Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1560
Peer-review started: November 25, 2022
First decision: December 13, 2022
Revised: January 7, 2023
Accepted: February 10, 2023
Article in press: February 10, 2023
Published online: March 6, 2023
Processing time: 96 Days and 19.9 Hours
Cerebral mucormycosis is an infectious disease of the brain caused by fungi of the order Mucorales. These infections are rarely encountered in clinical practice and are often misdiagnosed as cerebral infarction or brain abscess. Increased mortality due to cerebral mucormycosis is closely related to delayed diagnosis and treatment, both of which present unique challenges for clinicians.
Cerebral mucormycosis is generally secondary to sinus disease or other disseminated disease. However, in this retrospective study, we report and analyze a case of isolated cerebral mucormycosis.
The constellation of symptoms including headaches, fever, hemiplegia, and changes in mental status taken together with clinical findings of cerebral infarction and brain abscess should raise the possibility of a brain fungal infection. Early diagnosis and prompt initiation of antifungal therapy along with surgery can improve patient survival.
Core Tip: Cerebral mucormycosis is an infectious disease of the brain caused by fungi of the order Mucorales. These infections are rarely encountered in clinical practice and often misdiagnosed as cerebral infarction or brain abscess. Diagnosis and treatment are challenging, and as such this disease is often associated with a high mortality rate. Through this case study, we aim to help further understand the pathophysiology of cerebral mucormycosis and suggest strategies for the improvement of clinical diagnosis and treatment thereof.
- Citation: Chen CH, Chen JN, Du HG, Guo DL. Isolated cerebral mucormycosis that looks like stroke and brain abscess: A case report and review of the literature. World J Clin Cases 2023; 11(7): 1560-1568
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1560.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1560
Cerebral mucormycosis is a life-threatening fungal infection[1]. Although modern technology advances and various imaging techniques are available to aid its diagnosis and treatment, this disease still carries a mortality rate as high as 62%[2-4]. Mucormycosis can affect various areas of the body, including rhino-cerebral tissue, lung, skin, and gastrointestinal tract, and can even present as a disseminated infection. Cerebral mucormycosis is the most common presentation of infection caused by Mucorales fungi and can occur via direct invasion of rhino-orbital structures or by hematogenous spread to the brain[5].
Cerebral mucormycosis cases can be found across the globe, with 47 countries reporting this infection. North America accounts for the majority of reported cases (130/345, 37.68%), followed by Asia (87/345, 25.22%). The incidence of cerebral mucormycosis is higher in males (60%) and the age distribution of affected patients is wide (2 mo to 89 years)[6]. Cerebral mucormycosis is generally diagnosed in patients with underlying medical conditions, with diabetes being the most common[7]. Amphotericin B deoxycholate (AmB) antifungal therapy and surgical debridement are the most commonly utilized methods for the treatment of cerebral mucormycosis.
Isolated cerebral mucormycosis is very rare. Due to the lack of specific clinical manifestations, the misdiagnosis rate is high in the early stages of the disease. Up to 90% of cases are reported to go undiagnosed and untreated[8,9]. The most common symptoms are orbital and neurological, followed by headache. Neurological symptoms are often associated with other extracerebral symptoms, with fever reported in greater than 1 in 5 cases[10,11]. Therefore, the presence of any of these symptoms in immunocompromised patients requires a high degree of suspicion for brain fungal infection. Here, we present a case of isolated cerebral mucormycosis followed by discussion and review of the current literature.
A 49-year-old female patient presented to the outpatient clinic with headache for 11 d, and left upper and lower extremity weakness accompanied by fever for 1 wk.
The patient had persistent pain mainly due to right frontal and parietal headache, which could not be relieved after rest. The patient was hospitalized at the Department of Neurology with a body temperature of 39.2 ℃.
The patient had type 2 diabetes. A diagnostic consultation did not identify any foci of infection. She had no past history of otitis media, sinusitis, or head trauma.
The patient denied having any other relevant personal history and her familial history was unremarkable.
The physical examination revealed intact consciousness with a Glasgow coma scale (GCS) score of 15, a soft neck, left upper and lower extremity muscular strength (1 out of 5), a shallow left nasolabial fold, and intact deep and superficial sensation.
A complete blood count with differential revealed a white blood cell count of 13.4 × 109/L (normal reference range: 4.0–10.0 × 109/L), 80.0% neutrophils (normal reference range: 50%–70%), 12.1% lymphocytes (normal reference range: 20%–40%), and C-reactive protein level of 50.1 mg/L (normal reference range: 0–10 mg/L). Urinalysis revealed a white blood cell count of 229/μg (normal reference range: 0-26/μg). Urine culture grew Enterococcus faecalis. Blood and sputum cultures were negative.
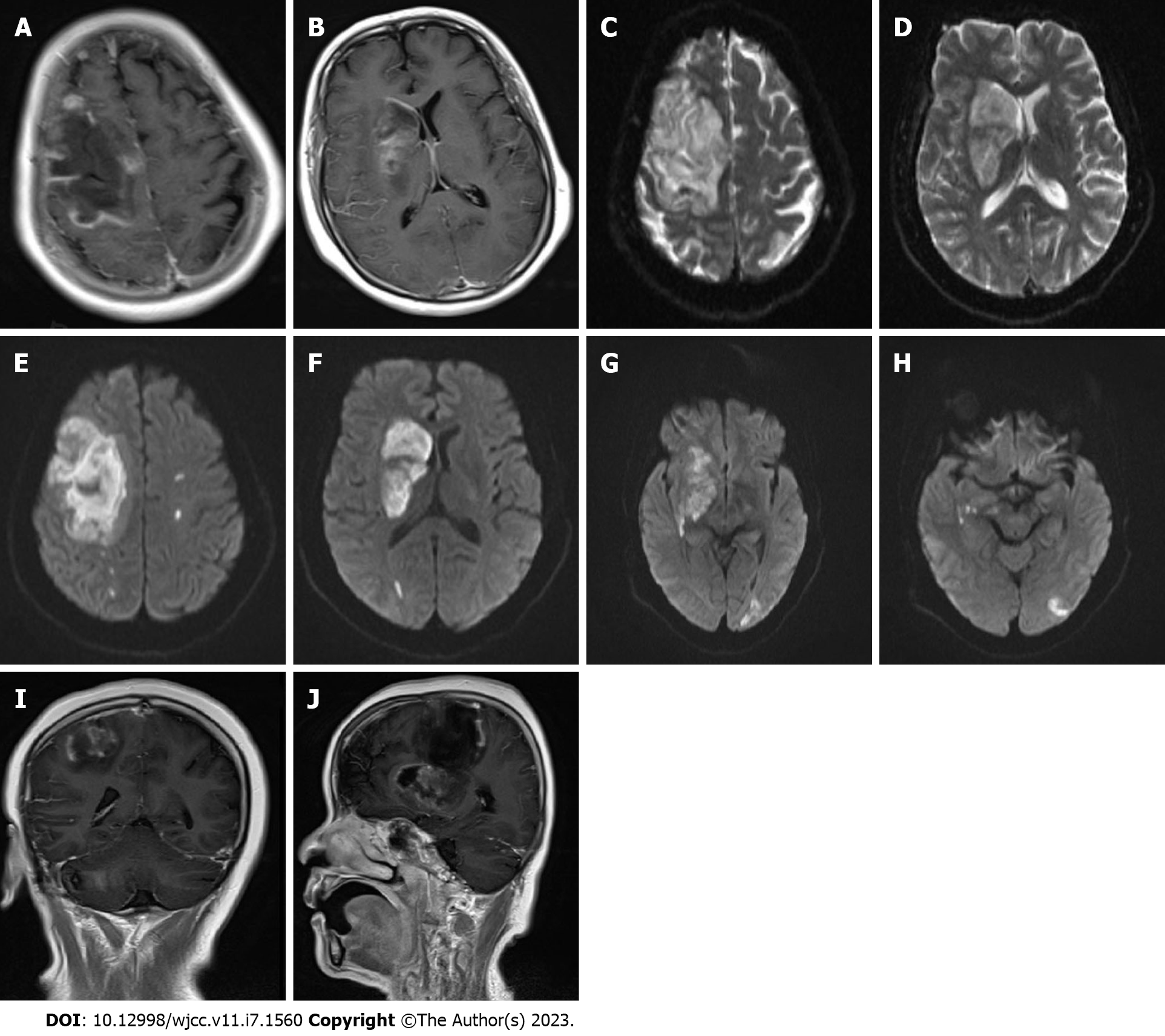
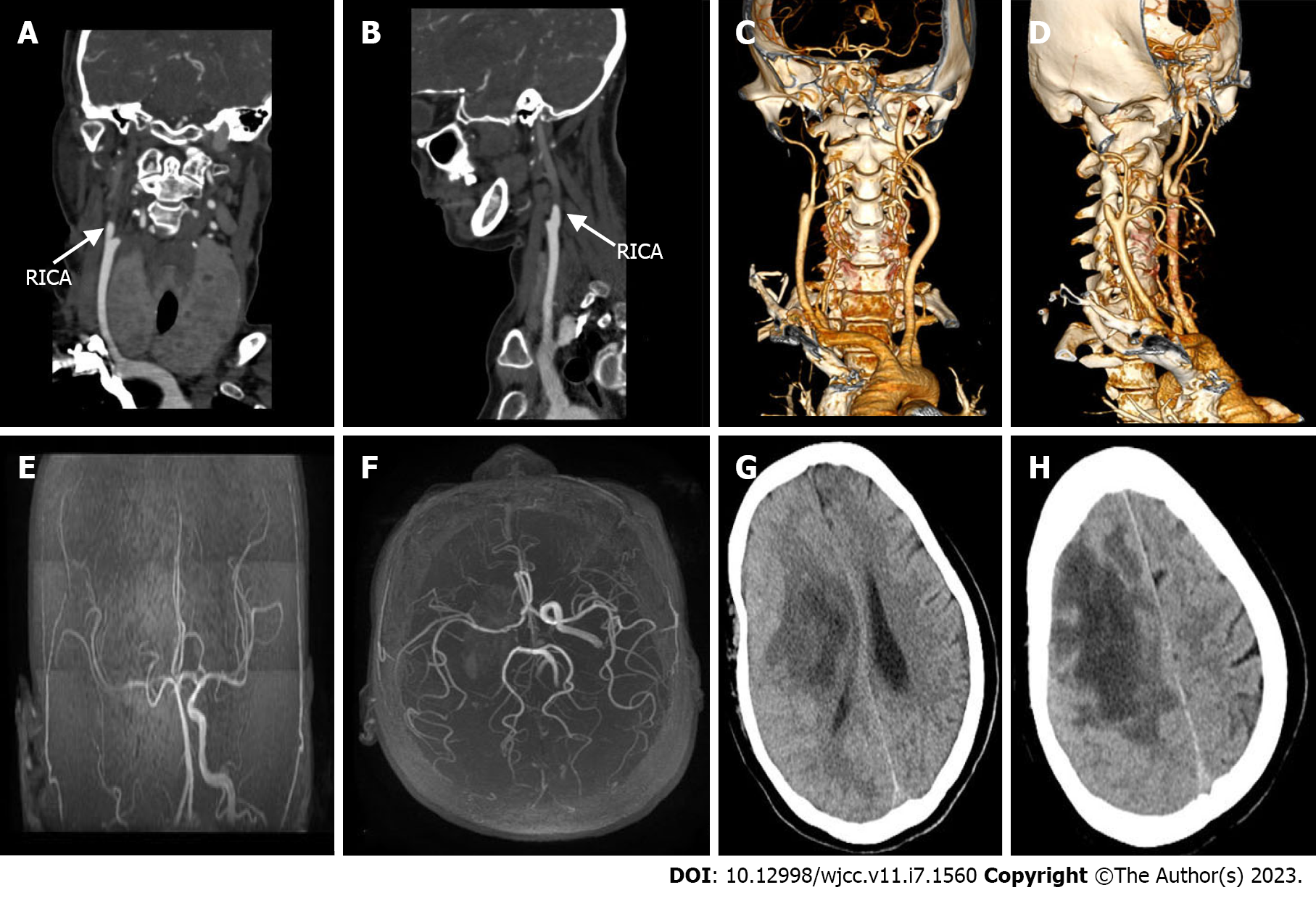
No obvious abnormalities were observed in a computed tomography (CT) scan of the chest. Cardiac Doppler ultrasound revealed mild mitral regurgitation. Magnetic resonance imaging (MRI) of the head with and without contrast demonstrated the following: (1) Large-scale abnormal signal lesions in the right frontal lobe and right basal ganglia with low signal on T1-weigted imaging, slightly higher signal on T2-weigted imaging, and high signal on diffusion-weighted imaging (DWI); (2) Enhancement of the gyrus around the lesion without significant space-occupying effect; and (3) Multiple long T2 signals and enhanced DWI signals in the left cerebral hemisphere (Figure 1). CT angiography of cervical arteries demonstrated occlusion of the right internal carotid artery (Figure 2A–D). Further examination of the arteries by magnetic resonance angiography showed that there was no development of the right internal carotid artery skull base and intracranial segments, and the anterior and posterior cerebral arteries were supplied by the traffic branch (Figure 2E and F).
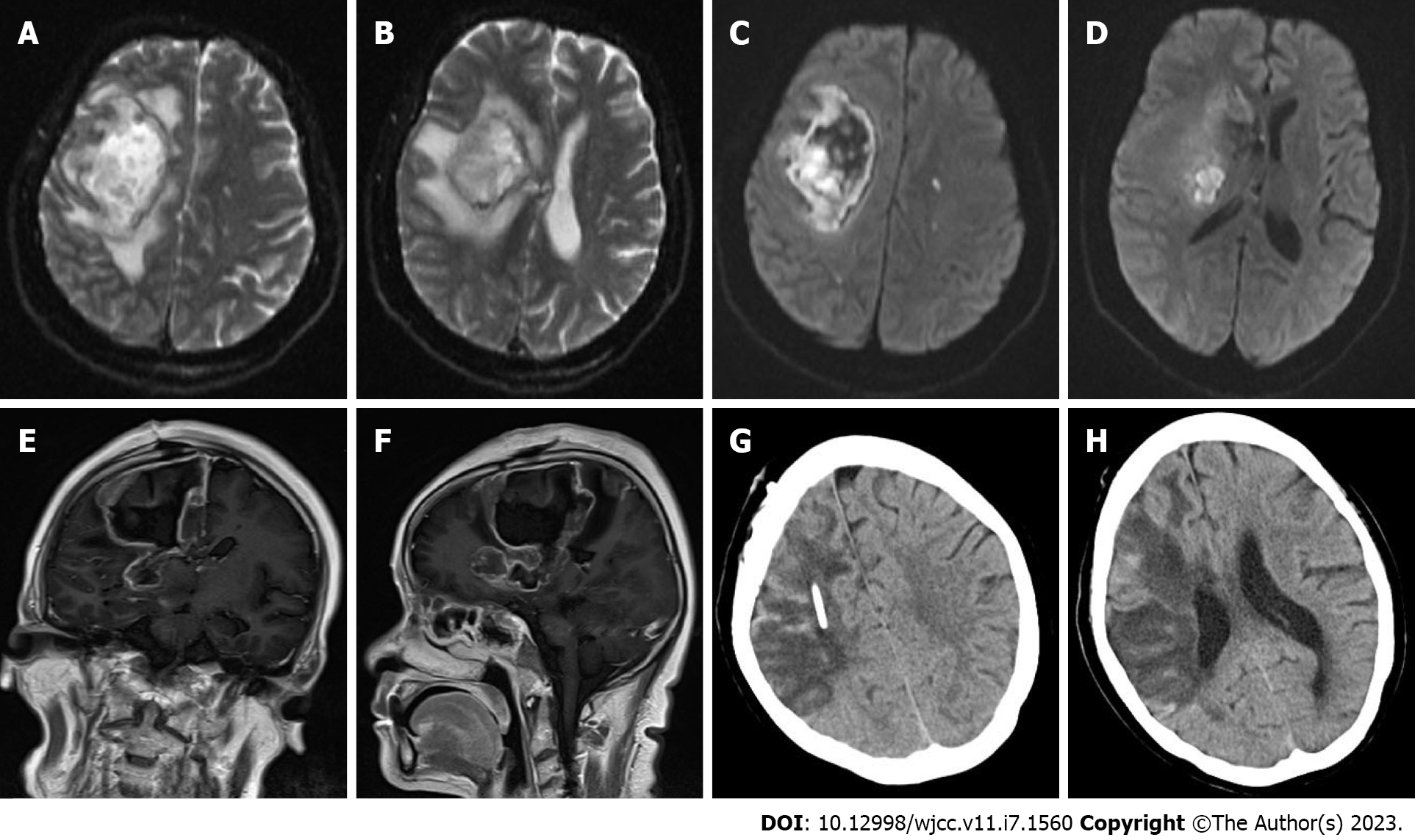
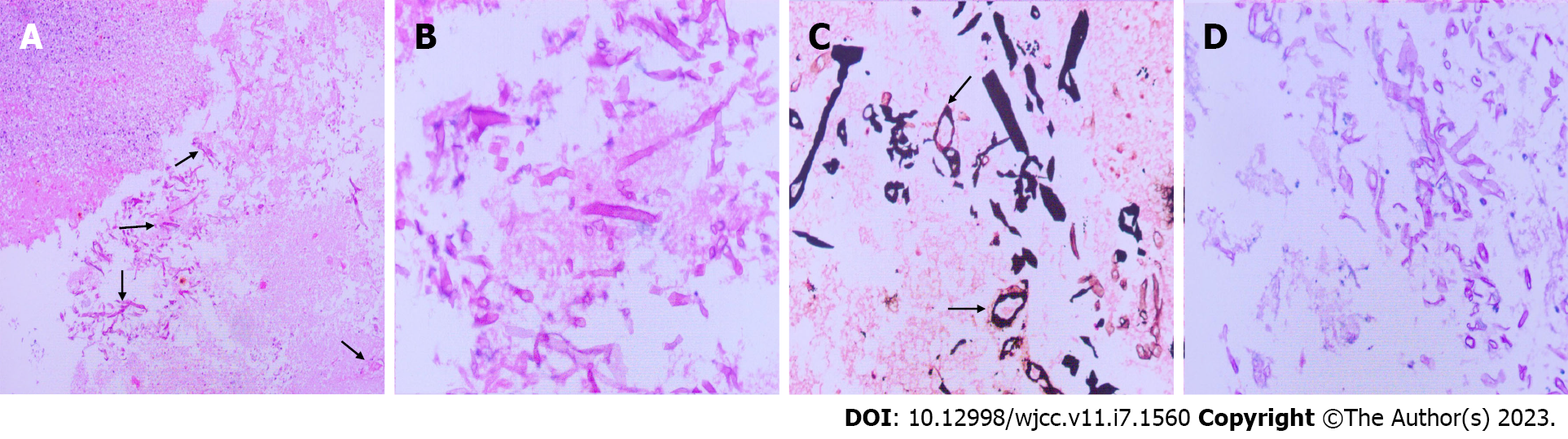
All available clinical evidence up to this point suggested cerebral infarction, with the only unexplained symptoms being persistent fever and headache. Per antibacterial susceptibility testing of the urine culture, piperacillin and tazobactam were administered intravenously, in addition to the initiation of standard treatment for cerebral infarction. In the following week, the patient's temperature fluctuated between 38 ℃ and 39 ℃, and her headache did not improve significantly. Two consecutive cerebrospinal fluid tests revealed the following: (1) Greater than 100 nucleated cells, most of which were neutrophils; (2) Slightly elevated glucose and protein levels; (3) Normal chloride levels; and (4) Opening pressures of 170 mmH2O and 210 mmH2O. No bacteria or fungi were found in cultures of the cerebrospinal fluid. Considering the possibility of brain abscess, the patient was empirically treated with meropenem and vancomycin. However, as discussed above, a CT scan of the head showed a low-density shadow in the right frontal lobe and right basal ganglia. The right basal ganglia showed focal enhancement, the right lateral ventricle was slightly compressed, and there was slight midline shift to the left. In light of these findings, 100 mL intravenous mannitol, 250 mL intravenous glycerol fructose, and intravenous sodium chloride were infused every 12 h. The patient's temperature continued to fluctuate between 37 ℃ and 38 ℃, and her headache persisted. Approximately 1 wk later, the patient's temperature suddenly rose to 39 ℃. A review of the head CT revealed a large area of infarction corresponding to the right middle cerebral artery. The density of some areas was slightly higher, the midline was shifted, and cerebral palsy developed (Figure 2G and H). Accordingly, right craniotomy and drainage were performed, with turbid serosanguineous fluid and no signs of abscess observed intraoperatively. After 1 wk, the patient's level of consciousness decreased (GCS, E3V4M4). Repeat MRI of the head was still consistent with cerebral infarction (Figure 3A–F). However, after communicating with the patient's family, resection of the right brain lesion was performed and tissue was sent for gross pathologic and histologic examination. Findings reported by pathology included purulent brain tissue with visible fungal structures revealed by special staining to be consistent with mucormycosis (Figure 4). On the 10th d after the brain lesion resection, repeat head CT showed a large low-density shadow, and the midline returned to normal (Figure 3G and H).
A final diagnosis of cerebral mucormycosis was made following histopathological examination of the resected brain lesion.
Following surgical resection of the right cerebral lesion, the patient received therapy with intravenous amphotericin B.
There were signs of improvement in the first 3 d following resection of the lesion. However, the patient gradually deteriorated and brain death was pronounced 1 mo later.
Cerebral mucormycosis is a life-threatening acute or chronic central nervous system disease caused by Mucorales fungi. In most cases, cerebral mucormycosis is caused by the spread of infection from the sinuses, and isolated cerebral mucormycosis is extremely rare. In a meta-analysis of 929 cases of mucormycosis, 30% described central nervous system disease, of which only 16% were confined to the central nervous system[2]. In other studies, isolated cerebral mucormycosis was found to account for up to 8% of all cases of mucormycosis[12-14]. Several risk factors are described in the literature, including intravenous drug use, diabetes mellitus, malignancy, solid and bone marrow transplantation, and iron overload status. Intravenous drug use is the most important risk factor for the development of isolated cerebral mucormycosis[15,16]. Verma et al[17] reported 30 cases of isolated cerebral mucormycosis, of which 17 were notable for history of intravenous drug abuse. Diabetes mellitus is the strongest risk factor for development of other mucormycosis infections, and in the meta-analysis of 929 cases mentioned above, 36% of patients with mucormycosis had a history of diabetes. It is believed that diabetic patients are susceptible to mucormycosis due to the fungal ketoreductase system allowing the organisms to metabolize ketone bodies. In addition, the hyperglycemia and acidosis often found in the setting of diabetes reduce neutrophil chemotaxis and adhesion to fungal hyphae as well as impair the inhibition of Mucorales spores and mycelia by alveolar macrophages[18]. In the present case, the patient had a history of poorly controlled diabetes mellitus, and as such carried an increased susceptibility to mucormycosis infection due to these immunosuppressive mechanisms.
As mentioned previously, the most common symptoms of cerebral mucormycosis are orbital and neurological, followed by headache. Neurological symptoms are often related to other extracerebral symptoms such as fever. In our case, the symptoms demonstrated in the patient were headache, hemiplegia, and fever. In a review of 13 cases of isolated cerebral mucormycosis, altered mental status (54%) and headache (51%) were common[6]. Considering the presenting symptoms of our patient and those of patients discussed in the literature, it is apparent that these nonspecific signs and symptoms are largely responsible for the challenges faced by clinicians in diagnosing cerebral mucormycosis, as many other conditions must be considered in the differential diagnosis. To further add to these challenges, imaging findings can also vary widely. For example, Kursun et al[10] report cavernous sinus invol
In addition to the nonspecific presenting symptoms and imaging findings discussed above, many reported cerebrospinal fluid cultures are essentially negative, and culture conditions necessary for the growth of Mucorales fungi are inconsistently reported[19]. Histopathological examination of involved tissue can confirm the diagnosis; however, this requires invasive surgery. Taken together, these difficulties underscore the importance of a high clinical suspicion of cerebral mucormycosis and careful differential diagnosis.
Histopathological visualization of broad, ribbon-like, pauciseptate hyphae with wide-angle branching supports the diagnosis of mucormycosis. Acute inflammation with predominant neutrophilia is usually present, but may be difficult to identify in patients with underlying neutropenia. In addition, histopathological identification of mucormycosis does not provide genus and species information nor antifungal susceptibility data, and has limited ability to detect mixed fungal infections[20,21]. New diagnostic methods, including matrix-assisted laser desorption/ionization-time of flight (commonly known as MALDI-TOF) testing of serum and cryosections, can be used to aid diagnosis[22]. Unfortunately, molecular techniques may not be available at all institutions, and histopathological examination remains the gold standard for the diagnosis of isolated cerebral mucormycosis[23,24]. In our case, the histologic diagnosis was confirmed by hematoxylin-eosin staining and periodic acid-silver methenamine staining of resected brain tissue.
Mucorales fungi are extremely invasive, and their hyphae can invade blood vessels. The growth of hyphae in the lumen of the blood vessels with associated damage to the endothelium can cause vascular occlusion, and formed fungal emboli can easily cause cerebral infarction. The blood vessels usually involved are the basilar artery and carotid artery, and the extravasation of fungal elements can lead to brain abscess formation[25,26]. In our case, the right internal carotid artery was completely occluded, and the MRI of the head demonstrated infarction in the right frontal lobe and right basal ganglia, likely due to impediment of blood flow by fungal emboli. However, infarction may also be a consequence of abscess formation.
The treatment of patients with mucormycosis is challenging, and even in adequately treated patients, the associated mortality rate is still high. Standard treatments include AmB and its lipid formulation and surgical debridement[27,28]. In most retrospective studies, AmB and its lipid formulation are the preferred treatment and are part of any potential combination therapy. Posaconazole in combination with AmB is reported to offer treatment benefits when compared to antifungal monotherapy[29]. In some reports, posaconazole may be a more advantageous treatment strategy when used as the first line treatment for cerebral mucormycosis in diabetic patients with antifungal-resistant infections[30,31]. Moreover, vascular invasion by Mucorales fungi leads to vascular occlusion and necrosis, in turn significantly reducing drug distribution to target tissue. This emphasizes the importance of surgical debridement in the treatment of cerebral mucormycosis, especially in the setting of vascular occlusion[32-34]. Debridement can aid in treatment by reducing the microbiological load and altering anaerobic and microaerobic conditions conducive to fungal reproduction. However, in many case studies, death occurred despite surgical debridement and adequate antifungal treatment. These deaths are largely attributed to the rapid invasion of the brain by Mucorales fungi, delayed surgical treatment, and progressive infections[35,36]. The combination of surgery and medication is superior to either treatment alone. Roden et al[2] reported in a review of 929 cases that the survival rate was 61% (324 of 532) in the AmB alone treatment group, 57% (51 of 90) in the surgery alone treatment group, and 70% (328 of 470) in the antifungal and surgical combined treatment group.
A literature review suggests that the risk factors for death of patients with cerebral mucormycosis can be categorized as follows: (1) Susceptibility to infection (e.g., history of diabetes and long-term use of broad-spectrum antibiotics); (2) Site of infection (e.g., Chandra et al[37] found in a retrospective analysis of 68 cases that basal ganglia lesions carried increased mortality due to higher iron availability in that region); and (3) Delay in diagnosis and treatment (e.g., studies finding that a treatment delay of longer than 6 d[38] or 1 wk[39] was associated with increased mortality).
Isolated cerebral mucormycosis is rare and diagnosis is challenging and requires a high degree of clinical suspicion. When a patient presents with headache, fever, hemiplegia, and altered mental status, his/her history and physical examination results should be closely followed by an investigation of the possibility of fungal infection. Histopathology can reveal the pathological characteristics of Mucorales fungi, and molecular methods (MALDI-TOF testing of serum and cryosections) may help to confirm the diagnosis and identification of pathogenic species. Early diagnosis and initiation of antifungal therapy and surgery can improve patient survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaushik P, India; Liu Y, China S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Fan JR
| 1. | Kennedy KJ, Daveson K, Slavin MA, van Hal SJ, Sorrell TC, Lee A, Marriott DJ, Chapman B, Halliday CL, Hajkowicz K, Athan E, Bak N, Cheong E, Heath CH, Morrissey CO, Kidd S, Beresford R, Blyth C, Korman TM, Robinson JO, Meyer W, Chen SC; Australia and New Zealand Mycoses Interest Group of the Australasian Society for Infectious Diseases. Mucormycosis in Australia: contemporary epidemiology and outcomes. Clin Microbiol Infect. 2016;22:775-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1793] [Cited by in RCA: 1966] [Article Influence: 98.3] [Reference Citation Analysis (1)] |
| 3. | Kontoyiannis DP, Azie N, Franks B, Horn DL. Prospective antifungal therapy (PATH) alliance(®) : focus on mucormycosis. Mycoses. 2014;57:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Skiada A, Pagano L, Groll A, Zimmerli S, Dupont B, Lagrou K, Lass-Florl C, Bouza E, Klimko N, Gaustad P, Richardson M, Hamal P, Akova M, Meis JF, Rodriguez-Tudela JL, Roilides E, Mitrousia-Ziouva A, Petrikkos G; European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17:1859-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 511] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 5. | Ermak D, Kanekar S, Specht CS, Wojnar M, Lowden M. Looks like a stroke, acts like a stroke, but it's more than a stroke: a case of cerebral mucormycosis. J Stroke Cerebrovasc Dis. 2014;23:e403-e404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Salehi M, Mahmoudi S, Rezahosseini O, Hashemi SJ, Ahmadikia K, Aala F, Khajavirad N, Alijani N, Izadi A, Getso MI, Abdollahi A, Salami A, Khatami SR, Adibimehr A, Hedayat Yaghoobi M, Sabahi M, Pazooki B, Yazdi F, Zebardast J, Saifi A, Hasan Nezhad M, Mardani M, Khodavaisy S. The Epidemiological, Clinical, Mycological, and Pathological Features of Rhino-cerebral Mucormycosis: A Systematic Review. Iran J Pathol. 2022;17:112-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 7. | Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, Chen SC. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 548] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 8. | Dong N, Jordan AE, Shen X, Wu X, Guo X, Zhao H, Wang Y, Wang D, Fang Q. Rhino-Orbital Cerebral Mucormycosis in a Patient With Diabetic Ketoacidosis: A Case Report and Literature Review. Front Neurol. 2022;13:815902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Walsh TJ, Gamaletsou MN, McGinnis MR, Hayden RT, Kontoyiannis DP. Early clinical and laboratory diagnosis of invasive pulmonary, extrapulmonary, and disseminated mucormycosis (zygomycosis). Clin Infect Dis. 2012;54 Suppl 1:S55-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 10. | Kursun E, Turunc T, Demiroglu YZ, Alışkan HE, Arslan AH. Evaluation of 28 cases of mucormycosis. Mycoses. 2015;58:82-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Chikley A, Ben-Ami R, Kontoyiannis DP. Mucormycosis of the Central Nervous System. J Fungi (Basel). 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Zhang GJ, Zhang SK, Wang Z, Zhu YX, Kong J, Huang LL, Guo YJ, Wang YJ, Zou RC, Xie CM. Fatal and Rapid Progressive Isolated Cerebral Mucormycosis Involving the Bilateral Basal Ganglia: A Case Report. Front Neurol. 2020;11:295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Meyerowitz EA, Sanchez S, Mansour MK, Triant VA, Goldberg MB. Isolated Cerebral Mucormycosis in Immunocompetent Adults who Inject Drugs: Case Reports and Systematic Review of the Literature. Open Forum Infect Dis. 2020;7:ofaa552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Mamali V, Koutserimpas C, Zarkotou O, Vrioni G, Samonis G. Isolated Cerebral Mucormycosis Caused by Lichtheimia Species in a Polytrauma Patient. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Ha NT, Lowery M, Woo J, Mehta Y, Bhanot N. Brain lesion in a recreational drug user: Isolated cerebral mucormycosis. IDCases. 2020;22:e00979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 16. | Kerezoudis P, Watts CR, Bydon M, Dababneh AS, Deyo CN, Frye JM, Kelley PC, Kemp AM, Palraj BV, Pupillo GT. Diagnosis and Treatment of Isolated Cerebral Mucormycosis: Patient-Level Data Meta-Analysis and Mayo Clinic Experience. World Neurosurg. 2019;123:425-434.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Verma A, Brozman B, Petito CK. Isolated cerebral mucormycosis: report of a case and review of the literature. J Neurol Sci. 2006;240:65-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Blitzer A, Lawson W. Fungal infections of the nose and paranasal sinuses. Part I. Otolaryngol Clin North Am. 1993;26:1007-1035. [PubMed] |
| 19. | Farid S, AbuSaleh O, Liesman R, Sohail MR. Isolated cerebral mucormycosis caused by Rhizomucor pusillus. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 20. | Sangoi AR, Rogers WM, Longacre TA, Montoya JG, Baron EJ, Banaei N. Challenges and pitfalls of morphologic identification of fungal infections in histologic and cytologic specimens: a ten-year retrospective review at a single institution. Am J Clin Pathol. 2009;131:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 171] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 544] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 22. | Tissot F, Agrawal S, Pagano L, Petrikkos G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C, Herbrecht R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica. 2017;102:433-444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 324] [Cited by in RCA: 422] [Article Influence: 46.9] [Reference Citation Analysis (1)] |
| 23. | Yang M, Lee JH, Kim YK, Ki CS, Huh HJ, Lee NY. Identification of mucorales from clinical specimens: a 4-year experience in a single institution. Ann Lab Med. 2016;36:60-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Al-Otaibi F, Albloushi M, Alhindi H, Timms MS. Carotid artery occlusion by rhinoorbitocerebral mucormycosis. Case Rep Surg. 2012;2012:812420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Fu KA, Nguyen PL, Sanossian N. Basilar artery territory stroke secondary to invasive fungal sphenoid sinusitis: a case report and review of the literature. Case Rep Neurol. 2015;7:51-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Lanternier F, Dannaoui E, Morizot G, Elie C, Garcia-Hermoso D, Huerre M, Bitar D, Dromer F, Lortholary O; French Mycosis Study Group. A global analysis of mucormycosis in France: the RetroZygo Study (2005-2007). Clin Infect Dis. 2012;54 Suppl 1:S35-S43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 28. | Cornely OA, Arikan-Akdagli S, Dannaoui E, Groll AH, Lagrou K, Chakrabarti A, Lanternier F, Pagano L, Skiada A, Akova M, Arendrup MC, Boekhout T, Chowdhary A, Cuenca-Estrella M, Freiberger T, Guinea J, Guarro J, de Hoog S, Hope W, Johnson E, Kathuria S, Lackner M, Lass-Flörl C, Lortholary O, Meis JF, Meletiadis J, Muñoz P, Richardson M, Roilides E, Tortorano AM, Ullmann AJ, van Diepeningen A, Verweij P, Petrikkos G; European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group; European Confederation of Medical Mycology. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin Microbiol Infect. 2014;20 Suppl 3:5-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 475] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 29. | Biswas D, Kotwal A, Kakati B, Ahmad S. Amphotericin B Resistant Apophysomyces elegans Causing Rhino-oculo-Cerebral Mucormycosis in an Immunocompetent Host. J Clin Diagn Res. 2015;9:DD01-DD02. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 30. | Tarani L, Costantino F, Notheis G, Wintergerst U, Venditti M, Di Biasi C, Friederici D, Pasquino AM. Long-term posaconazole treatment and follow-up of rhino-orbital-cerebral mucormycosis in a diabetic girl. Pediatr Diabetes. 2009;10:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Anders UM, Taylor EJ, Martel JR, Martel JB. Acute orbital apex syndrome and rhino-orbito-cerebral mucormycosis. Int Med Case Rep J. 2015;8:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Garas G, Choudhury N, Farrell R. Invasive fatal rhino-orbito-cerebral mucormycosis in diabetic ketoacidosis. JRSM Short Rep. 2010;1:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 33. | Angali RK, Jeshtadi A, Namala VA, Gannepalli A. Fatal rhino-orbito-cerebral mucormycosis in a healthy individual. J Oral Maxillofac Pathol. 2014;18:460-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Chegini Z, Didehdar M, Khoshbayan A, Rajaeih S, Salehi M, Shariati A. Epidemiology, clinical features, diagnosis and treatment of cerebral mucormycosis in diabetic patients: A systematic review of case reports and case series. Mycoses. 2020;63:1264-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Bavikar P, Mehta V. Rhino-Orbital-Cerebral Mucormycosis: A Fatal Complication of Uncontrolled Diabetes Mellitus. Cureus. 2017;9:e1841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 36. | Raffa LH. Rhino-orbito-cerebral mucormycosis following penetrating keratoplasty. J Surg Case Rep. 2019;2019:rjz314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Chandra S, Sharma S, Vats R, Pandey S. Isolated cerebral mucormycosis masquerading as a tumor in an immunocompetent patient. Autops Case Rep. 2021;11:e2020233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Yohai RA, Bullock JD, Aziz AA, Markert RJ. Survival factors in rhino-orbital-cerebral mucormycosis. Surv Ophthalmol. 1994;39:3-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 282] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Cano P, Horseman MA, Surani S. Rhinocerebral mucormycosis complicated by bacterial brain abscess. Am J Med Sci. 2010;340:507-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |