Published online Mar 6, 2023. doi: 10.12998/wjcc.v11.i7.1506
Peer-review started: September 13, 2022
First decision: October 28, 2022
Revised: November 11, 2022
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: March 6, 2023
Processing time: 170 Days and 3.8 Hours
Secondary hypertension is a relatively rare condition most commonly caused by renovascular disease due to atherosclerotic vascular disease or fibromuscular dysplasia. Although accessory renal arteries are frequent, to date, only six cases of secondary hypertension determined by their existence have been reported.
We describe a case of a 39-year-old female who came to the emergency depart
To the best of our knowledge, there are controversies regarding accessory renal arteries as a potential etiology for secondary hypertension, but the seven similar cases already described, along with the current case, could reinforce the necessity of more studies concerning this subject.
Core Tip: Unfortunately, nowadays, there are still a lot of young patients with hypertension that receive medical treatment, without any further research of secondary causes of hypertension. Also, secondary hypertension caused by the presence of accessory renal arteries is a controversial subject in the medical literature because there are studies confirming this association and studies that have found no association.
- Citation: Calinoiu A, Guluta EC, Rusu A, Minca A, Minca D, Tomescu L, Gheorghita V, Minca DG, Negreanu L. Accessory renal arteries - a source of hypertension: A case report. World J Clin Cases 2023; 11(7): 1506-1512
- URL: https://www.wjgnet.com/2307-8960/full/v11/i7/1506.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i7.1506
Hypertension is a frequent medical condition that is increasingly observed around the world. In 5%-15% of cases, hypertension arises as a result of an identifiable cause – secondary hypertension. The most common pathological process leading to secondary hypertension is renovascular disease, which is the result of atherosclerotic renovascular disease or fibromuscular dysplasia in most of these cases. No practical benefits have been found from screening all hypertensive patients for secondary hypertension. However, looking for a potential cause of hypertension might be of great importance in patients younger than age 40 and in patients with severe (grade 3) hypertension or a hypertensive emergency[1]. We report a case of secondary hypertension caused by stenosis of an accessory left renal artery; a very rare condition in the medical literature.
A 39-year-old white female who resided in the countryside presented to the emergency department with vertigo and a fronto-occipital headache.
Her symptoms started 3 months prior and had progressively worsened.
The patient’s medical history comprised severe hypertensive disease with a maximum systolic blood pressure of 230 mmHg, first diagnosed at the age of 20. She inconsistently took bisoprolol 5 mg per day, candesartan 32 mg per day and indapamide 1.5 mg per day, but she had interrupted her treatment 4 months prior.
The patient denied smoking, consuming alcohol, and substance abuse and claimed that her mother also suffered from hypertension.
The physical examination revealed an overweight woman (BMI = 29 kg/m2) with a right arm blood pressure (BP) of 280/140 mmHg and a left arm BP of 275/140 mmHg, normal breath sounds, normal respiratory rate, normal heart sounds, no vascular murmurs, normal symmetric peripheral pulse and normal neurological examination results.
Laboratory findings (creatinine, blood urea nitrogen, sodium, potassium, blood count, urinalysis, free triiodothyronine, thyroid-stimulating hormone) were unremarkable except for mild hypercholesterolemia.
Direct ophthalmoscopy performed in the ER revealed some flame-shaped hemorrhages and hard exudates at the inferior temporal arcade in the right eye and a macular hemorrhage and some flame-shaped bleeding along the temporal arcades in the left eye. Electrocardiography showed sinus rhythm, a heart rate of 90, left ventricular hypertrophy and negative T waves in DI and aVL. Further echocardiography in the ER displayed mild left ventricular hypertrophy, normal systolic and diastolic function, normal wall movement and the absence of hemodynamically significant valvulopathies.
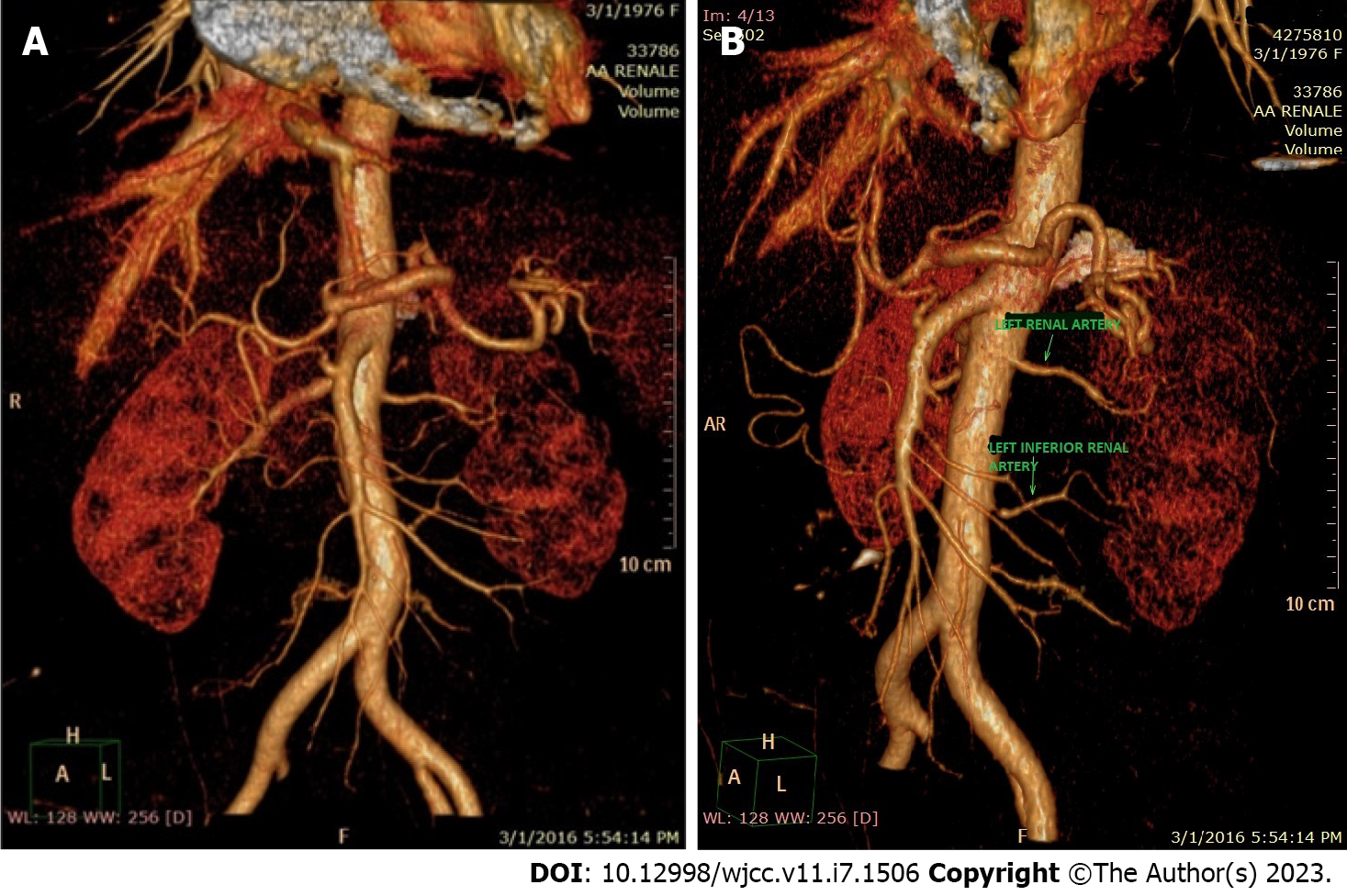
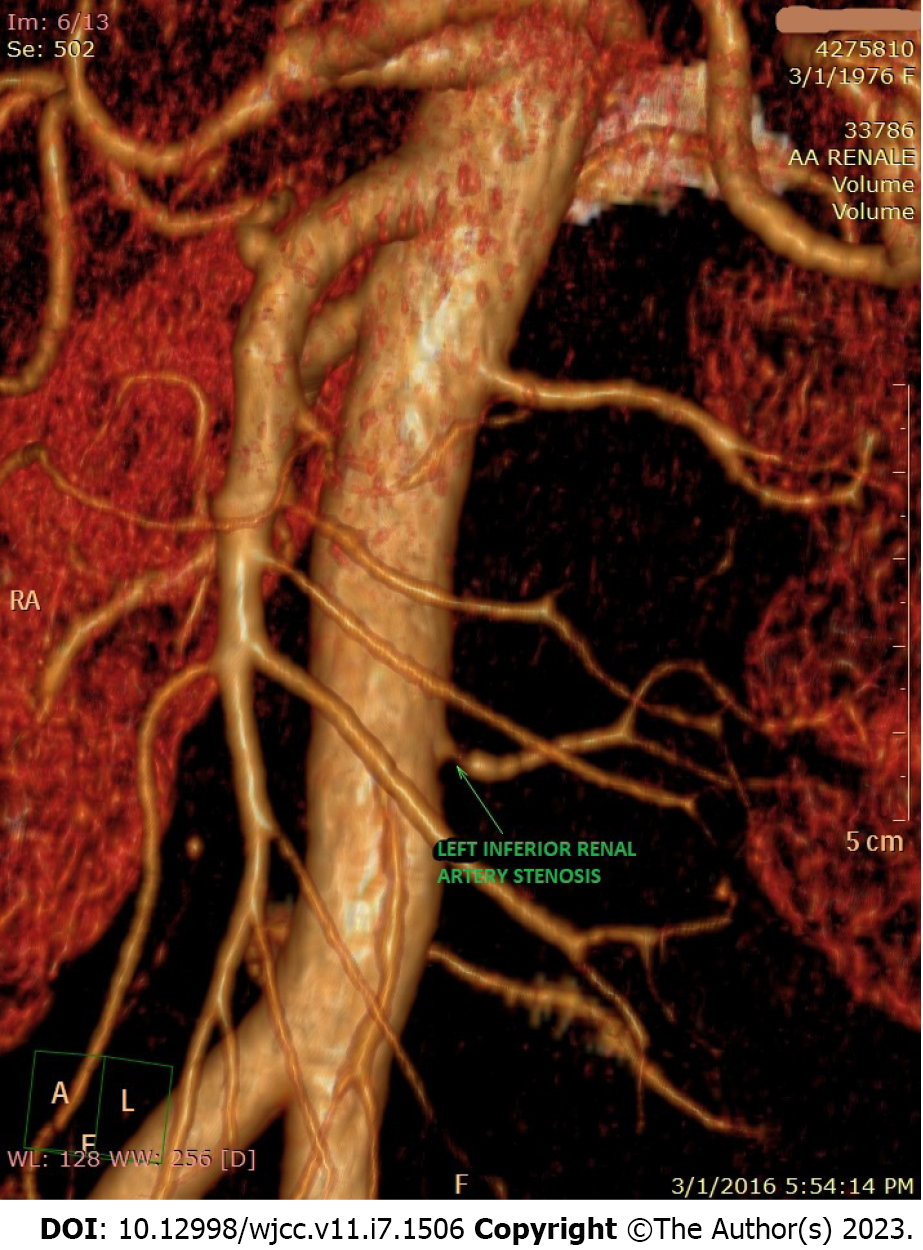
Suspecting a secondary hypertensive disease, we admitted the patient to the Internal Medicine Department to further seek the etiology and to stabilize her blood pressure. Abdominal echography and Doppler renal artery ultrasonography revealed a left kidney considerably smaller than the right kidney (100/53 mm vs 118/50 mm), a left renal sinus with a duplex collecting system, and normal flow in the extraparenchymal renal arteries. Additional computed tomography (CT) angiography revealed an aberrant extrarenal artery originating from the abdominal aorta and supplying the inferior pole of the left kidney (Figure 1). This structure presented stenosis of approximately 50% of the diameter at its origin in the abdominal aorta (Figure 2). To demonstrate whether the newfound renal artery stenosis was responsible for the patient’s high blood pressure values, we measured the level of plasma renin. Laboratory tests showed extremely high hyperreninemia (1300 μUI/mL; normal range, 4.4-46.1 μUI/mL), which confirmed our theory.
Regarding the differential diagnosis for renovascular hypertension in our patient, we considered the following potential causes of secondary hypertension: (1) Pheochromocytoma: Patients usually present with flushing, headache, tachycardia, and episodic uncontrolled hypertension, symptoms that were not present in our patient. Additionally, the CT scan did not reveal any suprarenal masses; (2) Primary hyperaldosteronism: Patients present with persistent hypokalemia and metabolic alkalosis. Our patient had normal blood potassium levels; (3) Obstructive sleep apnea: This is usually seen in obese males with an increased neck circumference and a history of snoring, which was not applicable in our case; (4) Coarctation of the aorta: Patients usually have a systolic murmur, radio-femoral delay, and upper extremity hypertension, which were not present in our patient. Additionally, the CT scan did not reveal narrowing in the aorta; and (5) Cushing syndrome: This is associated with discriminatory physical features such as moon facies, buffalo hump, proximal myopathy, glucose intolerance, abdominal striae, and central obesity, which our patient did not show at clinical examination.
Severe secondary hypertension caused by stenosis of an accessory left renal artery.
Considering the patient’s urgent hypertensive crisis and hypertensive encephalopathy, the patient immediately received intravenous furosemide and oral nifedipine and clonidine before undergoing further investigation for a diagnosis, and her blood pressure dropped to 160/80 mmHg. Regarding renal artery stenosis, its permeability, and the absence of pathological changes in the left and right renal arteries, we decided to adopt conservative management in this case.
Consequently, the patient was discharged home with a recommendation for a low-sodium diet and medical treatment, including perindopril 10 mg per day, amlodipine 10 mg per day and indapamide 1.5 mg per day. One month later, she maintained adequate BP control (120/60 mmHg), a normal heart rate (72 bpm) and normal laboratory findings (serum creatinine, blood urea nitrogen, sodium and potassium) without pathological changes.
An accessory renal artery is a vestige from the intrauterine development period, with a prevalence ranging from 24% to 42%[2]. In a cadaver dissection study, the incidence of an accessory renal artery was slightly higher in males. Additionally, a higher frequency of these structures was observed on the right side than on the left side, and there was a higher probability of encountering a superior polar artery than an inferior one in cases with a single accessory renal artery[3-4]. Our female patient was born with a rare anatomical variation – an inferior left polar renal artery. The chief pathophysiological mechanism underlying renovascular hypertension involves the hypersecretion of renin, which accelerates the conversion of angiotensin I to angiotensin II and enhances the adrenal release of aldosterone. The result is profound angiotensin-mediated vasoconstriction and aldosterone-induced sodium and water retention. The ensuing cascade of events varies, depending on the presence of a functioning contralateral kidney[5].
When two kidneys are present, aldosterone-mediated sodium and water retention is handled properly by the nonstenotic kidney, precluding volume from contributing to angiotensin II–mediated hypertension. In contrast, a solitary ischemic kidney has little or no capacity for sodium and water excretion, allowing volume to play an additive role in hypertension[6].
When the contralateral kidney is functional, such as in the case of our patient, volume expansion is avoided, and renin levels remain high. The two kidneys are in opposition; the stenotic kidney avidly retains sodium and produces excess renin in response to renal ischemia, while the nonstenotic kidney excretes sodium and water to maintain euvolemia, and the renin production decreases. The end result is systemic hypertension that is mediated by both renin and angiotensin[7].
In this way, the patient presented with secondary hypertension at a very young age.
Not adhering to the treatment was an aggravating factor for her disease, resulting in complications in other organs, such as hypertensive retinopathy. Considering the permeability of the left inferior polar artery, our first therapeutic option was conservative management of the disease to avoid surgery and its possible complications, such as vascular injuries to a functional accessory renal artery, a situation that might result in complications such as kidney failure[8]. During our research, we identified only 6 similar cases. They are summarized in Table 1[9-12].
| Ref. | Clinic | Imaging | Treatment | Follow-up |
| [13] | 5-year-old boy with severe hypertension (BP 190/130 mmHg) partially uncontrolled with propranolol, diuretics and spironolactone | Arteriogram: an elongated, nonstenotic aberrant artery arising from the common iliac artery feeding the lower pole of the right kidney | Partial nephrectomy and resection of the aberrant artery at its origin | One month later: BP 120/70 mmHg without any medication |
| [13] | 16-year-old girl with severe hypertension (BP 220/115 mmHg) partially controlled with metoprolol 100 mg/day and hydrochlorothiazide 50 mg/day) | Arteriogram: a nonstenotic aberrant artery arising from the lower aorta supplying the lower pole of the left kidney | Medical treatment with captopril, diuretics and a ß-blocker | Generally, well controlled under medical treatment;Lost to follow-up at 19 years old |
| [15] | 29-year-old patient with hypertension uncontrolled with amlodipine 10 mg and atenolol 50 mg daily (BP 160/100 mmHg) | Digital subtraction angiography: left accessory renal artery entrapped by the diaphragmatic crus with 90% stenosis of the proximal ostial segment | Medical treatment | Close monitoring of the patient’s BP and consideration of further invasive and aggressive treatment in case of prolonged uncontrolled hypertension |
| [12] | 21-year-old female with severe hypertension (BP 220/142 mmHg) without relevant previous medical history | Renal magnetic resonance angiography: bilateral accessory renal arteries were seen superior to the main renal arteries; Renal angiography: no stenosis in the main or accessory arteries bilaterally | Medical treatment with spironolactone 75 mg and amlodipine 10 mg daily | BP control achieved with medication |
| [12] | 41-year-old woman with history of hypertension for 3 years partially controlled with amlodipine 5 mg daily (BP 145/100 mmHg) | Renal magnetic resonance angiography: bilateral small accessory left renal arteries supplying the upper pole of the kidney; Renal angiography: no stenosis in the accessory arteries | Medical treatment with spironolactone 50 mg and oral potassium chloride 1.2 mg daily | BP control achieved with medication |
| [14] | 31-year-old female with reported history of elevated blood over the past 7 years (BP 150/100 mmHg) | Renal ultrasound: left accessory renal artery; Renal CT: ostial stenosis of the left accessory renal artery | Medical treatment with amlodipine 10 mg and lisinopril 5 mg | BP control was achieved with lisinopril 10 mg, and amlodipine was discontinued |
As might be observed in the Table, like our case, the majority of the patients with secondary hypertension associated with the presence of an accessory renal artery were females under 45 years of age. Additionally, the accessory renal artery was more frequently located on the left side.
Secondary hypertension caused by the presence of accessory renal arteries is a controversial subject in the medical literature because there are studies confirming this association and studies that have found no association[13-15]. Thus, although renal arteries are related to higher blood pressure in middle-aged patients with primary hypertension, a recent retrospective study concluded that an accessory renal artery is an independent risk factor for developing increased blood pressure[16]. Not only did our patient have an accessory artery, but the artery had a 50% stenosis at its origin from the aorta causing a significant hypersecretion of renin, which led to severe and uncontrolled hypertension.
We consider that the difficulty of this case was choosing the optimal therapeutic intervention for our patient – pharmacological treatment vs percutaneous revascularization. Usually, revascularization is reserved for patients with hypertension refractory to antihypertensive medications, progressive worsening of renal function, or a degree of renal artery stenosis greater than 80% to 85%[17,18].
An increasing number of studies have proven that accessory renal arteries are common in the general population. This case, along with the few similar cases already published in the medical literature of an accessory renal artery as the cause of secondary hypertension, proves that every clinician should be aware of this potential etiology when treating a patient with secondary hypertension, especially when significant asymmetry in kidney size is seen on ultrasound or other imaging scans. Furthermore, these cases reinforce the necessity for future multicenter studies on this subject.
We are grateful to our patient for permitting us to write this article using her private information, even if it is anonymously published.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Romanian Society of Gastroenterology & Hepatology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Romania
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: He Z, China; Wondmagegn H, Ethiopia S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; Authors/Task Force Members. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36:1953-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 1945] [Article Influence: 277.9] [Reference Citation Analysis (0)] |
| 2. | Yufa A, Mikael A, Lara G, Nurick H, Andacheh I. Accessory renal arteries involved in atherosclerotic occlusive disease at the aortic bifurcation. J Vasc Surg Cases Innov Tech. 2020;6:425-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Jamkar AA, Khan B, Joshi DS. Anatomical study of renal and accessory renal arteries. Saudi J Kidney Dis Transpl. 2017;28:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Sykes D. The arterial supply of the human kidney with special reference to accessory renal arteries. Br J Surg. 1963;50:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Persu A, Canning C, Prejbisz A, Dobrowolski P, Amar L, Chrysochou C, Kądziela J, Litwin M, van Twist D, Van der Niepen P, Wuerzner G, de Leeuw P, Azizi M, Januszewicz M, Januszewicz A. Beyond Atherosclerosis and Fibromuscular Dysplasia: Rare Causes of Renovascular Hypertension. Hypertension. 2021;78:898-911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (78)] |
| 6. | Textor SC, Lerman L. Renovascular hypertension and ischemic nephropathy. Am J Hypertens. 2010;23:1159-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Viera AJ, Neutze DM. Diagnosis of secondary hypertension: an age-based approach. Am Fam Physician. 2010;82:1471-1478. [PubMed] |
| 8. | Glodny B, Cromme S, Reimer P, Lennarz M, Winde G, Vetter H. Hypertension associated with multiple renal arteries may be renin-dependent. J Hypertens. 2000;18:1437-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 9. | Chung AA, Millner PR. Accessory Renal Artery Stenosis and Secondary Hypertension. Case Rep Nephrol. 2020;2020:8879165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 10. | Ozmen CA, Hazirolan T, Canyigit M, Peynircioglu B, Cil BE. An unusual reason for renovascular hypertension: entrapment of an accessory renal artery by the diaphragmatic crus. J Vasc Interv Radiol. 2006;17:1713-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Kang K, Ma Y, Jia C, Cheng Y, Yang Y, Wang L, Jiang Y, Lu Y. Relationship between Accessory Renal Artery and Clinical Characteristics of Middle-Aged Patients with Primary Hypertension. Int J Hypertens. 2020;2020:7109502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Gupta A, Tello R. Accessory renal arteries are not related to hypertension risk: a review of MR angiography data. AJR Am J Roentgenol. 2004;182:1521-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Davies ER, Sutton D. Hypertension and Multiple Renal Arteries. Lancet. 1965;1:341-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Chan PL, Tan FHS. Renin dependent hypertension caused by accessory renal arteries. Clin Hypertens. 2018;24:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Kem DC, Lyons DF, Wenzl J, Halverstadt D, Yu X. Renin-dependent hypertension caused by nonfocal stenotic aberrant renal arteries: proof of a new syndrome. Hypertension. 2005;46:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Shen J, Lyu L, Wu X, Ji J, Zeng C, Li S, Zhao Y, Xu J, Lin L, Lu C, Mao W, Wei T. Correlation between Renal Artery Anatomy and Hypertension: A Retrospective Analysis of 3000 Patients. Evid Based Complement Alternat Med. 2021;2021:9957361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 17. | Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 750] [Cited by in RCA: 632] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 18. | Iwashima Y, Ishimitsu T. How should we define appropriate patients for percutaneous transluminal renal angioplasty treatment? Hypertens Res. 2020;43:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |