Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1549
Peer-review started: October 19, 2022
First decision: December 26, 2022
Revised: January 13, 2023
Accepted: February 15, 2023
Article in press: February 15, 2023
Published online: March 6, 2023
Processing time: 133 Days and 22 Hours
Hyperthyroidism often leads to tachycardia, but there are also sporadic reports of hyperthyroidism with severe bradycardia, such as sick sinus syndrome (SSS) and atrioventricular block. These disorders are a challenge for clinicians.
We describe three cases of hyperthyroidism with SSS and found 31 similar cases in a PubMed literature search. Through the analysis of these 34 cases, we found 21 cases of atrioventricular block and 13 cases of SSS, with 67.6% of the patients experiencing bradycardia symptoms. After drug treatment, temporary pacemaker implantation, or anti-hyperthyroidism treatment, the bradycardia of 27 patients (79.4%) was relieved, and the median recovery time was 5.5 (2-8) d. Only 7 cases (20.6%) needed permanent pacemaker implantation.
Patients with hyperthyroidism should be aware of the risk of severe bradycardia. In most cases, drug treatment or temporary pacemaker placement is recommended for initial treatment. If the bradycardia does not improve after 1 wk, a permanent pacemaker should be implanted.
Core Tip: Severe bradycardia, such as sick sinus syndrome and atrioventricular block, can occasionally be encountered in patients with hyperthyroidism. These pose a challenge for physicians. We report three cases of hyperthyroidism with severe bradycardia and identified an additional 31 cases indexed in PubMed. We found that hyperthyroidism with severe bradycardia may require 1 wk of observation before deciding whether to implant a permanent pacemaker. The use of drugs (e.g., atropine, isoproterenol, and/or anti-hyperthyroidism treatment), implantation of temporary pacemakers, and correcting the electrolyte disorder are recommended before permanent pacemaker implantation.
- Citation: He YL, Xu WX, Fang TY, Zeng M. Hyperthyroidism and severe bradycardia: Report of three cases and review of the literature. World J Clin Cases 2023; 11(7): 1549-1559
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1549.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1549
Hyperthyroidism refers to a group of clinical syndromes characterized by increased excitability and hypermetabolism of the nervous, circulatory, and digestive systems caused by excessive serum thyroid hormone levels[1]. Hyperthyroidism is a common disease. In the United States, the prevalence is estimated to be 1.20%[2]. In China, the prevalence of overt hyperthyroidism is 0.78% and that of subclinical hyperthyroidism is 0.44%[3].
Hyperthyroidism affects the heart in the following ways[4,5]: (1) It increases the sensitivity of heart β-receptors to catecholamine; (2) it directly acts on myocardial contractile protein and enhances the positive inotropic effect of the myocardium; and (3) it leads to the expansion of peripheral blood vessels, an increase in the resistance of peripheral blood vessels, and a subsequent compensatory increase in cardiac output. Therefore, patients with hyperthyroidism often develop tachycardia (such as sinus tachycardia and rapid atrial fibrillation). However, severe bradycardia, such as sick sinus syndrome (SSS) and atrioventricular block, are occasionally encountered in patients with hyperthyroidism, which pose a challenge for physicians.
It is unclear whether anti-hyperthyroidism treatment will reduce the patient’s heart rate. Hyperthyroidism is also known to induce rapid atrial fibrillation, but the treatment of rapid atrial fibrillation along with bradycardia is also unclear. Specifically, it is unknown whether patients with hyperthyroidism and bradycardia need permanent pacemakers and when such pacemakers should be placed. In the present report, we share our experience with three patients with hyperthyroidism combined with severe bradycardia who were treated at our hospital since 2019. We also review similar cases published in PubMed and summarize the features and treatment strategies. These findings will be useful for the diagnosis and treatment of hyperthyroidism with severe bradycardia in the future.
A 63-year-old woman suffered from hunger and weight loss for 15 years. Her condition worsened and was accompanied by palpitations and syncope in the 4 mo prior to presentation.
In 2004, the patient was diagnosed with hyperthyroidism based on frequent hunger and weight loss and was prescribed medication for hyperthyroidism (information about the treatment was not available). She adhered to the drug regimen for more than 1 year, but she later discontinued the treatment and started self-treatment with Chinese herbal medicines (the drugs are unknown). She experienced some symptom relief from the alternative treatment. However, since December 2018, her symptoms reappeared, and she repeatedly experienced palpitations and syncope.
The patient had no previous medical history.
The patient was a housewife and denied any relevant family history.
On physical examination, the patient’s blood pressure was 143/71 mmHg. Slight protrusion of the eyeballs (bilateral) was observed, and the thyroid gland showed degree II swelling and moved with swallowing. The heart rate was 99 beats/min and regular, and no noise was heard during auscultation. Her hands trembled slightly.
Triiodothyronine (TT3) level was 5.39 nmol/L (normal range: 0.88-2.44 nmol/L). Thyroxine (TT4) was > 308.88 nmol/L (normal range: 62.68-150.80 nmol/L). Free triiodothyronine (FT3) was > 46.08 pmol/L (normal range: 2.63-5.70 pmol/L). Free thyroxine (FT4) was 58.59 pmol/L (normal range: 9.01-19.05 pmol/L). Thyroid-stimulating hormone (TSH) was < 0.0025 mIU/L (normal range: 0.35-4.94 mIU/L). Thyrotrophin receptor antibody (TRAb) was 39.56 U/L (normal level, ≤ 1.75 U/L). Thyroid peroxidase antibody (TPOAb) was 69.52 IU/L (normal level, < 5.61 IU/L). Blood potassium, troponin, brain natriuretic peptide, routine blood parameters, transaminase, and creatinine levels were normal.
Echocardiography showed no abnormality. Continuous electrocardiogram (ECG) monitoring showed paroxysmal atrial fibrillation that was automatically converted into a sinus rhythm. During rhythm conversion, sinus arrest for 4 s was observed.
The patient was diagnosed as hyperthyroidism and SSS.
The day after hospitalization, the patient experienced palpitations. Her heart rate was 130-140 beats/min and irregular. ECG revealed atrial fibrillation. She was intravenously administered with 0.2 mg of cedilanide. After 4 h, the patient felt her heart stop beating, and this was accompanied by dizziness and fatigue. Her blood pressure was 140/80 mmHg, and she had arrhythmia, with her heart rate fluctuating between 50 beats/min and 160 beats/min. She was transferred to the cardiology intensive care unit.
Continuous ECG monitoring showed that her heart rate fluctuated between 70 beats/min and 180 beats/min, and it was accompanied by paroxysmal atrial fibrillation that was automatically converted into a sinus rhythm. During rhythm conversion, sinus arrest for 4 s occurred, and the patient had syncope again. A temporary pacemaker was implanted.
She was diagnosed with hyperthyroidism and was treated with propylthiouracil (100 mg tid). She was also given metoprolol (25 mg bid) for paroxysmal rapid atrial fibrillation.
After 10 d of observation, a second laboratory examination showed that her thyroid hormone levels were normal (TT3 = 2.86 nmol/L, TT4 = 129.76 nmol/L, FT3 = 5.81 pmol/L, FT4 = 7.87 pmol/L, and TSH = 0.02 mIU/L). However, rapid atrial fibrillation, sinus rhythm, and pacing rhythm still occurred alternately according to the findings of ECG monitoring. A permanent dual-chamber pacemaker was implanted, and she continued treatment with propylthiouracil (300 mg/d) and metoprolol sustained-release tablets (95 mg/d).
No syncope occurred during the 2-year follow-up.
A 76-year-old woman suffered from repeated episodes of palpitations and syncope for more than 6 mo.
Since March 2019, the patient had been experiencing repeated episodes of dizziness, palpitations, and syncope. In August 2019, she was diagnosed with SSS at a local hospital, and a temporary pacemaker was implanted. After 1 wk, her symptoms improved and the temporary pacemaker was removed. However, 3 d prior to admission to our hospital, she experienced syncope again.
The patient was diagnosed with hyperthyroidism in 1999 and was treated with methimazole. She had a history of hypertension for more than 7 years and was treated with amlodipine. However, she did not monitor it regularly.
The patient was previously employed and retired 18 years prior. She denied any relevant family history.
On physical examination, the patient’s blood pressure was 178/80 mmHg. Her heart rate was 84 beats/min. Auscultation revealed regular and normal sounds. No goiter, exophthalmos, or tremor of the hands was observed.
TT3 level was 1.35 nmol/L (normal range: 0.88-2.44 nmol/L). TT4 was 107.75 nmol/L (normal range: 62.68-150.80 nmol/L). FT3 was 3.38 pmol/L (normal range: 2.63-5.70 pmol/L). FT4 was 14.32 pmol/L (normal range: 9.01-19.05 pmol/L). TSH was < 0.0025 mIU/L (normal range: 0.35-4.94 mIU/L). TRAb was 9.18 IU/L (normal level ≤ 1.75 IU/L). TPOAb was 899.86 IU/L (normal level: < 5.61 IU/L).
Cardiac ultrasound showed local calcification and mild regurgitation in the posterior mitral valve, as well as mild regurgitation of the tricuspid valve and aortic valve. Holter ECG revealed sinus arrest, and the longest RR interval was 3.77 s.
The patient was diagnosed as SSS and subclinical hyperthyroidism.
A permanent pacemaker was implanted, and treatment with methimazole (10 mg/d) was continued.
No dizziness or syncope occurred after implantation of the pacemaker during the 2-year follow-up.
A 60-year-old man was admitted because he had been experiencing palpitations for 6 mo and dizziness for 2 mo.
The patient reported that he had started experiencing repeated episodes of palpitations and chest tightness in the past 6 mo. ECG showed paroxysmal atrial fibrillation, and he was treated with metoprolol (25 mg bid) intermittently. Two months prior to presentation, the patient had recurrent dizziness accompanied by amaurosis that resolved spontaneously within a few seconds. The patient had discontinued metoprolol 1 mo prior. However, repeated episodes of dizziness and palpitations occurred. Since the onset of the disease, his body weight had decreased by 10 kg.
The patient had a history of hypertension for more than 1 year, but he did not take any medicine.
The patient had just retired from administrative work and denied any relevant family history.
At the time of admission to our hospital, his blood pressure was 150/80 mmHg. The heart rate was 55 beats/min, and regular sounds were heard during auscultation. His hands trembled slightly, but no goiter or exophthalmos was observed.
TT3 Level was 4.12 nmol/L (normal range: 0.88-2.44 nmol/L). TT4 was 208.65 nmol/L (normal range: 62.68-150.80 nmol/L). FT3 was 15.34 pmol/L (normal range: 2.63-5.70 pmol/L). FT4 was 29.00 pmol/L (normal range: 9.01-19.05 pmol/L). TSH was < 0.0025 mIU/L (normal range: 0.35-4.94 mIU/L). TRAb was 9.28 IU/L (normal level: ≤ 1.75 IU/L). TPOAb was 4.02 IU/L (normal level: < 5.61 IU/L).
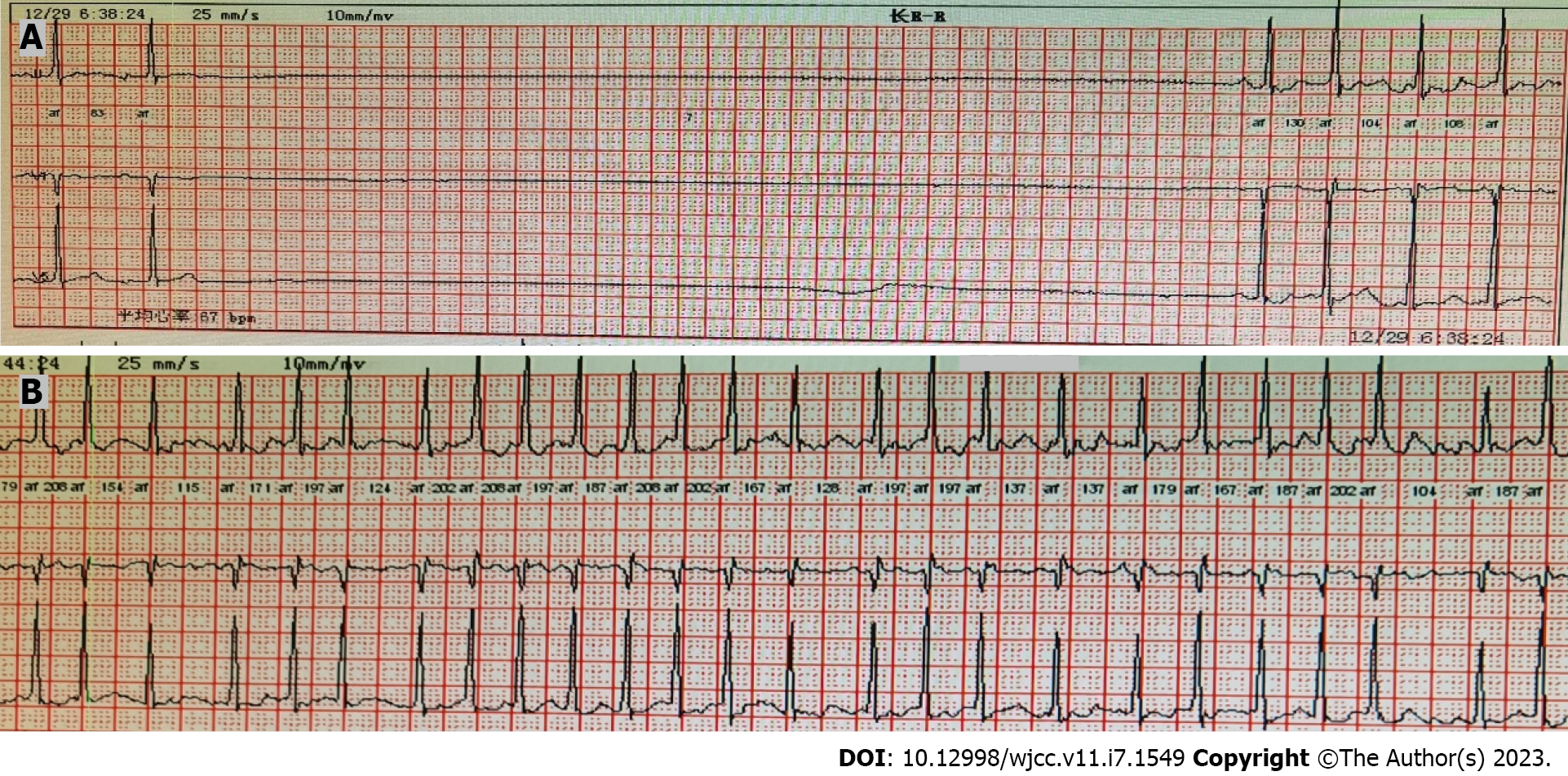
Cardiac ultrasound showed mild regurgitation of the tricuspid valve, mitral valve, and aortic valve and mild pulmonary hypertension (estimated pulmonary artery systolic pressure, 40 mmHg). Twelve-lead ECG taken at admission indicated sinus bradycardia with a heart rate of 55 beats/min. However, Holter ECG indicated atrial fibrillation, and the fastest heart rate was 200 beats/min, accompanied by sinus arrest (Figure 1). Sinus arrest for more than 3 s was observed 16 times, and the longest arrest time was 8.1 s.
The patient was diagnosed as Hyperthyroidism and SSS.
A permanent dual-chamber pacemaker was implanted, and treatment with methimazole (10 mg bid) was started.
The patient recovered well and did not have any episodes of syncope during the 1-year follow-up.
We searched PubMed for similar cases. The search query was (hyperthyroidism OR hyperthyroid OR Graves OR Basedow) AND (bradycardia OR sick sinus syndrome OR atrioventricular block). This search retrieved 31 similar cases. With the three cases that we report here, we analyze 34 cases in total.
The features of all 34 cases are summarized in Table 1. The age of the patients ranged from 10 to 76 years. In 22 cases, the patients were less than 60 years of age, and in 12 cases, the patients were ≥ 60 years. With regard to sex distribution, 24 (70.6%) patients were female, and 10 (29.4%) were male. It appeared that hyperthyroidism combined with bradycardia was more likely to occur in patients under 60 years of age and in females. Among the 34 patients, 21 (61.8%) had atrioventricular block and 13 (38.2%) had SSS.
| Number | Reference | Year | Age in yr | Sex | Bradycardia type |
| 1 | [6] | 1970 | 22 | F | Complete AVB |
| 2 | [7] | 1980 | 55 | F | 2nd degree AVB |
| 3 | [8] | 1985 | 55 | F | Complete AVB |
| 4 | [9] | 1985 | 54 | F | Complete AVB |
| 5 | [10] | 1990 | 29 | M | Complete AVB |
| 6 | [11] | 1998 | 16 | F | Complete AVB |
| 7 | [12] | 2001 | 60 | F | Complete AVB |
| 8 | [12] | 2001 | 30 | F | Complete AVB |
| 9 | [13] | 2005 | 50 | M | Complete AVB |
| 10 | [13] | 2005 | 68 | F | Complete AVB |
| 11 | [14] | 2006 | 67 | M | Complete AVB |
| 12 | [15] | 2007 | 40 | M | Complete AVB |
| 13 | [16] | 2008 | 59 | M | Complete AVB |
| 14 | [17] | 2009 | 28 | F | Complete AVB |
| 15 | [18] | 2011 | 55 | F | Complete AVB |
| 16 | [19] | 2012 | 37 | F | Complete AVB |
| 17 | [20] | 2012 | 71 | F | 2nd degree AVB |
| 18 | [21] | 2014 | 75 | F | 2nd degree AVB |
| 19 | [22] | 2015 | 30 | M | 2nd degree AVB |
| 20 | [23] | 2017 | 10 | F | 2nd degree AVB |
| 21 | [24] | 2020 | 28 | F | Complete AVB |
| 22 | [25] | 1972 | 21 | F | SSS |
| 23 | [25] | 1972 | 26 | M | SSS |
| 24 | [25] | 1972 | 63 | F | SSS |
| 25 | [26] | 1990 | 65 | F | SSS |
| 26 | [27] | 1995 | 50 | F | SSS |
| 27 | [28] | 1999 | 22 | M | SSS |
| 28 | [29] | 2006 | 36 | M | SSS |
| 29 | [30] | 2017 | 48 | F | SSS |
| 30 | [30] | 2017 | 63 | F | SSS |
| 31 | [30] | 2017 | 66 | F | SSS |
| 32 | This report | 2019 | 63 | F | SSS |
| 33 | This report | 2019 | 76 | F | SSS |
| 34 | This report | 2020 | 60 | M | SSS |
Table 2 summarizes the clinical presentation. Except for two cases not mentioned, five cases (14.7%) were hospitalized for bradycardia first, and hyperthyroidism was detected after a clinical workup. Nine cases (26.4%) had hyperthyroidism and bradycardia simultaneously. Eighteen cases (52.9%) were diagnosed with hyperthyroidism first, and bradycardia was found during hyperthyroidism treatment. These data showed that bradycardia may be either the first symptom of hyperthyroidism or may appear at any stage during the hyperthyroidism treatment. In 8 of 34 patients, β-receptor blocker or digitalis was used to control tachycardia caused by hyperthyroidism before bradycardia was detected. In terms of symptoms, 67.6% had symptoms related to bradycardia. The most common bradycardia symptom was syncope. Other symptoms of bradycardia were fatigue, palpitations, and dizziness. For the accompanying conditions, two patients had fever, two had hypokalemia, and one had acute renal insufficiency with hyperkalemia.
| Number | Symptoms of bradycardia | Sequence of occurrence of hyperthyroidism and bradycardia | Use of anti-arrhythmic drugs before onset of bradycardia | Accompanying conditions |
| 1 | - | Hyperthyroidism first | - | - |
| 2 | Syncope | Hyperthyroidism first | Propranolol | - |
| 3 | Syncope | Hyperthyroidism first | Digoxin and propranolol | - |
| 4 | Syncope | Hyperthyroidism first | - | - |
| 5 | - | - | - | Fever |
| 6 | - | - | - | Fever |
| 7 | Syncope | Hyperthyroidism first | - | - |
| 8 | Presyncope | Simultaneous | - | - |
| 9 | Syncope | Hyperthyroidism first | Propranolol | - |
| 10 | - | Bradycardia first | - | - |
| 11 | Near syncope | Bradycardia first | Carvedilol | - |
| 12 | Syncope | Bradycardia first | - | - |
| 13 | - | Simultaneous | - | Jaundice and febrile, creatinine 250 μmol/L |
| 14 | Syncope | Hyperthyroidism first | - | - |
| 15 | Syncope | Hyperthyroidism first | - | - |
| 16 | Syncope | Hyperthyroidism first | - | - |
| 17 | Syncope | Simultaneous | - | - |
| 18 | - | Simultaneous | - | - |
| 19 | - | Hyperthyroidism first | - | - |
| 20 | Dizziness | Hyperthyroidism first | Atenolol | - |
| 21 | Syncope | Bradycardia first | - | - |
| 22 | Syncope | Hyperthyroidism first | - | - |
| 23 | - | Simultaneous | - | Periodic paralysis |
| 24 | Syncope | Hyperthyroidism first | - | - |
| 25 | Syncope | Hyperthyroidism first | Propranolol, diltiazem | - |
| 26 | Fatigue | Bradycardia first | - | - |
| 27 | - | Simultaneous | - | - |
| 28 | Syncope | Simultaneous | - | Blood potassium 2.3 mmol/L |
| 29 | Palpitation | Hyperthyroidism first | Metoprolol | - |
| 30 | - | Simultaneous | - | - |
| 31 | - | Hyperthyroidism first | - | - |
| 32 | Syncope | Hyperthyroidism first | Cedilanide | - |
| 33 | Syncope | Hyperthyroidism first | - | - |
| 34 | Syncope | Simultaneous | - | - |
Table 3 shows the treatment and outcome of the 34 cases. In 11 cases (32.3%), bradycardia was treated with atropine, isoproterenol, or glucocorticoid. In 11 cases (32.3%), the patients were implanted with a temporary pacemaker. Most of the patients were given anti-hyperthyroidism treatment at the same time. Twenty-seven patients (79.4%) recovered or their condition significantly improved through treatment of their accompanying conditions (such as fever and electrolyte disorder) or implantation of a temporary pacemaker and hyperthyroidism treatment. The recovery time of bradycardia was reported in 16 cases. The median recovery time was 5.5 (2-8) d. Permanent pacemakers were implanted in 7 cases (20.6%), and the majority (85.7%) occurred in elderly patients.
| Number | Hyperthyroidism treatment | Bradycardia drug treatment | Temporary pacemaker | ECG changes after treatment1 | Bradycardia recovery time in d | Permanent pacemaker |
| 1 | Propylthiouracil | Isoproterenol, atropine, prednisone, | Yes | 1st degree AVB | 7 | No |
| 2 | Methimazole | Atropine | Yes | 1st degree AVB | 8 | No |
| 3 | Propylthiouracil, iodine-131 | Atropine, isoprenaline | No | Returned to normal | - | No |
| 4 | Methimazole | Prednisolone | Yes | Returned to normal | 1 | No |
| 5 | - | - | - | Returned to normal | - | - |
| 6 | Carbimazole, intravenous iodide a | Dexamethasone | No | Returned to normal | - | No |
| 7 | Methimazole | - | No | No recovery | No recovery | Yes |
| 8 | - | Prednisone | No | No recovery | No recovery | Yes |
| 9 | Propylthiouracil | - | No | 1st degree AVB | - | No |
| 10 | Propylthiouracil | - | No | Returned to normal | - | No |
| 11 | Methimazole | Atropine | Yes | No recovery | No recovery | Yes |
| 12 | Propylthiouracil | - | No | Returned to normal | 3 | No |
| 13 | Carbimazole | - | Yes | Returned to normal | 21 | No |
| 14 | Propylthiouracil | - | No | Returned to normal | 8 | No |
| 15 | Propylthiouracil, subtotal thyroidectomy | - | No | Returned to normal | 7 | No |
| 16 | Propylthiouracil | Steroid | No | 1st degree AVB | 7 | No |
| 17 | Radioactive iodine | - | No | Returned to normal | - | No |
| 18 | - | - | No | Returned to normal | - | No |
| 19 | - | - | No | Returned to normal | 2 | No |
| 20 | Carbimazole | - | No | 1st degree AVB | 2 | No |
| 21 | Propylthiouracil | - | Yes | Normal | 8 | No |
| 22 | Carbimazole | Atropine | Yes | Sinus bradycardia | 2 | No |
| 23 | - | - | No | Returned to normal | - | No |
| 24 | Carbimazole | Atropine | Yes | Returned to normal | 4 | No |
| 25 | Propylthiouracil and potassium iodine | - | No | Returned to normal | 10 | No |
| 26 | Methylmercaptoimidazole | Atropine | Yes | Returned to normal | 2 | No |
| 27 | Subtotal thyroidectomy | - | No | Returned to normal | - | No |
| 28 | Carbimazole | - | No | Returned to normal | 2 | No |
| 29 | Methimazole, surgical treatment | - | No | Returned to normal | - | No |
| 30 | Methimazole | - | No | No recovery | No recovery | Yes |
| 31 | Methimazole | - | No | Arrhythmias were fewer and shorter | - | No |
| 32 | Propylthiouracil | - | Yes | No recovery | No recovery | Yes |
| 33 | Methimazole | - | Yes | No recovery | No recovery | Yes |
| 34 | Propylthiouracil | - | No | / | / | Yes |
The exact mechanism of hyperthyroidism combined with bradycardia is unclear. Studies on the possible causes are primarily focused on the direct impact of hyperthyroidism on the conduction system[31], inflammation[32,33], and hypokalemia[22,25,29]. The molecular mechanism may involve the effect of T3 on phospholamban/sarco(endo)plasma reticulum Ca ATPase type 2A (SERCA2a). The regulation of Ca2+ is closely related to phospholamban and SERCA2a. SERCA2a is a Ca2+ enzyme that transfers Ca2+ from the cytoplasm of cells to the sarcoplasmic reticulum[34]. When SERCA2a activity decreases and the intracellular Ca2+ level increases, the pacing activity of the sinoatrial node is inhibited. This results in bradycardia[35]. Phospholamban is an allosteric inhibitor of the SERCA2a pump that reduces the affinity of the SERCA2a pump for Ca2+[36]. However, the expression of phospholamban can be inhibited by T3[37,38]. Therefore, during the treatment of hyperthyroidism, even a slight decrease in T3 Levels may affect phospholamban/SERCA2a and lead to bradycardia[39]. This may partly explain why some patients developed bradycardia after treatment for hyperthyroidism.
β-receptor blockers are also commonly used in the treatment of hyperthyroidism. However, only seven cases were treated with β-receptor blockers before bradycardia occurred (Table 2). It is possible that β-receptor blockers are involved in the occurrence of bradycardia, but they may not be the main cause of bradycardia.
As there are only a few reported cases of hyperthyroidism combined with severe bradycardia, large-scale evidence-based research on the disease course and treatment is difficult. In the clinical setting, it is unclear for physicians whether a patient should receive a permanent pacemaker and when a permanent pacemaker should ideally be implanted. Unfortunately, the findings from the cases reported so far are still inconsistent.
Most scholars believe that pacemaker implantation to treat severe bradycardia combined with hyperthyroidism should be delayed as much as possible. The need for a permanent pacemaker should be determined only after thyroid function stabilizes[7]. However, Ozcan et al[40] reported a series of 21 patients with hyperthyroidism (including subclinical hyperthyroidism) complicated with atrioventricular block. After hyperthyroidism treatment and follow-up, only three cases (14%) of bradycardia were found to be caused by hyperthyroidism. Based on their findings, they concluded that patients with thyroid dysfunction combined with second- or third-degree atrioventricular block almost always require permanent pacemaker implantation, even after the thyroid state returns to normal. However, based on our analysis of the 34 reported cases, we observed that most cases of hyper
We hypothesize that most elderly patients with hyperthyroidism and severe bradycardia may need permanent pacemaker implantation. Our analysis revealed that 6 of the 12 elderly patients required permanent pacemaker implantation. Elderly patients accounted for 85.7% (6/7) of all reported permanent pacemaker implantations. Elderly patients are more likely to need a permanent pacemaker because the cardiac conduction system undergoes a series of changes with age, such as a decrease in the number of pacing cells, an increase in fibrous tissue, and fat infiltration in the sinoatrial node. These events cause difficulty for the elderly to recover.
We directly implanted a permanent pacemaker without waiting for 1 wk in Case 3. The patient exhibited obvious alternation between rapid atrial fibrillation and sinus arrest within a day, and cardiac ultrasound showed changes in cardiac structure. In this case, we did not know if hyperthyroidism was the only reason for bradycardia. Therefore, we were unsure if treatment of hyperthyroidism would relieve the severe bradycardia or how long it would take. We do know that acute unstable bradycardia can lead to cardiac arrest, and this patient had multiple sinus arrests that could last for 8.1 s. By not directly treating bradycardia and treating hyperthyroidism instead, his risk of cardiac arrest would be higher. Therefore, his family chose to have a permanent pacemaker implanted immediately. He recovered well and did not have any episodes of syncope during the 1-year follow-up.
In most cases, hyperthyroidism with severe bradycardia may require 1 wk of observation before deciding whether to implant a permanent pacemaker. Using drugs, such as atropine and isoproterenol and/or anti-hyperthyroidism treatment, or implantation of temporary pacemakers to increase heart rate and assessment of blood potassium levels need to be completed before permanent pacemaker implantation. Moreover, we must remember that the probability of recovery of the elderly will decrease. Therefore, in special cases, such as elderly patients, an immediate permanent pacemaker implantation may be chosen.
Patients with hyperthyroidism carry a risk of severe bradycardia. For patients with hyperthyroidism accompanied by severe bradycardia, treatment with drugs or a temporary pacemaker for 1 wk is recommended before implantation of a permanent pacemaker. However, in special cases where the patient’s safety is at stake or in elderly patients who are at risk, permanent pacemaker implantation can be carried out immediately with the patient’s consent.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Junior JMA, Brazil; Ong H, Malaysia S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet. 2016;388:906-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 423] [Cited by in RCA: 505] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 2. | Doubleday AR, Sippel RS. Hyperthyroidism. Gland Surg. 2020;9:124-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Li Y, Teng D, Ba J, Chen B, Du J, He L, Lai X, Teng X, Shi X, Li Y, Chi H, Liao E, Liu C, Liu L, Qin G, Qin Y, Quan H, Shi B, Sun H, Tang X, Tong N, Wang G, Zhang JA, Wang Y, Xue Y, Yan L, Yang J, Yang L, Yao Y, Ye Z, Zhang Q, Zhang L, Zhu J, Zhu M, Ning G, Mu Y, Zhao J, Shan Z, Teng W. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: Epidemiological Evidence from 31 Provinces of Mainland China. Thyroid. 2020;30:568-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 4. | Polikar R, Burger AG, Scherrer U, Nicod P. The thyroid and the heart. Circulation. 1993;87:1435-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 296] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Klein I, Ojamaa K. Thyroid hormone and the cardiovascular system. N Engl J Med. 2001;344:501-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1363] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 6. | Muggia AL, Stjernholm M, Houle T. Complete heart block with thyrotoxic myocarditis. Report of a case. N Engl J Med. 1970;283:1099-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Miller RH, Corcoran FH, Baker WP. Second and third degree atrioventricular block with Graves' disease: a case report and review of the literature. Pacing Clin Electrophysiol. 1980;3:702-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kramer MR, Shilo S, Hershko C. Atrioventricular and sinoatrial block in thyrotoxic crisis. Br Heart J. 1985;54:600-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Nakagawa S, Higa A, Kondoh H, Koiwaya Y, Tanaka K. Cyclic sinus node dysfunction in a patient with hyperthyroidism. Arch Intern Med. 1985;145:2126-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Sriussadaporn S, Vannasaeng S, Trisukosol D, Nitiyanant W, Piraphatdist T, Vichayanrat A. Complete heart block complicating hyperthyroidism: a case report. J Med Assoc Thai. 1990;73:53-57. [PubMed] |
| 11. | Ho SC, Eng PH, Ding ZP, Fok AC, Khoo DH. Thyroid storm presenting as jaundice and complete heart block. Ann Acad Med Singap. 1998;27:748-751. [PubMed] |
| 12. | Vassiliou V. Thyrotoxicosis with heart block. J R Soc Med. 2001;94:552-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 13. | Topaloglu S, Topaloglu OY, Ozdemir O, Soylu M, Demir AD, Korkmaz S. Hyperthyroidism and complete atrioventricular block--a report of 2 cases with electrophysiologic assessment. Angiology. 2005;56:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kuo YC, Tseng YT, Lee TI, Hsieh MH. Chronic bifascicular block with intermittent complete atrioventricular block induced by hyperthyroidism. Int J Cardiol. 2006;110:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 15. | Ozaydin M, Türker Y, Doğan A, Varol E, Aslan SM, Altinbaş A. An unusual cause of syncope: hyperthyroidism. Anadolu Kardiyol Derg. 2007;7:453-454. [PubMed] |
| 16. | Krishnamoorthy S, Narain R, Creamer J. Unusual presentation of thyrotoxicosis as complete heart block and renal failure: a case report. J Med Case Rep. 2009;3:9303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Karakaş CY, Topaloğlu C, Canbolant E, Seyfeli E, Akgül F. Hyperthyroidism as a rare cause of complete AV block. Anadolu Kardiyol Derg. 2009;9:67-68. [PubMed] |
| 18. | Aritürk Y, Islamoglu E, Tekbas. An unusual presentation of hyperthyroidism: atrioventricular complete heart block. Acta Endocrinologica (Buc). 2011;7:405-409. [DOI] [Full Text] |
| 19. | Al Bannay R, Husain A, Khalaf S. Complete heart block in thyrotoxicosis, is it a manifestation of thyroid storm? Case Rep Endocrinol. 2012;2012:318398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Kausel A, Korniyenko A, Sandhu G. Bradyarrhythmia as a presenting feature of subclinical hyperthyroidism. QJM. 2012;105:461-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | illogo GR, Konaté L, Samandoulougou A. Transient atrioventricular block in multinodular goiter: report of a case. Pan Afr Med J. 2014;19:53. [DOI] [Full Text] |
| 22. | Upta A, Arora S. Atrioventricular Heart Blocks in Thyrotoxicosis. JACC. 2015;65:A738. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Adesokan A, Vigneswaran T, Ajzensztejn M, Mathur S. Atrioventricular block: an unusual complication of Graves' disease. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Eom YS, Oh PC. Graves' Disease Presenting with Complete Atrioventricular Block. Case Rep Endocrinol. 2020;2020:6656875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 25. | Wan SH, Lee GS, Toh CC. The sick sinus syndrome. A study of 15 cases. Br Heart J. 1972;34:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Lubitz RM, Acker JJ. Thyrotoxicosis induced sick sinus syndrome: medical therapy may avoid permanent pacing. Pacing Clin Electrophysiol. 1990;13:700-702. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 27. | Namura M, Kanaya H, Ikeda M, Shibayama S, Ohka T. Hyperthyroidism complicated with sick sinus syndrome. Jpn Circ J. 1995;59:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Moustaghfir A, Kharchafi A, Mahassin F, Chaari J, Ghafir D, Ohayon V, Archane MI. [Sinus dysfunction in Basedow's disease. A case report]. Rev Med Interne. 1999;20:804-805. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 29. | Lee G, McGavigan AD, Hillock RJ, Roberts-Thomson KC, Stevenson IH, Mond HG. A grave case of bradycardia. Pacing Clin Electrophysiol. 2006;29:788-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Tudoran M, Tudoran C. Hyperthyroidism and sick sinus syndrome, a rare but challenging association: A study of three cases. Niger J Clin Pract. 2017;20:1046-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Talwar KK, Gupta V, Kaul U, Ahuja MM, Bhatia ML. Electrophysiological studies in thyrotoxicosis with and without associated sick sinus syndrome. Clin Cardiol. 1987;10:249-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Ortmann C, Pfeiffer H, Du Chesne A, Brinkmann B. Inflammation of the cardiac conduction system in a case of hyperthyroidism. Int J Legal Med. 1999;112:271-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 33. | Biondi B, Kahaly GJ. Cardiovascular involvement in patients with different causes of hyperthyroidism. Nat Rev Endocrinol. 2010;6:431-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 34. | Chen PS, Joung B, Shinohara T, Das M, Chen Z, Lin SF. The initiation of the heart beat. Circ J. 2010;74:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Wang Q, Wang D, Yan G, Qiao Y, Sun L, Zhu B, Wang X, Tang C. SERCA2a was serotonylated and may regulate sino-atrial node pacemaker activity. Biochem Biophys Res Commun. 2016;480:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | . Retraction. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. Am J Physiol Cell Physiol. 2010 Mar;298(3):C521-9. doi: 10.1152/ajpcell.00479.2009. Am J Physiol Cell Physiol. 2012;303:C1000. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 37. | Holt E, Sjaastad I, Lunde PK, Christensen G, Sejersted OM. Thyroid hormone control of contraction and the Ca(2+)-ATPase/phospholamban complex in adult rat ventricular myocytes. J Mol Cell Cardiol. 1999;31:645-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 38. | Kimura Y, Otsu K, Nishida K, Kuzuya T, Tada M. Thyroid hormone enhances Ca2+ pumping activity of the cardiac sarcoplasmic reticulum by increasing Ca2+ ATPase and decreasing phospholamban expression. J Mol Cell Cardiol. 1994;26:1145-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 54] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 39. | Hoefig CS, Harder L, Oelkrug R, Meusel M, Vennström B, Brabant G, Mittag J. Thermoregulatory and Cardiovascular Consequences of a Transient Thyrotoxicosis and Recovery in Male Mice. Endocrinology. 2016;157:2957-2967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ozcan KS, Osmonov D, Erdinler I, Altay S, Yildirim E, Turkkan C, Hasdemir H, Cakmak N, Alper AT, Satilmis S, Gurkan K. Atrioventricular block in patients with thyroid dysfunction: prognosis after treatment with hormone supplementation or antithyroid medication. J Cardiol. 2012;60:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |