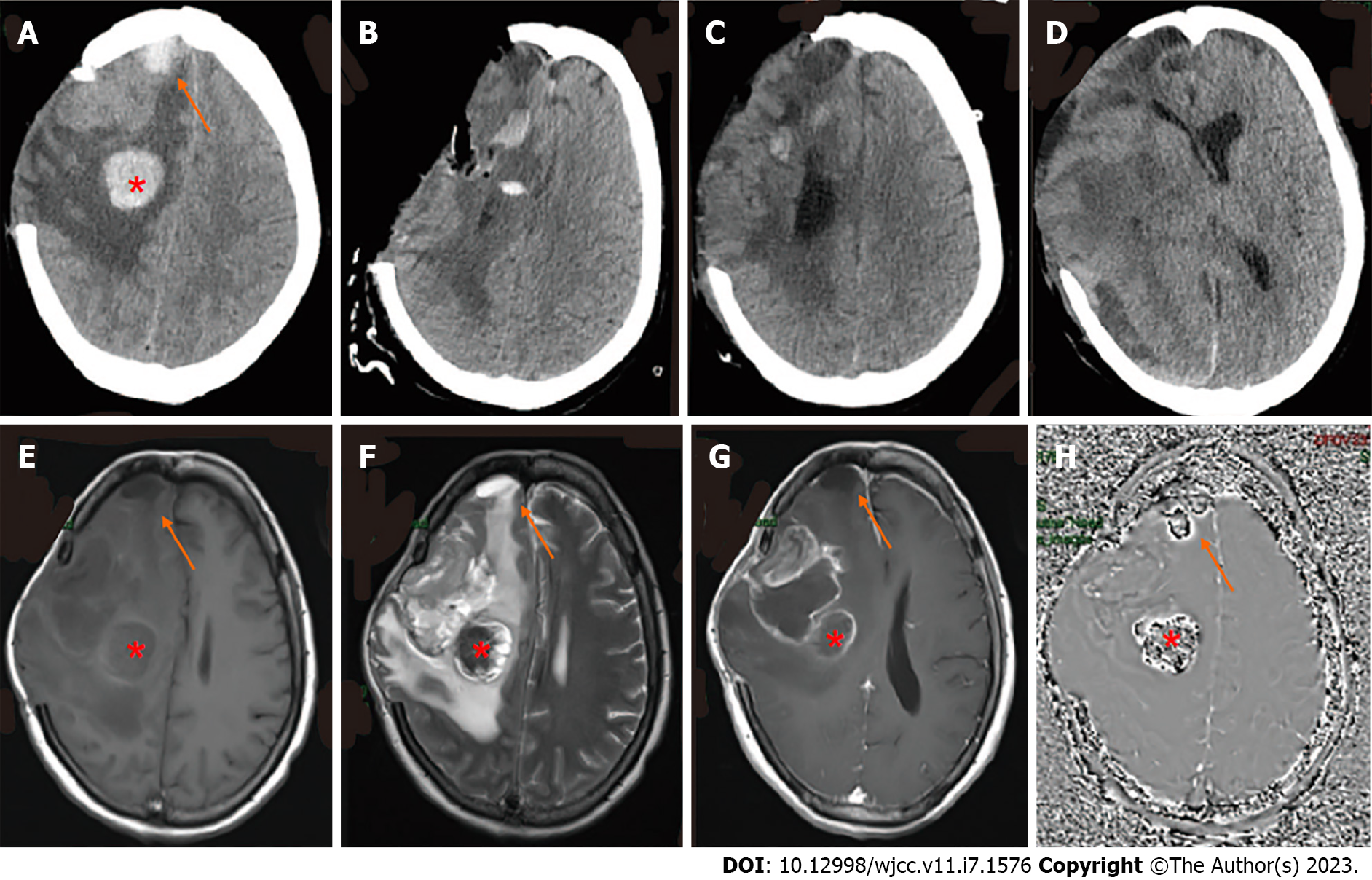
CT showed a new cerebral hemorrhage in the right craniocerebral operation area as well as in the frontal lobe (Figure 3A). Subsequent MRI indicated the presence of a residual cavity in the right frontoparietal lobe and basal ganglia with marginal enhancement (Figure 3E-G). To obtain the susceptibility-weighted imaging (SWI) of the hemorrhage of the frontal lobe and craniocerebral operation area (Figure 3H), we performed neuronavigation-assisted resection of the right frontotemporal parietal lesion plus extended flap decompression. As shown in Figure 3B, the patient had obvious swelling of the right frontotemporal parietal lobe.
During the operation, a neoplasm was detected under the meninges of the right inferior frontal gyrus and parietal lobe, which appeared gray and yellow with a brittle texture. A hematoma with a complete capsule and a thick, tough texture was present in the right temporo-parietal frontal lobe.
After surgery, the patient was continuously conscious but the scalp in the bone window of the right surgical area gradually bulged due to high tension and eventually ruptured multiple times. On the 23rd d after surgery, the tumor was observed again in the intracranial surgical area and had enlarged (Figure 3C). Due to the failed surgery, the patient’s family refused consent for another operation. On the 50th d after the operation, CT showed rapid tumor growth accompanied by brain hernia (Figure 3D). The patient was discharged and died 3 d later.
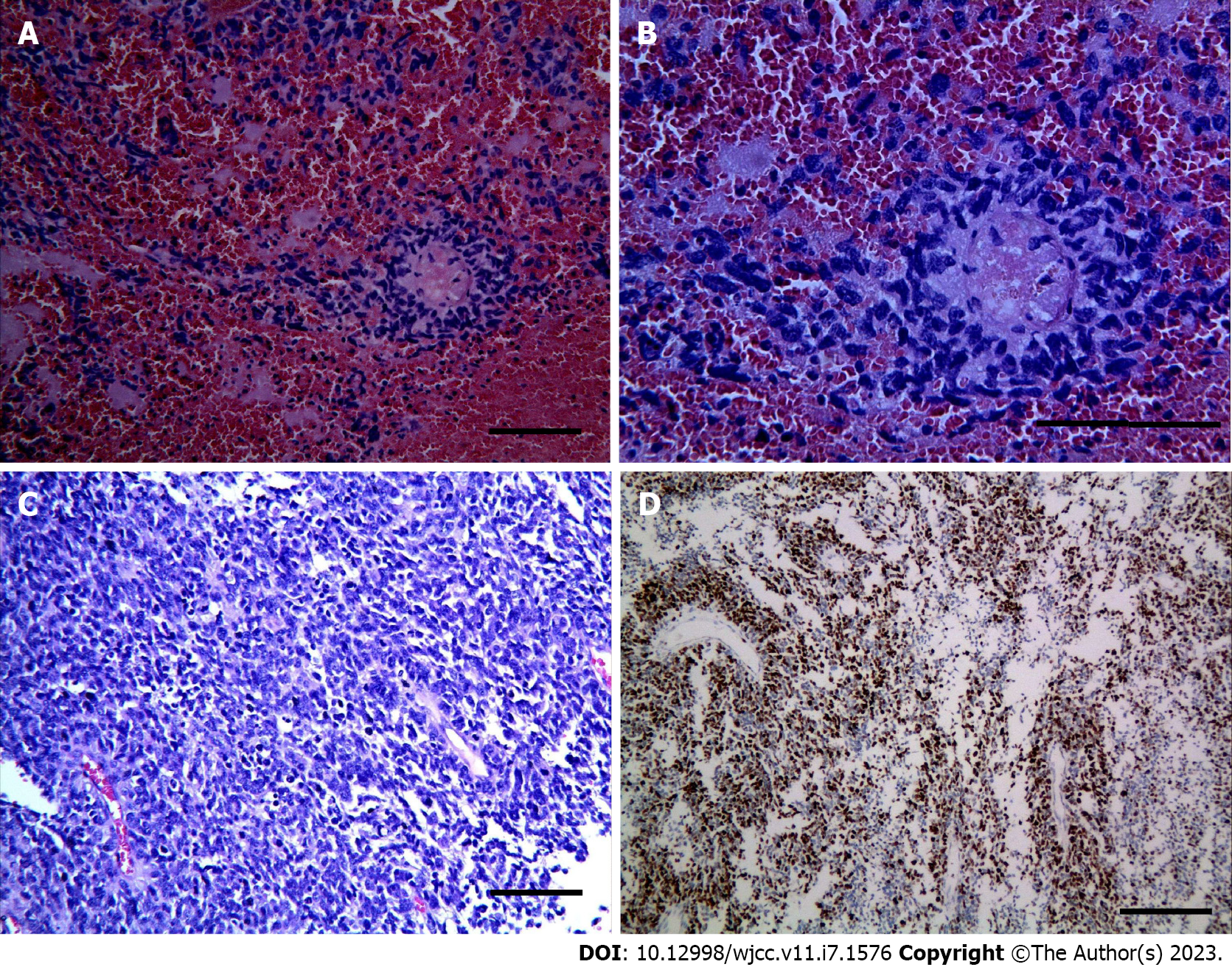
The right temporo-parietal frontal lobe was removed. Grossly, the mass was suspected to be glioblastoma (WHO IV) with extensive necrosis and hemorrhage, which was confirmed on histology. The tumor cells were round and diffusely arranged with perinuclear haloes, akin to that seen in oligodendroglioma (Figure 4A-C). Immunohistochemical staining showed that 70% of the tumor cells were positive for Ki-67 (Figure 4D). Staining for GFAP, S-100, p53, IDH1/2, OLig-2, cytokeratin (CK), CD3, CD5 CD10, CD20, Bcl-6, C-MYC, EMA, Mum-1, EBER, Synaptophysin, CD99 and chromogranin A (CgA) were negative. The samples were sent to Jiangsu Shihe Gene, Nanjing for high-throughput sequencing (reference genome: GRCh37/ HG19). The results indicated that the tumor was negative for chromosomal 1p/19q co-deletion, ATRX mutation, BRAF mutation or fusion, IDH1 or IDH2 mutation, MGMT methylation, TERT promoter mutation, and TP53 mutation or copy number variation. The tumor was positive for amplification of MYCN and deletion of CDKN2A.
Here, we present a case of glioma that initially manifested as spontaneous intracranial hemorrhage (ICH), which in a separate case had the same cerebral region of lesion before. The patient previously had spontaneous bleeding in the same region (basal ganglia) prior to the second and third hospital admissions. During the patient’s third admission, we detected hemorrhage in the frontal lobe, corresponding to the site of a pilocytic astrocytoma that was resected 25 years earlier. Spontaneous ICH is a rare clinical presentation of intracranial tumors and has been reported in only 0.5%-3.4% of published cases[3,4]. The bleeding of gliomas may be the result of direct pressure on vessels by brain tumor cells, or due to proliferation of malformed vasculature in the setting of hypoxia, tumor necrosis, and tumor coagulopathy[8]. A study of 23 patients with glioma initially presented as intracerebral hemorrhage[3,9] in which the presence of intracranial tumors or transformed intracranial tumors was not previously established. Intratumoral hemorrhage and abnormal coagulation can increase the risk of spontaneous ICH[3], and hypertension is responsible for 8% of cases of parenchymal hemorrhage in cancer patients[10].
It is challenging to diagnose tumors that initially present with hemorrhage. One study showed that diagnostic delay occurs in up to 2/3 of such cases, with a median of 60 d from presentation to diagnosis (range: 0–280 d)[4]. In our case, the patient had ICH with brain herniation, which requires emergency care and as such did not receive a complete MRI. The hematoma was large and covered the tumor, making it impossible to identify the tumor on CT alone. MRI can also be ineffective in detecting tumors or associated ICH in such cases[5], however it does have the highest efficacy in the identification of glioma and other malignancies that present with spontaneous ICH, especially in adult patients[4]. In our case, following the emergent surgery, MRI still did not identify the tumor. We hypothesized that the hemorrhage was related to glioma, as the perihematomal edema was larger than normal as a result of the 3 h span of active intracerebral hemorrhage. Edema associated with brain tumors is typically vasogenic, which frequently leads to loss of integrity of the blood-brain barrier (BBB) prior to the secondary incidence of ICH. Due to the accumulation of serum proteins and thrombin in the presence of inflammation, the BBB is often compromised resulting in extensive perihematomal edema. However, the formation of perihematomal edema in ICH is mainly caused by hematoma-induced direct tissue injury in the absence of preexisting edema. Additionally, the breakdown of hemoglobin, thrombin, and iron causes additional inflammation and neurotoxicity, which exacerbates the perihematomal edema[5]. In our case, the volume of perihematomal edema was greater than that of typical ICH. Therefore, it is doubtful that the tumor formed and presented as ICH at the second admission. MRI was performed 11 d after the second surgery, and showed marginal enhancement of the right frontal lobe and the residual cavity in the basal ganglia; these findings may have indicated a tumor, but we misdiagnosed it as glial hyperplasia. One and a half months after the operation, the patient was admitted for the third time. She had developed another hematoma within the basal ganglia, on which there was a previous operation. The frontal lobe hematoma was verified by SWI. The patient had a pilocytic astrocytoma in the frontal lobe 25 years earlier, which had been surgically removed and did not rebleed for 25 years. Spontaneous hemorrhage has been observed in 8% to 24% of cases of pilocytic astrocytoma[11]. Pilocytic astrocytomas occur primarily in the pediatric population, and so far only 59 cases of hemorrhagic pilocytic astrocytoma have been reported in adults[7], of which only 20 cases include supportive radiological data[12]. Delayed hemorrhage of pilocytic astrocytoma is very rare, and only one case has been reported in which the hemorrhage occurred 18 years after initial tumor resection[12].
The degree of malignancy in brain tumors strongly correlates with the risk of hemorrhage. Most ICH cases occur in high-grade gliomas, which are characterized by rapid growth associated with the formation of abnormal blood vessels[13]. However, in our case, the pilocytic astrocytoma was initially classified as WHO grade 1 rather than a benign, slow-growing type[6]. Therefore, our patient showed good recovery and gave birth to a son several years later. Spontaneous bleeding is rare in patients with LGG, including pilocytic astrocytoma, but can be lethal despite the low-grade tumor histology.
Most LGG patients with hemorrhage do not undergo genetic testing. One study reported 2 cases of cerebellar pilocytic astrocytoma that were tested for the BRAF V600E mutation and for other BRAF fusion variants[14]. Additionally, FGFR1 mutations are frequent in pediatric and young adult LGG patients with spontaneous bleeding, and FGFR1 mutated pilocytic astrocytomas are typically located in the midline, including in the diencephalon[11]. However, no significant differences have been reported between hemorrhagic and non-hemorrhagic glioma patients with respect to age, sex, location, Ki-67 expression, or degree of microvascular proliferation[15]. None of the above mutations was detected in our patient.
Pilocytic astrocytoma hemorrhage is likely associated with vascular changes due to densely hyalinized thick-walled vessels, vascular endothelial hyperplasia, and thin-walled ectatic vessels[16]. For example, a stretched vessel may be disrupted by a large cystic tumor component after only minor trauma[17]. Another hypothesis is that vascular changes determine the alteration of bloodflow dynamics, with the consequent formation of microaneurysms which may eventually rupture. Rapid tumor growth may also lead to local hypoxia due to overexpression of VEGF and altered bloodflow dynamics and hydrostatic pressure, resulting in microhemorrhages during Valsalva-like maneuvers; this may also lead to extensive hemorrhage[14]. However, some studies suggest that microvascular proliferation differs significantly between hemorrhagic and non-hemorrhagic cases of pilocytic astrocytoma[15]. There are also reports of hemorrhage that occurs years after radiation therapy and chemotherapy[18]. Radiotherapy-induced intra-tumoral hemorrhage result from repeated micro-bleeds in regressive alterations[19]. However, none of the pilocytic astrocytomas treated with radiation therapy presented with re-bleeding during follow-up[7]. In our case, the patient did not receive radiation or chemotherapy, which excluded these as risk factors for hemorrhage. Therefore, it can be assumed that pilocytic astrocytoma may portend a slightly worse prognosis, resulting in a higher rate of bleeding, in spite of its benign nature[20].
Our patient had tumor recurrence or progression in the right craniocerebral resection site and frontal lobe, which we misdiagnosed as glial hyperplasia. MRI showed a complete capsule and residual cavity in the operated area, which we identified as abscess or simply rebleeding. The diagnosis of glioma was confirmed only after histological examination, which revealed oligodendroglioma-like features. The tumor cells were negative for GFAP, S-100, p53, IDH1/2, OLig-2, CK, CD3, CD5 CD10, CD20, Bcl-6, C-MYC, EMA, Mum-1, EBER, Syn, CD99 and CgA, and positive for the proliferation marker Ki-67[21]. The high degree of proliferation coincided with rapid tumor growth and brain hernia formation within 50 d after resection.
Genetic analysis identified MYCN amplification and CDKN2A deletion in tumor samples, whereas chromosomal 1p/19q co-deletion, ATRX mutation, BRAF mutation or fusion, IDH1 or IDH2 mutation, MGMT methylation, TERT promoter mutation, and TP53 mutation or copy number variation were not detected. Gliomas with 1p/19q co-deletion as well as mutations in IDH1 are generally characterized as oligodendrogliomas, and respond well to radiation and chemotherapy[6]. IDH1 mutation is an early event in glioma formation, and typically appears before TP53 mutation or 1p/19q co-deletion. IDH mutations have been detected in 80% of LGGs and in 76% of secondary GBM[22]. Consequently, our case was diagnosed as glioblastoma with oligodendroglial characteristics and atypical molecular structure.
A population study showed that the mean duration of progression from LGG to GBM was 5.3 yr and that from anaplastic astrocytoma to GBM was 1.4 yr[23]. Although LGG progresses slowly during the initial phase, its growth accelerates once it progresses to a higher grade. Furthermore, its progression pattern may change following therapy, particularly after surgical resection[23]. Prognosis is substantially worse during tumor recurrence or progression, and LGG can be fatal once it undergoes malignant progression. One study of 44 LGG patients found an average of 3.15 somatic gene alterations during recurrence or progression, but not necessarily in the primary tumor. The genes most frequently mutated in LGG include NF1, CDKN2A, BAX, CCND2, EGFR, CREB3L2, GNAS, MYCN, PLAG1, PTK6, RIM2, RNF213 and WNK2[24]. Furthermore, the CDKN2A-CDKN2B tumor suppressor locus is deleted in 44% of gliomas. However, neither MYC amplification nor CDKN2A-CDKN2B deletion are commonly found in oligodendroglial or astrocytic glioma subtypes[25]. In our case, both alterations may have been involved in tumor recurrence or progression. There is evidence that MYCN amplification and homozygous deletion of CDKN2A/B are associated with worse overall survival[26].
Some histological and molecular features of this case were consistent with glioblastoma with a primitive neuronal component, although they were not entirely typical of this diagnostic entity. Glioblastoma with primitive neuronal component is a newly recognized pattern in the WHO 2016 classification that includes otherwise classical high-grade diffuse gliomas with one or more foci of sharply demarcated primitive nodules showing neuronal differentiation[27]. Similar to primitive neuronal cells that constitute embryonal neoplasms of the central nervous system (CNS), these foci show immunoreactivity to synaptophysin, loss of GFAP expression, and a high Ki-67 proliferation index, the latter of which was also observed in our case. Two particular features of this pattern are its high rate of cerebrospinal fluid (CSF) dissemination and frequent MYCN or MYC gene amplification[28]. However, we did not observe CSF dissemination or synaptophysin positivity, which contradicts the presence of a primitive neuronal component.
Diffuse glioneuronal tumor with oligodendroglioma-like features and nuclear clusters (DGONC)[29] is a newly-defined CNS tumor characterized by DNA methylation of low-grade glioneuronal tumors with recurrent monosomy 14, oligodendroglioma-like characteristics, and nuclear clusters. The histological profile of the DGONC tumor class overlaps substantially with other CNS tumor entities, including the presence of clear cell morphology, vascular proliferation, and GFAP-negativity, which are commonly observed in (anaplastic) oligodendroglioma, pediatric oligodendroglioma, or neurocytic tumors[30]. The histological and molecular characteristics of these tumors are similar to those of our case, but this entity is not currently represented in the WHO classification system.
We report a case of glioma that initially presented as ICH, which was misdiagnosed and not yet verified histologically. The patient had repeated bleeding in the site of pilocytic astrocytoma that had been resected 25 years earlier. The hemorrhage was later confirmed to be a malignant progression of the glioma.
There were several limitations in our case. The patient underwent tumor resection at another hospital, and we received only a summary of the postoperative pathological results at discharge. Furthermore, the hemorrhage that occurred on the second admission required emergent surgery, which precluded the identification of the tumor until after further diagnostic testing.
ICH may be the initial manifestation of glioma, and atypical perihematomal edema may be a clue to this diagnosis. Certain histological and molecular features in this case are reminiscent of the newly-defined DGONC, a glioblastoma subtype with a primitive neuronal component, which, however, is not currently recognized by the WHO.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Paparoupa M, Germany; Znegui T, Tunisia; S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Schrag M, Kirshner H. Management of Intracerebral Hemorrhage: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:1819-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Kapoor A, Savardekar A, Tewari MK, Chatterjee D, Radotra BD. Spontaneous hemorrhages in pediatric supratentorial pilocytic astrocytomas. Malignant presentation of a benign entity. Childs Nerv Syst. 2015;31:1617-1620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Koivunen RJ, Satopää J, Meretoja A, Strbian D, Haapaniemi E, Niemelä M, Tatlisumak T, Putaala J. Incidence, risk factors, etiology, severity and short-term outcome of non-traumatic intracerebral hemorrhage in young adults. Eur J Neurol. 2015;22:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Joseph DM, O'Neill AH, Chandra RV, Lai LT. Glioblastoma presenting as spontaneous intracranial haemorrhage: Case report and review of the literature. J Clin Neurosci. 2017;40:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Nawabi J, Hanning U, Broocks G, Schön G, Schneider T, Fiehler J, Thaler C, Gellissen S. Neoplastic and Non-Neoplastic Causes of Acute Intracerebral Hemorrhage on CT : The Diagnostic Value of Perihematomal Edema. Clin Neuroradiol. 2020;30:271-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G, Soffietti R, von Deimling A, Ellison DW. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro Oncol. 2021;23:1231-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4523] [Cited by in RCA: 6490] [Article Influence: 1622.5] [Reference Citation Analysis (1)] |
| 7. | Pagano A, Novegno F, Ferlosio A, Lunardi P. Repeat Bleeding 18 Years After Hemorrhagic Pilocytic Astrocytoma: Prognostic Implications of Conservative Management-Case Report and Literature Review. World Neurosurg. 2019;123:328-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Broka A, Hysenaj Z, Sharma S, Rehmani R. Lion in Sheep's Clothing: Glioblastoma Mimicking Intracranial Hemorrhage. Cureus. 2021;13:e14212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 9. | Huang G, Chen L, Qin C, Cheng D, Lu Q, Yu L, Liang Z. Cerebral hemorrhage as the initial manifestation in patients with systemic cancer. Int J Neurosci. 2018;128:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Navi BB, Reichman JS, Berlin D, Reiner AS, Panageas KS, Segal AZ, DeAngelis LM. Intracerebral and subarachnoid hemorrhage in patients with cancer. Neurology. 2010;74:494-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ishi Y, Yamaguchi S, Hatanaka KC, Okamoto M, Motegi H, Kobayashi H, Terasaka S, Houkin K. Association of the FGFR1 mutation with spontaneous hemorrhage in low-grade gliomas in pediatric and young adult patients. J Neurosurg. 2020;134:733-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Boukobza M, Goutagny S, Cazals-Hatem D, Laissy JP. Hemorrhagic presentation of frontal partially calcified pilocytic astrocytoma in an 18-year-old woman: A case report and literature review as "clinical case". Neurochirurgie. 2019;65:32-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Demirci Otluoğlu G, Özek MM. A rare clinical presentation: a pleomorphic xanthoastrocytoma presenting with intracerebral haemorrhage and metastasizing vigorously-case report and review of the literature. Childs Nerv Syst. 2019;35:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Wilson MP, Johnson ES, Hawkins C, Atkins K, Alshaya W, Pugh JA. Hemorrhagic presentations of cerebellar pilocytic astrocytomas in children resulting in death: report of 2 cases. J Neurosurg Pediatr. 2016;17:446-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Shibahara I, Kanamori M, Kumabe T, Endo H, Sonoda Y, Ogawa Y, Watanabe M, Tominaga T. Hemorrhagic onset of pilocytic astrocytoma and pilomyxoid astrocytoma. Brain Tumor Pathol. 2009;26:1-5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | White JB, Piepgras DG, Scheithauer BW, Parisi JE. Rate of spontaneous hemorrhage in histologically proven cases of pilocytic astrocytoma. J Neurosurg. 2008;108:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Sun S, Zhou H, Ding ZZ, Shi H. Cerebellar pilocytic astrocytomas with spontaneous intratumoral hemorrhage in the elderly: A case report and review of the literature. Medicine (Baltimore). 2018;97:e11329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Hillemanns A, Kortmann RD, Herrlinger U, Skalej M, Krapf H. Recurrent delayed brain hemorrhage over years after irradiation and chemotherapy for astrocytoma. Eur Radiol. 2003;13:1891-1894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Ramdurg SR, Maitra J. A rare case of infantile cerebellar pilocytic astrocytoma and thrombocytopenia presenting with intratumoral hemorrhage. J Pediatr Neurosci. 2016;11:249-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Prasad GL, Nandeesh BN, Menon GR. Hemorrhagic presentation of intracranial pilocytic astrocytomas: literature review. Neurosurg Rev. 2019;42:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Remnant L, Kochanova NY, Reid C, Cisneros-Soberanis F, Earnshaw WC. The intrinsically disorderly story of Ki-67. Open Biol. 2021;11:210120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 22. | Zhang L, Liu Y, Wang M, Wu Z, Li N, Zhang J, Yang C. EZH2-, CHD4-, and IDH-linked epigenetic perturbation and its association with survival in glioma patients. J Mol Cell Biol. 2017;9:477-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol. 2005;64:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 904] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 24. | Chen F, Chandrashekar DS, Scheurer ME, Varambally S, Creighton CJ. Global molecular alterations involving recurrence or progression of pediatric brain tumors. Neoplasia. 2022;24:22-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Bai H, Harmancı AS, Erson-Omay EZ, Li J, Coşkun S, Simon M, Krischek B, Özduman K, Omay SB, Sorensen EA, Turcan Ş, Bakırcığlu M, Carrión-Grant G, Murray PB, Clark VE, Ercan-Sencicek AG, Knight J, Sencar L, Altınok S, Kaulen LD, Gülez B, Timmer M, Schramm J, Mishra-Gorur K, Henegariu O, Moliterno J, Louvi A, Chan TA, Tannheimer SL, Pamir MN, Vortmeyer AO, Bilguvar K, Yasuno K, Günel M. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat Genet. 2016;48:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 238] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 26. | Shirahata M, Ono T, Stichel D, Schrimpf D, Reuss DE, Sahm F, Koelsche C, Wefers A, Reinhardt A, Huang K, Sievers P, Shimizu H, Nanjo H, Kobayashi Y, Miyake Y, Suzuki T, Adachi JI, Mishima K, Sasaki A, Nishikawa R, Bewerunge-Hudler M, Ryzhova M, Absalyamova O, Golanov A, Sinn P, Platten M, Jungk C, Winkler F, Wick A, Hänggi D, Unterberg A, Pfister SM, Jones DTW, van den Bent M, Hegi M, French P, Baumert BG, Stupp R, Gorlia T, Weller M, Capper D, Korshunov A, Herold-Mende C, Wick W, Louis DN, von Deimling A. Novel, improved grading system(s) for IDH-mutant astrocytic gliomas. Acta Neuropathol. 2018;136:153-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 304] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 27. | Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10993] [Cited by in RCA: 10852] [Article Influence: 1205.8] [Reference Citation Analysis (0)] |
| 28. | Romo CG, Palsgrove DN, Sivakumar A, Elledge CR, Kleinberg LR, Chaichana KL, Gocke CD, Rodriguez FJ, Holdhoff M. Widely metastatic IDH1-mutant glioblastoma with oligodendroglial features and atypical molecular findings: a case report and review of current challenges in molecular diagnostics. Diagn Pathol. 2019;14:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Pickles JC, Mankad K, Aizpurua M, Paine SM, Bridges LR, Carceller F, Szychot E, Walker M, Fairchild AR, Mistry T, Ogunbiyi O, Rolland A, Stone TJ, Dryden C, Addy D, Garimberti E, Chalker J, Sahm F, Jones DT, Hargrave D, Jacques TS. A case series of Diffuse Glioneuronal Tumours with Oligodendroglioma-like features and Nuclear Clusters (DGONC). Neuropathol Appl Neurobiol. 2021;47:464-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Deng MY, Sill M, Sturm D, Stichel D, Witt H, Ecker J, Wittmann A, Schittenhelm J, Ebinger M, Schuhmann MU, Figarella-Branger D, Aronica E, Staszewski O, Preusser M, Haberler C, Lauten M, Schüller U, Hartmann C, Snuderl M, Dunham C, Jabado N, Wesseling P, Deckert M, Keyvani K, Gottardo N, Giangaspero F, von Hoff K, Ellison DW, Pietsch T, Herold-Mende C, Milde T, Witt O, Kool M, Korshunov A, Wick W, von Deimling A, Pfister SM, Jones DTW, Sahm F. Diffuse glioneuronal tumour with oligodendroglioma-like features and nuclear clusters (DGONC) - a molecularly defined glioneuronal CNS tumour class displaying recurrent monosomy 14. Neuropathol Appl Neurobiol. 2020;46:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |