Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1426
Peer-review started: December 18, 2022
First decision: January 3, 2023
Revised: January 7, 2023
Accepted: February 7, 2023
Article in press: February 7, 2023
Published online: February 26, 2023
Processing time: 68 Days and 4.2 Hours
Primary malignant melanoma of the esophagus (PMME) is a rare malignant disease whose clinical and molecular pathological features, origin and pathoge
In this paper, we report a case of a 73-year-old male with PMME. The patient complained of progressive dysphagia accompanied by substantial weight loss. Gastroscopy revealed a purple black bulging-type mass in the lower esophagus with easy bleeding on contact and scattered satellite lesions in the stomach. Histopathological biopsy revealed melanocytes in the esophageal mucosa. Physical examination and multidisciplinary consultation led to diagnostic exclusion of melanoma originating in other organs, such as the skin. Through this case report and literature review, we aimed to describe the clinical and molecular pathological features of PMME and summarize possible pathways of pathoge
PMME is a rare malignancy of the esophagus with a poor prognosis. Clinicians should raise their awareness and be able to identify early lesions.
Core Tip: We report a case of a 73-year-old male with primary malignant melanoma of the esophagus (PMME) and extensive systemic multiple metastases, with an unavoidable mortality outcome despite aggressive diagnosis and treatment. Through this case report and literature review, we aimed to further improve clinicians' understanding of the clinical and molecular pathological features, origin and pathogenesis of PMME, to avoid misdiagnosis and missed diagnoses, to present cutting-edge treatment advances and to develop individualized and comprehensive treatment plans tailored to patients in an effort to improve their clinical outcomes.
- Citation: Wang QQ, Li YM, Qin G, Liu F, Xu YY. Primary malignant melanoma of the esophagus: A case report. World J Clin Cases 2023; 11(6): 1426-1433
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1426.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1426
Malignant melanoma is a malignant tumor with extremely high malignancy and associated with a poor prognosis caused by the malignant transformation of melanocytes distributed in the stroma. It occurs mainly in the skin but also in the adjacent mucous membranes of the skin, such as the nasopharynx, oral cavity, and conjunctiva, and in the gastrointestinal tract, including the rectum and anus. However, malignant melanoma originating in the esophagus is extremely rare[1], and there are only 400 published case reports worldwide[2].
Primary malignant melanoma of the esophagus (PMME) accounts for approximately 0.1%-0.2% of primary esophageal malignancies[3], with an estimated incidence of 0.0036 cases per million/year[2]. It is difficult to diagnosis and is characterized by rapid progression, high recurrence and metastasis rates, and poor prognosis. The rarity of PMME, limited awareness of clinicians, and diverse histomorphology of the disease have been shown to lead to a low presurgical confirmation rate and frequent misdiagnosis as esophageal cancer, hemangioma, lymphoma, hematoma or other disease at initial presentation.
Therefore, there is an urgent need to better understand the characteristics of PMME. We report a case of PMME in a 73-year-old male, along with a literature review of previous cases, to summarize the epidemiological features and clinicopathological characteristics of PMME, explore potential pathogenic mechanisms, and report on cutting-edge advances in biologic therapy.
A 73-year-old Chinese male complained of progressive dysphagia for 7 mo.
Seven months prior, the patient began to have dysphagia with no obvious cause that was initially obvious when eating solid food, which required water intake; his condition worsened, and eventually the patient could eat only liquid food and suffered substantial weight loss of approximately 9 kg; there was no chest pain, nausea, vomiting, vomiting blood, black stool or other discomfort. He then consulted a local hospital, and gastroscopy revealed a purple black mass in the lower part of the esophagus (30-39 cm from the incisor); when touched, red blood flowed from this mass. The local hospital proposed a diagnosis of hematoma or hemangioma, and given the risk of bleeding associated with biopsy, no aggressive biopsy was performed to obtain pathology, and the patient was referred to our hospital for treatment.
The patient had a history of fish spikes that had been stuck in the esophagus 1 year prior, a condition that resolved on its own without endoscopy. Forty years previously, he was diagnosed with tuberculous pleurisy and was cured with standardized antituberculosis treatment for 3 years.
The patient denied any familial history of malignancy.
The admission vital signs were as follows: Body temperature, 36.1 °C; blood pressure, 14.1/9.8 KPa; heart rate, 81 beats per min; and respiratory rate, 21 breaths per min. In addition, the patient was lean with a body mass index of 18.5 kg/m2; there was painless enlargement of axillary and inguinal lymph nodes along with abdominal tenderness unaccompanied by pressure pain or rebound pain; no abnormal masses were palpated. Manual anal examination was not performed.
Hemoglobin levels were normal. Serum tumor markers were normal (including carcinoembryonic antigen, carbohydrate associated antigen 19-9, and alpha-fetoprotein). The fecal occult blood test was negative. The coagulation function D-dimer level was 0.82 μg/mL (reference value less than 0.5 μg/mL). The remaining routine blood and urine analysis showed no abnormalities.
Enhanced computed tomography (CT) of the chest, abdomen and pelvis showed a soft tissue mass in the middle and lower esophagus, suggesting esophageal cancer, and multiple enlarged lymph nodes on the gastric lesser curvature and retroperitoneum. Enhanced CT of the chest showed a clear intrapulmonary artery filling defect, and pulmonary thromboembolism was considered. Upon cranial magnetic resonance imaging (MRI), no important abnormal signal was seen in the brain parenchyma.
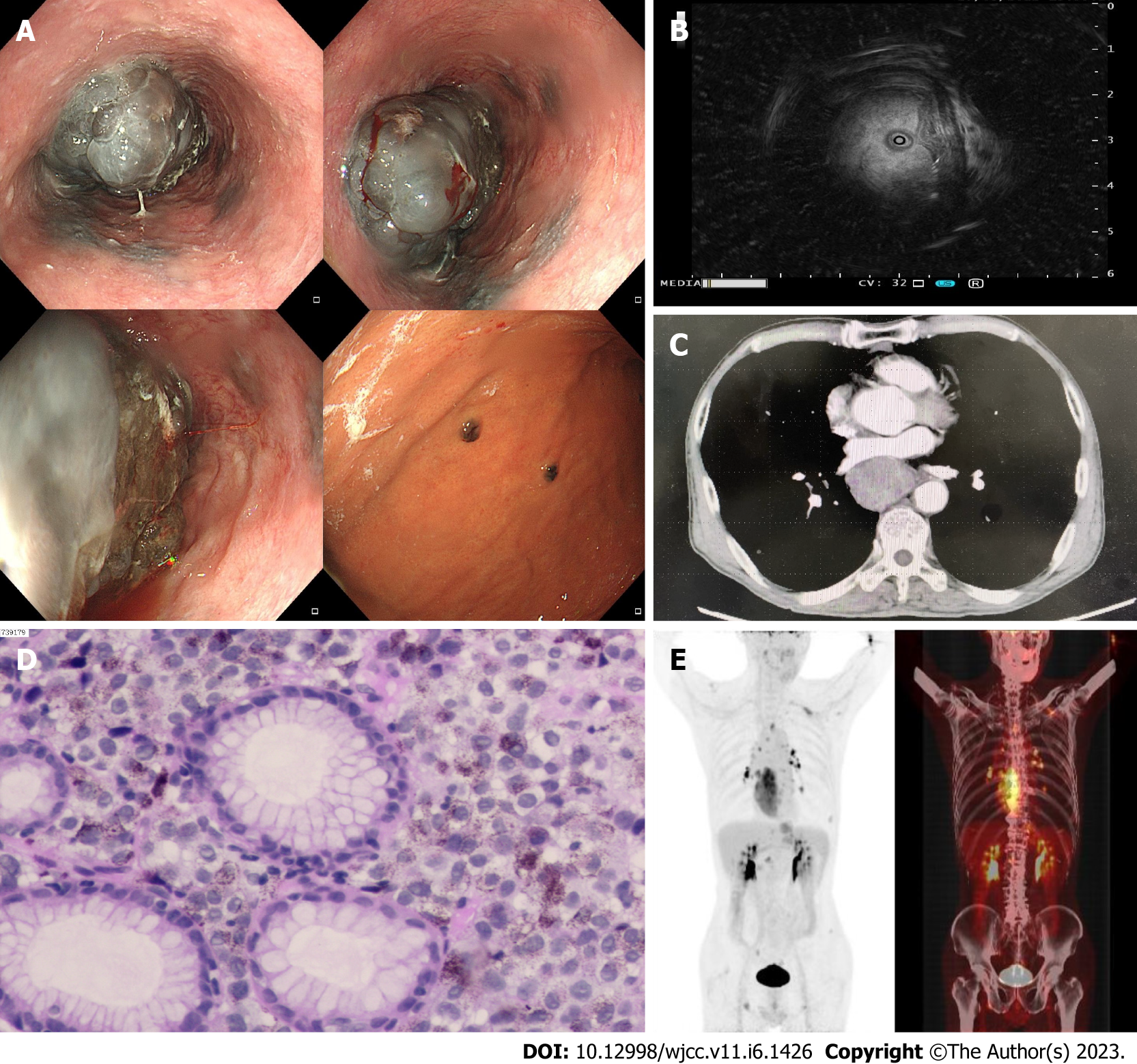
Gastroscopy was repeated and showed a bulging mass in the esophagus (30-40 cm from the incisor) characterized by a blackish color, rough surface, seeming erosion in the middle of the lesion, and easy bleeding on contact; it caused a narrowing of the esophageal lumen, but an endoscope could pass by it. The gastric fundus and mucosa of the gastric body were scattered with multiple flat elevated lesions, approximately 0.4-0.6 cm in size and blackish in color (Figure 1A). Ultrasound endoscopy revealed that the lesion had mixed echogenicity, predominantly hypoechogenicity, and involved the entire wall of the duct, with enlarged lymph nodes visible outside the wall (Figure 1B). Gastroscopy and ultrasound endoscopy further validated the enhanced CT findings (Figure 1C). A diagnosis of malignant melanoma of the esophagus was considered with a high possibility.
Upon histopathological examination, the combined morphological and immunohistochemical findings were consistent with malignant melanoma [immunohistochemistry: S-100 monoclonal (+), human melanoma black antibody (HMB45) (+),melanoma antigen protein (Melan-A) (+), Ki67 (MIB-1) (80% +), Sry-related HMg-Box gene 10 (SOX-10) (+), CK (AE1/AE3) (-)] (Figure 1D).
Whole-body positron emission tomography (PET)-CT revealed hypermetabolic occupancy in the lower and middle esophagus, multiple hypermetabolic lymph nodes throughout the body, multiple hypermetabolic foci in bone, and malignant lesions with multiple metastases in lymph nodes and bone. A ground-glass shadow in the lower lobe of the left lung with mild metabolic increase was seen and cited for close follow-up (Figure 1E).
The patient had a brown swelling of approximately 0.5 cm in diameter behind the right ear, which was diagnosed as seborrheic keratosis on dermoscopy.
Given the patient's medical history and the results of adjuvant examinations, the final diagnosis was PMME with gastric metastasis, lymph node metastasis, bone metastasis, possible pulmonary metastasis, and acute pulmonary thromboembolism (low- to moderate-risk)[4].
Considering the patient's tumor stage and physical status, the oncology specialist was invited to develop a first-line treatment plan: Chemotherapy + immunotherapy, including dacarbazine and programmed death 1 (PD-1) (pablizumab). Considering the patient's risk of bleeding from esophageal tumors, the prescribed anticoagulation regimen was edoxaban 30 mg per day orally for pulmonary thromboembolism. In addition, due to the patient's significant dysphagia and weight loss, we gave him individualized intravenous nutrition infusion, including glucose, essential amino acids, fat emulsion and electrolytes.
Telephone follow-up was performed 3 mo after the diagnosis, and the patient had died.
In this paper, we report a rare case of PMME that included extensive systemic metastases and concomitant tumor-associated pulmonary thromboembolism and resulted in patient death as the final outcome. PMME is very rare and accounts for approximately 0.1%-0.2% of primary esophageal malignancies[5,6]. It is mostly seen in elderly people approximately 70 years of age and is more common in men than in women, with a male to female ratio of approximately 2:1[7]. It is primarily found in the lower and middle esophagus (> 90% of cases), with the lowest incidence in the upper segment[2,8]. The clinical manifestations are atypical, do not attract sufficient attention and vigilance, and may include progressive dysphagia, retrosternal pain, and weight loss[9,10], with a small number of cases being identified incidentally on physical examination[11]. PMME is highly aggressive and progresses rapidly clinically, with distant metastases observed at the time of diagnosis in 18.4% of patients[6] and lymph node metastases occurring most frequently, followed by liver, lung, and brain metastases[5]. According to previous studies, the median survival time of PMME patients is only 10-20 mo, with an overall 5-year survival rate of 5%[2] to 20%[3]. Early and accurate diagnosis and individualized comprehensive treatment may prolong the survival time of PMME patients.
Early and accurate diagnosis of PMME relies on imaging examinations such as endoscopy, ultrasound endoscopy, CT, MRI, and PET-CT, but histopathological examination remains the gold standard for confirming the diagnosis. Possible findings are summarized in the following list according to examination modality. Endoscopic findings are as follows: Wide basal polyp-like, myxoid mass with black, brown, gray or dark brown surface due to different degrees of pigmentation; some lesions may have ulcers on the surface and bleed easily when touched; most lesions are solitary, a few are multiple, and surrounding satellite lesions may also be seen. Ultrasound endoscopic findings are as follows: Mainly originating from the mucosal layer; showing a heterogeneous hypoechoic shadow. Histopathological findings are as follows: Gross pathology may show polypoid, myxoid, or nodular elevations with or without melanin deposition. Routine hematoxylin and eosin staining can reveal melanin granules; microscopically, tumor cells infiltrate to varying depths and can infiltrate the plasma membrane with nested clusters, sheets, epithelial-like distribution, and rich interstitial vasculature; tumor cell infiltration can be accompanied by varying degrees of lymphocytic infiltration; melanocytes vary in size and volume and are round, polygonal, or irregular in shape, with large nuclei, partially visible eosinophilic nucleoli, easily seen pathological nuclear divisions, and abundant cytoplasm[12]. Immunohistochemical findings are as follows: Positive expression of proteins such as HMB45, Melan-A, S-100, vimentin, and SOX-10 but not epithelial tumor markers, myogenic markers or lymphoma markers, etc.[10].
Among them, HMB45 and Melan-A have shown better specificity[13,14]. Therefore, the Allen-Spitz diagnostic criteria are currently the more accepted diagnostic criteria for PMME[2], which include: (1) Tumors originating from areas of junctional changes within the esophageal squamous epithelium; (2) histology with typical melanoma structure and the presence of melanin granules within the tumor cells; (3) positive immunohistochemical staining for HMB45 and/or S-100; and (4) exclusion of metastasis of primary lesions in melanoma-prone sites such as skin, eye, anus, and rectum.
Patients with PMME have a short survival time and high mortality rate, and the exploration of their treatment options has never stopped. Scholars worldwide have not reached a consensus regarding PMME treatment options, which are mainly derived from treatment experience with cutaneous malignant melanoma. It is currently believed that radical surgical excision remains the treatment of choice for patients with early operable PMME. However, one study found multiple postoperative tumor recurrences despite radical esophagectomy, and further analysis of driver gene heterogeneity and clonality revealed recurrent subclonal drivers originating from the initial tumor, suggesting that early spread already existed beyond the scope of surgical resection[6]. This finding needs to be validated by additional studies and replication in patients with different tumor stages. If migration and implantation of subclones is confirmed to have occurred at an early stage of the tumor, in addition to early diagnosis and early surgical resection, more aggressive adjuvant therapy will be required postoperatively. Postoperative adjuvant therapy can be considered a multidisciplinary combination of radiotherapy, chemotherapy, targeted therapy, immunotherapy, etc. However, there is a wide divergence in the findings of previous studies as to whether combination therapy will considerably prolong the survival of patients. A large retrospective study in China showed that radiotherapy was an independent influencing factor on the overall survival of PMME patients, and the longest overall survival time among patients who received radiotherapy was up to 8.4 years, suggesting that individualized radiotherapy may be a key direction for future study regarding PMME treatment. In contrast, some studies have shown that radiotherapy and chemotherapy have limited therapeutic effects on PMME patients and do not prolong their survival time[2,15].
In recent years, the widespread use of molecular biology technologies such as second-generation gene sequencing has made it possible to explore PMME at the level of gene mutations and to develop individualized targeted therapies and immunotherapy[1,3]. For example, Li et al[16] found genetic mutations in the MAPK signaling pathway in 55% of PMME samples, suggesting that MEK/MAPK inhibitors may be suitable for the treatment of some PMME patients and help improve their clinical outcomes[16]. In addition, immunotherapy is an emerging area of research in the treatment of PMME[3,17]. PD-1 inhibitors such as pablizumab and nabritumomab and cytotoxic T-cell-associated antigen 4 inhibitors such as ipilimumab have been shown to significantly prolong overall survival in metastatic cutaneous melanoma when combined[2]. Indeed, immune checkpoint inhibitors are currently po
The origin of PMME has been the focus of debate, and its pathogenic mechanism is still unclear. The following hypotheses have been proposed: (1) PMME originates from melanocytes in the basal layer of the esophageal mucosal epithelium, and injury factors and overstimulation of growth factors produced by local cells in the mucosa contribute to the development of PMME; (2) in previous reports, melanocytes were not observed microscopically in some patients, whereas black granules were visible in the adjacent squamous epithelium of nonpigmented melanoma tissue, and melanin markers were positively expressed on the surface of the black granules, thus presumably suggesting an associated pathogenesis that involves black granules near highly proliferating malignant melanoma tissue in normal squamous epithelium[20]. It has also been shown that both pigmented and nonpigmented malignant melanomas are derived from black granules in adjacent squamous epithelium; (3) in addition, it has been proposed that PMME originates from ectodermal neural crest melanocytes and that neural crest melanocytes migrate or become disoriented during embryonic development and remain in the esophageal epithelium, leading to PMME; (4) molecular biology has provided some clues to the occurrence and progression of PMME[3,16]. In one study, whole-exome testing of primary tumor, recurrent tumor, and normal control tissues from the same PMME patient revealed ARHGAP35 gene mutations throughout tumorigenesis and recurrence and indicated that ARHGAP35 gene mutations play an important role in promoting tumor recurrence and metastasis as well as the immunosuppressive microenvironment[6]; and (5) in addition, a series of driver gene mutations, such as BRAF, NRAS, PTEN, and TP53, have been observed in PMME samples with a high degree of microsatellite instability, suggesting the involvement of the MEK/MAPK signaling pathway in PMME development[1,2,16,21]. The findings of another study are consistent with this finding, showing a high degree of genetic instability and intratumoral heterogeneity in PMME samples and showing from gene clustering analysis that MAPK, transforming growth factor-β and other signaling pathways are involved in the progression of PMME[6]. However, a study in which the genetic profiles of 20 PMME patients were analyzed did not find mutations in genes common to melanoma, such as BRAF and TP53[9]. Therefore, the molecular genetic profile of PMME still needs to be analyzed and validated with a larger sample size. In addition to the question of pathogenic mechanism, the metastatic pathway of PMME is also an aspect we need to consider. The metastatic pathways of malignant tumors include direct infiltration, lymphatic metastasis and hematogenous metastasis. However, some patients with PMME have double or multiple primary tumors (approximately 13%)[2], and endoscopic examination mostly shows satellite foci in the stomach and duodenum, which does not exclude the presence of very early tumor cell migration and implantation[6], submucosal dissemination[1], longitudinal spread[22], and tumors with multifocal origin[23].
Compared with previous case reports, our study also has strengths and weaknesses. While previous reports focused on the clinicopathological features of PMME and tumor infiltration and metastasis, our study comprehensively addressed multiple aspects of pathogenesis, early diagnosis, and cutting-edge therapeutic advances in addition to the clinicopathological features and malignant biological behavior of the tumor. Moreover, while previous studies mentioned complications such as pericardial effusion and third-degree atrioventricular block, our study highlights for the first time that pulmonary thromboembolism, a tumor-associated complication, can increase the complexity of the patient's condition and limit more aggressive comprehensive treatment, advocating that clinicians should also enhance the diagnosis and management of tumor-associated complications. The shortcoming of our study is that the patient in our report declined molecular tests such as BRAF and NRAS due to economic factors, and the presence of meaningful driver mutations could not be clarified, creating a certain obstacle to the development of individualized targeted and immunotherapy regimens. Unfortunately, the patient experienced a fatal outcome.
PMME is a rare nonepithelium-derived malignancy of the esophagus with poor prognosis. Due to the atypical clinical presentation and diverse histomorphology, the accuracy of preoperative diagnosis is low. Clinicians and endoscopists should be aware of rare esophageal diseases, increase their vigilance regarding these disease, and identify early lesions in a timely manner. Standardized surgical resection combined with adjuvant therapy is still the mainstream treatment pathway for PMME, and more promising therapeutic targets, radiotherapy and immunotherapy options need to be explored in the future to improve the prognosis of PMME patients.
All authors thank the patient and his family for valuable support.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferreira GSA, Brazil; Scriba MF, South Africa S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Li J, Yan S, Liu Z, Zhou Y, Pan Y, Yuan W, Liu M, Tan Q, Tian G, Dong B, Cai H, Wu N, Ke Y. Multiregional Sequencing Reveals Genomic Alterations and Clonal Dynamics in Primary Malignant Melanoma of the Esophagus. Cancer Res. 2018;78:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 2. | Cazzato G, Cascardi E, Colagrande A, Lettini T, Resta L, Bizzoca C, Arezzo F, Loizzi V, Dellino M, Cormio G, Casatta N, Lupo C, Scillimati A, Scacco S, Parente P, Lospalluti L, Ingravallo G. The Thousand Faces of Malignant Melanoma: A Systematic Review of the Primary Malignant Melanoma of the Esophagus. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 3. | Williams E, Bolger JC, Darling G. Radical Resection in an Era of Immune Therapy for Primary Esophageal Melanoma. Ann Thorac Surg. 2022;114:e423-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | Konstantinides SV, Meyer G, Becattini C, Bueno H, Geersing GJ, Harjola VP, Huisman MV, Humbert M, Jennings CS, Jiménez D, Kucher N, Lang IM, Lankeit M, Lorusso R, Mazzolai L, Meneveau N, Áinle FN, Prandoni P, Pruszczyk P, Righini M, Torbicki A, Van Belle E, Zamorano JL; The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS): The Task Force for the diagnosis and management of acute pulmonary embolism of the European Society of Cardiology (ESC). Eur Respir J. 2019;54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 816] [Article Influence: 136.0] [Reference Citation Analysis (0)] |
| 5. | Sun H, Gong L, Zhao G, Zhan H, Meng B, Yu Z, Pan Z. Clinicopathological characteristics, staging classification, and survival outcomes of primary malignant melanoma of the esophagus. J Surg Oncol. 2018;117:588-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Li J, Guan W, Ren W, Liu Z, Wu H, Chen Y, Liu S, Quan X, Yang Z, Jiang C, He J, Xiao X, Ye Q. Longitudinal genomic alternations and clonal dynamics analysis of primary malignant melanoma of the esophagus. Neoplasia. 2022;30:100811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Wallis G, Sehgal V, Haider A, Bridgewater J, Novelli M, Dawas K, Haidry R. Primary malignant melanoma of the esophagus. Endoscopy. 2015;47 Suppl 1 UCTN:E81-E82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Choudhary NS, Puri R, Goel R, Sud R. Primary malignant melanoma involving the whole esophagus: a rare case with rarer presentation. Endoscopy. 2014;46 Suppl 1 UCTN:E621-E622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Lasota J, Kowalik A, Felisiak-Golabek A, Zięba S, Waloszczyk P, Masiuk M, Wejman J, Szumilo J, Miettinen M. Primary malignant melanoma of esophagus: clinicopathologic characterization of 20 cases including molecular genetic profiling of 15 tumors. Mod Pathol. 2019;32:957-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 10. | Hashimoto T, Makino T, Yamasaki M, Tanaka K, Miyazaki Y, Takahashi T, Kurokawa Y, Motoori M, Kimura Y, Nakajima K, Morii E, Mori M, Doki Y. Clinicopathological characteristics and survival of primary malignant melanoma of the esophagus. Oncol Lett. 2019;18:1872-1880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Fukunaga H, Kaneda H, Kumakawa H, Takahashi Y. Asymptomatic Primary Malignant Melanoma of the Gastroesophageal Junction. Intern Med. 2016;55:709-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Iwanuma Y, Tomita N, Amano T, Isayama F, Tsurumaru M, Hayashi T, Kajiyama Y. Current status of primary malignant melanoma of the esophagus: clinical features, pathology, management and prognosis. J Gastroenterol. 2012;47:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 13. | Cheng L, Guo ZY, Lei L, Wang WX, Xu CW, Fang MY. Treatment and prognosis of primary malignant melanoma of the esophagus. Transl Cancer Res. 2020;9:4141-4147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Dai L, Wang ZM, Xue ZQ, He M, Yuan Y, Shang XQ, Chen KN; Chinese Cooperative Primary Malignant Melanoma of the Esophagus Group (CCPMMEG). Results of surgical treatment for primary malignant melanoma of the esophagus: A multicenter retrospective study. J Thorac Cardiovasc Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Imai S, Suzuki A, Yamamoto Y, Koyama M, Sugiyama S, Kitazawa M, Miyagawa Y, Miyagawa S. Primary malignant melanoma of esophagus following chemoradiotherapy for esophageal squamous cell carcinoma: report of a case. Clin J Gastroenterol. 2017;10:336-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Li J, Liu B, Ye Q, Xiao X, Yan S, Guan W, He L, Wang C, Yu Z, Tai Z, Pei S, Ma Y, Li S, Wang Y, Wu N. Comprehensive genomic analysis of primary malignant melanoma of the esophagus reveals similar genetic patterns compared with epithelium-associated melanomas. Mod Pathol. 2022;35:1596-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Chacón M, Pfluger Y, Angel M, Waisberg F, Enrico D. Uncommon Subtypes of Malignant Melanomas: A Review Based on Clinical and Molecular Perspectives. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 18. | Board R, Smittenaar R, Lawton S, Liu H, Juwa B, Chao D, Corrie P. Metastatic melanoma patient outcomes since introduction of immune checkpoint inhibitors in England between 2014 and 2018. Int J Cancer. 2021;148:868-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Wang X, Kong Y, Chi Z, Sheng X, Cui C, Mao L, Lian B, Tang B, Yan X, Si L, Guo J. Primary malignant melanoma of the esophagus: A retrospective analysis of clinical features, management, and survival of 76 patients. Thorac Cancer. 2019;10:950-956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Ueyama H, Yao T, Matsumoto K, Nakagawa Y, Takeda T, Nagahara A, Watanabe S. Flat-type primary malignant melanoma of the esophagus. Endosc Int Open. 2016;4:E687-E689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Tsuyama S, Kohsaka S, Hayashi T, Suehara Y, Hashimoto T, Kajiyama Y, Tsurumaru M, Ueno T, Mano H, Yao T, Saito T. Comprehensive clinicopathological and molecular analysis of primary malignant melanoma of the oesophagus. Histopathology. 2021;78:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Schizas D, Mylonas KS, Bagias G, Mastoraki A, Ioannidi M, Kanavidis P, Hasemaki N, Karavokyros I, Theodorou D, Liakakos T. Esophageal melanoma: a systematic review and exploratory recurrence and survival analysis. Dis Esophagus. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Zhou YB, Yuan Y, Hu B, Che GW. Image of the Month: Primary Multifocal Malignant Melanoma of Esophagus Co-Occurs With Esophagogastric Junction Adenocarcinoma. Am J Gastroenterol. 2016;111:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |