Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1349
Peer-review started: October 5, 2022
First decision: November 22, 2022
Revised: December 17, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: February 26, 2023
Processing time: 142 Days and 6.7 Hours
The aim of the present study was to enhance understanding of the diagnosis and treatment of atypical hereditary spherocytosis (HS), and to broaden the diagnostic thoughts of physicians for patients with jaundice.
A 28-year-old male presented with jaundice, bile duct stone, and splenomegaly, but without anemia. Other causes of jaundice were excluded, and gene se
Following a definitive diagnosis, genetic testing and response to treatment identified a gene variant site for a novel hemolytic anemia.
Core Tip: A novel mutation in the SPTB gene was identified in a patient with hemolytic anemia, which caused the patient to present with extremely high jaundice without obvious hemolysis. At the same time, there was no similar mutation in the patient’s family. Because medical treatment was ineffective, we finally performed splenectomy after communicating with the patient. After splenectomy, the patient’s liver function recovered. The patient’s liver function continued to be normal during follow-up.
- Citation: Jiang N, Mao WY, Peng BX, Yang TY, Mao XR. Clinical manifestations of adult hereditary spherocytosis with novel SPTB gene mutations and hyperjaundice: A case report. World J Clin Cases 2023; 11(6): 1349-1355
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1349.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1349
Hereditary spherocytosis (HS) is a type of hemolytic anemia caused by congenital membrane defects of red blood cells. HS is an autosomal dominant inheritance disease that predominantly affects infants and children[1]. Here, a case of adult HS with a novel heterozygous variant of the SPTB gene (c.1801C>T) is reported. The patient had severe jaundice [total bilirubin (TBIL): 1625 μmol/L] without anemia. The final diagnosis was confirmed based on clinical observation, gene sequencing, and response to treatment and is summarized below.
A 28-year-old male was admitted to the First Hospital of Lanzhou University due to jaundice and stomachache on March 8, 2021.
The patient experienced intermittent right upper abdominal distension and pain after eating greasy food for 6 mo before admission, which could be relieved by taking medicine. Symptoms worsened 5 d before admission, including abdominal pain and white clay stools. The local hospital checked for elevated BIL, and an abdominal magnetic resonance imaging (MRI) showed cholecystolithiasis and bile duct dilation. Consequently, the patient was transferred to our hospital. The diagnosis was biliary calculi; therefore, endoscopic retrograde cholangiopancreatography with sphincterotomy + balloon exploration and lithotomy + biliary stent angioplasty was performed. Postoperative BIL remained high, so the patient was transferred to the Department of Infectious Diseases for further diagnosis and treatment.
The patient had no history of past illness.
The patient was a cook without any notable unhealthy habits. He was not exposed to drugs or poisons and did not smoke or drink alcohol. The patient and his family members had no specific history of genetic diseases.
Body mass index 22.13 kg/m2, waist circumference 90 cm and hip circumference 93 cm, severe yellow staining of skin and mucous membranes, abdominal tenderness, Murphy’s sign positive, liver not palpable and spleen palpable three fingers under the costal.
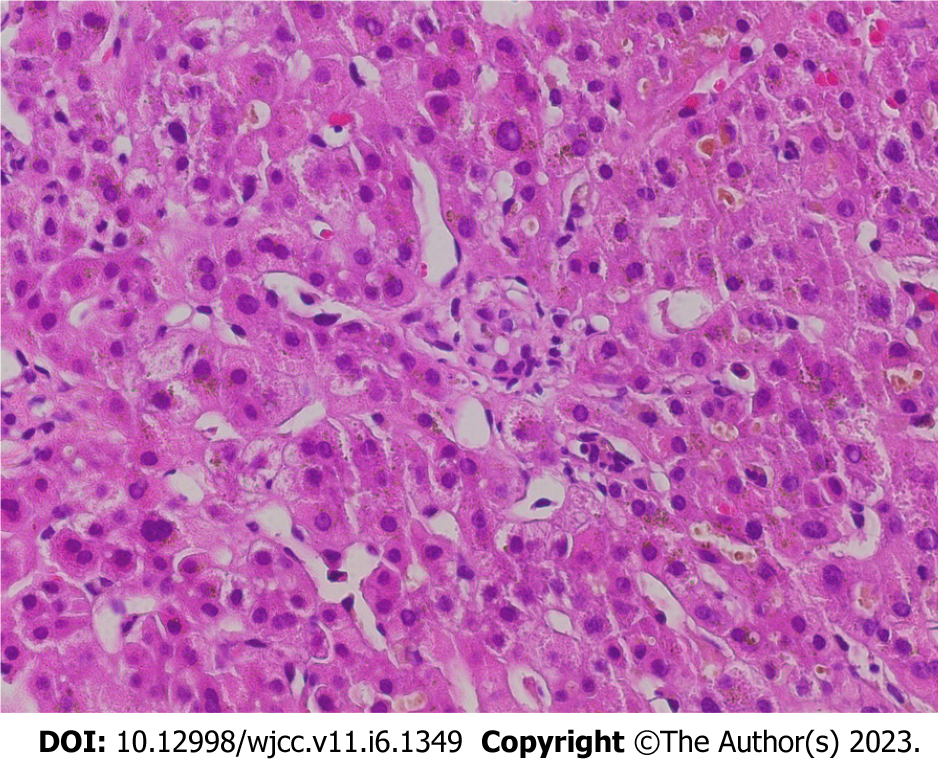
Hemoglobin (Hb) was 110 g/L, aspartate aminotransferase was 34 U/L, alanine aminotransferase was 70 U/L, TBIL was 1618 μmol/L, direct BIL (DBIL) was 998 μmol/L, and indirect BIL (IBIL) was 828 μmol/L. The cause of jaundice was actively investigated. The results of the infection index (hepatitis virus, non-heparophilic viruses, bacteria, fungi, and so on), immunological indicators, and iron and copper metabolism index were not obviously abnormal. Drugs, alcohol, parasites, and other factors were also excluded. Liver biopsy showed hepatic lobule structural disorder, widened portal area, fibrous tissue hyperplasia, infiltration of inflammatory cells dominated by lymphocytes, bile duct hyperplasia, punctate necrosis of some hepatocytes, and biliary pigment deposition in the cytoplasm of some cells. Immunohistochemical and specific staining results included net staining (indicating fibrosis in portal area), CK19 (small bile ducts 2+), CD45 (inflammatory cells 2+), CD38 (individual cells 1+), ki67 (18%), GPC -3(-), Masson’s trichrome stain (fibrosis in portal area), and CD34 (vascular 2+). The conclusion from the liver biopsy and staining was that the morphology was consistent with chronic inflammation with liver cholestasis and a tendency of liver cirrhosis (G2S2) (as shown in Figure 1).
In terms of hemolytic jaundice, the blood smear showed that the size of red blood cells was slightly different from normal, the filling was acceptable, no abnormal red blood cells were detected, and platelets were scattered and clustered; 39.0% of the blood cells were erythroid, predominantly middle and late immature erythrocytes, with no abnormal morphology, polychromatic erythrocytes were clearly visible and were basically the same size as mature erythrocytes, the filling was acceptable. These findings suggested that a diagnosis of proliferative anemia should be considered. Anemia screening assays (direct and indirect anti-human globules, anti-alkali Hb assay, Hb A2 assay, micro Hb electrophoresis, isopropanol, methemoglobin reduction test, G-6-PD fluorescent spot test, denatured globin body, H Inclusion bodies, and red blood cell osmotic fragility test) were negative. Bone marrow puncture considered hyperplastic anemia.
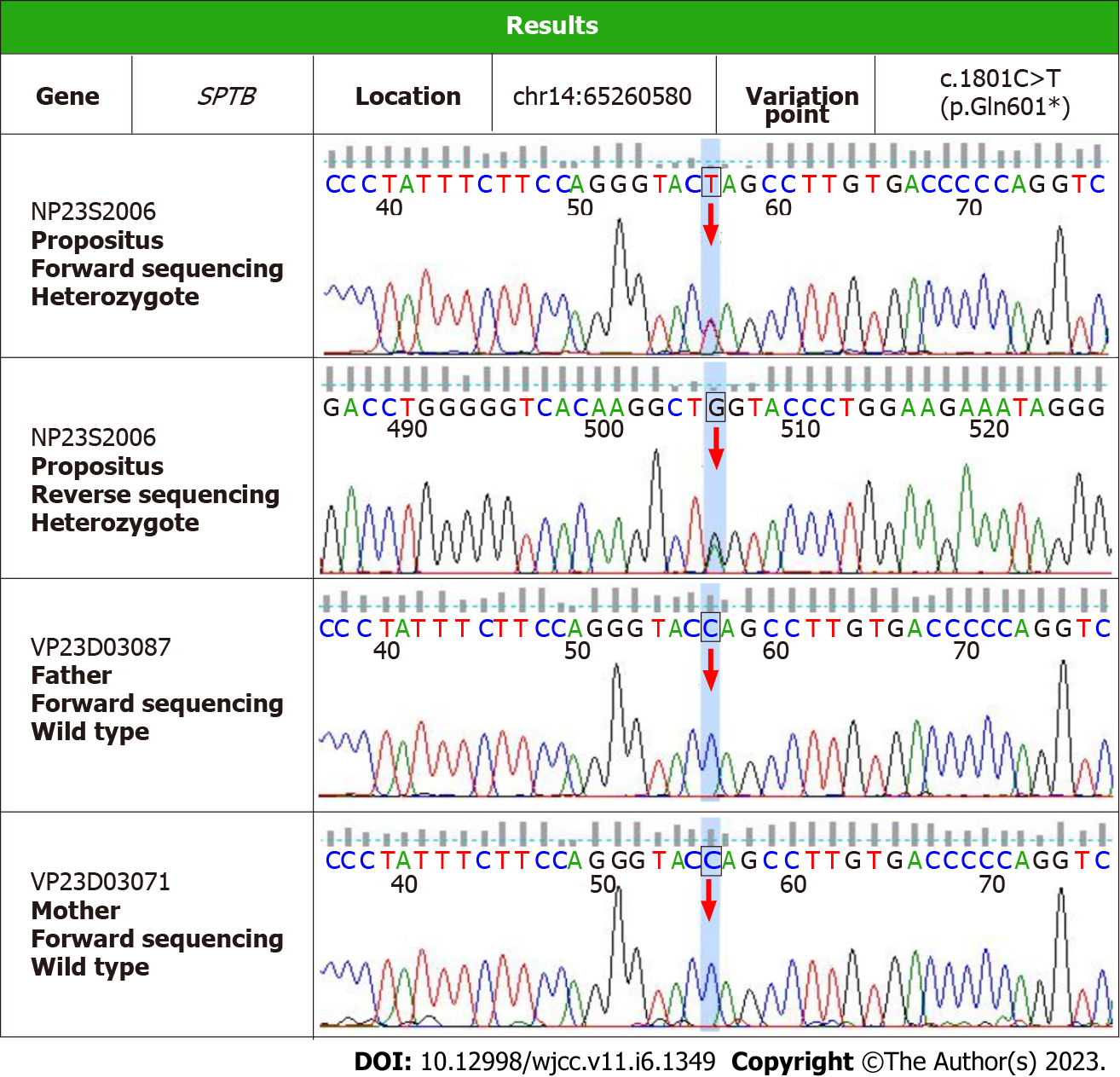
The patient was screened for hereditary liver disease. The detection was performed on an Illumina sequencing platform and the GATK software suite was used for sequencing data analysis. High-throughput sequencing of a liver panel showed that on chromosome 14 (chr14: 65260580), the SPTB gene in exon 14 had a mutation of cytosine to thymine at nucleotide 1801, resulting in a glutamine nonsense mutation at amino acid 601 to a stop codon [NM_001355436: exon14: c.1801C>T (p.Q601X)]. The variant that was identified in the high-throughput sequencing was validated by Sanger sequencing. This variant was suspected to cause HS. Parental genetic testing indicated that the variant was not found in the father or mother; in addition, the variant was not detected in the patient’s daughter (Figure 2).
The spleen (length × thickness) was 192 mm × 63 mm. Abdominal MRI + magnetic resonance cholangiopancreatography + diffusion-weighted imaging and abdominal CTA were not obviously abnormal.
The patient was diagnosed with HS.
While the cause of jaundice was investigated in the patient, he was given treatment for liver protection, BIL regression, and symptoms. As the treatment progressed, the liver function indexes of the patient improved; TBIL fluctuated between 250 and 400 μmol/L, DBIL was 140-300 μmol/L, and IBIL was 120-200 μmol/L. The spleen was progressively reduced to 167 mm × 60 mm. However, considering that the patient still had anemia and abnormal liver function, combined with the patient’s genetic report and the trend of fibrosis in the liver, to make a clear diagnosis and effectively control the anemia and jaundice and combined with the wishes of the patient and his family, a splenectomy was performed on December 31, 2021. Intraoperative pathological examination showed spleen capsule thickening, splenic corpuscle atrophy, splenic sinus dilatation, and congestion.
Three days after surgery, the patient’s BIL rapidly decreased to nearly normal levels. The patient’s clinical and laboratory tests continue to be good (Table 1). Therefore, the patient was diagnosed with HS, and we believe that the CHR14:65260580 SPTB gene NM_001355436: exon14: c.1801C>T (p.Q601X) variant is the pathogenic gene of HS, which has not been reported previously in the literature.
| Indicator | Before ERCP | After ERCP | During medication | Before removal of biliary stent | After removal of biliary stent | Before splenectomy | After splenectomy | Recent results |
| TBIL (μmol/L) | 1734.7 | 1618.1 | 442.9 | 83.5 | 96.4 | 105.1 | 31.5 | 22.5 |
| DBIL (μmol/L) | 1146.4 | 998.0 | 316.7 | 13.0 | 14.8 | 14.9 | 7.8 | 5.5 |
| IBIL (μmol/L) | 588.3 | 828.5 | 126.2 | 70.5 | 81.6 | 90.2 | 23.7 | 17.0 |
| Hb (g/L) | 122 | 110 | 94 | 76 | 72 | 109 | 122 | 139 |
| MCH (pg) | 33.1 | 32.1 | 34.5 | 32 | 35.0 | 32.0 | 31 | |
| RDW-SD (fL) | 66.2 | 66.4 | 51.9 | 64.4 | 57.2 | 58.6 | 55.3 | 52.7 |
| RET# (× 1012/L) | 0.235 | 0.254 | 0.260 | 0.368 | 0.249 | 0.231 | 0.026 | 0.043 |
| AST (U/L) | 37 | 34 | 34 | 14 | 12 | 31 | 47 | 22.5 |
| ALT (U/L) | 103 | 70 | 70 | 18 | 12 | 60 | 54 | 32.3 |
| ALP (U/L) | 359 | 299.9 | 299.9 | 102.0 | 105.0 | 169.4 | 171.7 | 214.5 |
| GGT (U/L) | 264.6 | 154.2 | 154.2 | 29.0 | 26.7 | 72.1 | 176.9 | 40.2 |
| ALB (g/L) | 43.2 | 39.0 | 39 | 45.7 | 71.7 | 57.1 | 47.0 | 50.1 |
| Crea (μmol/L) | 61 | 91.3 | 91.3 | 109.6 | 107.5 | 108.6 | 93.9 | 81.8 |
| UA (μmol/L) | 88 | 64 | 64 | 596 | 649 | 620 | 435 | 447 |
| TG (mmol/L) | 1.96 | 2.76 | 2.76 | 0.95 | 1.04 | 1.85 | 1.50 | 1.75 |
| LDL-C (mmol/L) | 1.91 | 1.83 | 1.83 | 0.94 | 1.16 | 1.21 | 2.21 | 2.18 |
HS is a rare genetic disorder with a global distribution but is the most common cause of hemolytic anemia caused by erythrocyte membrane defects. The incidence of HS in Northern Europe and North America is 1/5000 and 1/2000, respectively, while the predicted incidence of HS in China is (1.27-1.49)/100000[2].
The main pathogenesis of HS is the abnormality of the protein encoding the bilayer between the inner membrane skeleton and the outer lipid of erythrocytes, which leads to a decrease in the stability of the erythrocyte membrane. These defects damage the elasticity of erythrocytes so that they become spherical, and they can be destroyed by the spleen, resulting in hemolytic anemia. Simultaneously, erythrocytes are exposed to the adverse environment of a large spleen, including acidification and oxidation conditions, which aggravate hemolysis[3].
There is an obvious family history of HS and there are five common gene abnormalities that are known to cause HS: SPTA1 gene encoding α spectrin, SPTB gene encoding β spectrin, ANK1 gene encoding ankyrin, SLC4A1 gene encoding band 3 protein, and EPB42 gene encoding protein 4.2. HS mainly occurs through autosomal dominant inheritance, accounting for 75% of cases; the remaining cases are due to autosomal recessive inheritance, among which the SPTA1 and EPB42 genes are more common. New variants have also been reported, but their relative frequency has not yet been determined[4].
The clinical characteristics of HS include: (1) It can appear at any age, the onset time is usually early, and some patients have a positive family history of HS; (2) There are various clinical manifestations and severity. The main manifestations of patients with moderate and severe HS are anemia, jaundice, spleen enlargement, acute hemolysis, and biliary calculi. Once obstructive jaundice occurs, temporary liver obstruction can lead to dyslipidemia, the surface area of the erythrocyte membrane increases, and hemolysis can be alleviated in a short time; and (3) The anti-human globulin test is negative in HS patients. Most patients with HS have high mean Hb levels and spherical erythrocytes on peripheral blood smears[5]. Genetic testing has marked advantages over biochemistry and cell morphology in the diagnosis of HS, especially when the clinical symptoms are atypical, the family history is negative, or routine laboratory tests cannot confirm the diagnosis[6].
In the current study, the patient was monitored for mutation of cytosine to thymine at nucleotide 1801 of exon 14 of the SPTB gene on chromosome 14. The SPTB gene has a crucial role in maintaining cell membrane organization and stability and is a major component of the cytoskeletal network beneath the red blood cell plasma membrane. The spectrin protein encoded by SPTB interacts with other proteins through a specific binding domain to maintain the biconcave shape of human erythrocytes[7]. Variants of this gene may cause red blood cells to fail to form their normal shape, leading to spherocytosis and elliptocytosis[8].
HS is treated to reduce hemolysis and anemia, and other complications. The decision to perform a splenectomy should consider the severity of hemolysis, the patient’s age, and surgical complications[9]. Splenectomy is feasible in the following cases: (1) Children over 6 years old who have transfusion dependence, severe anemia, or related severe symptoms; (2) Patients with severe hemolysis and/or severe symptoms (e.g., abdominal symptoms related to splenomegaly, discomfort related to jaundice), or delayed growth or extramedullary hematopoietic; and (3) When HS patients have severe and recurrent cholelithiasis (splenectomy can be used to reduce cholelithiasis). Some studies suggest that splenectomy and cholecystectomy can significantly improve the quality of life and prolong the survival time for HS patients with cholelithiasis[10]. After splenectomy in patients with HS, Hb and serum BIL levels can return to normal within a few days. Splenectomy is reported to be more effective in reducing hemolysis in HS patients with variants of SPTA1 and SPTB genes compared with other genes. This may be due to the varying degrees of reticuloendothelial clearance in the blood machinery of different individuals, related to the opsonization (natural antibody binding) of blood protein/protein albumin/protein monomeric erythrocytes[10].
The patient in the current study had no family history of HS and had acute onset as an adult. The patient’s clinical symptoms were severe jaundice and gallstones. However, when the stones were removed, the jaundice did not improve. In the early stage of the disease, the patient’s Hb was normal, the leucocytic screening was negative, and there were no spherical red blood cells on the peripheral blood smear. However, during the course of the disease, there were sufficient hematopoietic raw materials, no obvious blood loss, and Hb was repeatedly reduced; consequently, hemolytic disease could not be excluded. Full-exome sequencing for genetic diseases was conducted in the patient and indicated that SPTB gene NM_001355436: exon14: c.1801C>T (p.Q601X) of CHR14:65260580 was suspected as a pathogenic gene. The proband’s parents and daughter did not have this gene abnormality. The patient’s postoperative indicators rapidly returned to normal after splenectomy. Therefore, combined with the above characteristics, it is considered that the modified gene locus is mutated into a HS pathogenic gene. Furthermore, there are no previous case reports of this variant.
In conclusion, when HS is considered in patients without typical clinical symptoms, molecular detection of genes can be used to facilitate diagnosis, and clinical characteristics and response to treatment can be combined to clarify diagnosis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Naganathbabu O, India; VV R, India S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Manciu S, Matei E, Trandafir B. Hereditary Spherocytosis - Diagnosis, Surgical Treatment and Outcomes. A Literature Review. Chirurgia (Bucur). 2017;112:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 2. | Zheng LP, Bai LH, Huang H, Yi Y. [Progress on Laboratory Diagnosis of Hereditary Spherocytosis--Review]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2020;28:704-707. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Li H, Lu L, Li X, Buffet PA, Dao M, Karniadakis GE, Suresh S. Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders. Proc Natl Acad Sci U S A. 2018;115:9574-9579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | He BJ, Liao L, Deng ZF, Tao YF, Xu YC, Lin FQ. Molecular Genetic Mechanisms of Hereditary Spherocytosis: Current Perspectives. Acta Haematol. 2018;139:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | Sun XJ, Li HY, Li DP, Liu YZ, Zhang JY, Yin YK, Su MH, Pan H, Li QL, Hu B, Liu H, Shi J. [Clinical manifestations of erythrocyte membrane protein coding gene mutations in hereditary spherocytosis]. Zhonghua Xue Ye Xue Za Zhi. 2018;39:912-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Russo R, Andolfo I, Manna F, Gambale A, Marra R, Rosato BE, Caforio P, Pinto V, Pignataro P, Radhakrishnan K, Unal S, Tomaiuolo G, Forni GL, Iolascon A. Multi-gene panel testing improves diagnosis and management of patients with hereditary anemias. Am J Hematol. 2018;93:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 7. | Maddala R, Walters M, Brophy PJ, Bennett V, Rao PV. Ankyrin-B directs membrane tethering of periaxin and is required for maintenance of lens fiber cell hexagonal shape and mechanics. Am J Physiol Cell Physiol. 2016;310:C115-C126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 8. | Meng LL, Yuan SM, Tu CF, Lin G, Lu GX, Tan YQ. Next-generation sequencing identified a novel SPTB frameshift insertion causing hereditary spherocytosis in China. Ann Hematol. 2019;98:223-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Iolascon A, Andolfo I, Barcellini W, Corcione F, Garçon L, De Franceschi L, Pignata C, Graziadei G, Pospisilova D, Rees DC, de Montalembert M, Rivella S, Gambale A, Russo R, Ribeiro L, Vives-Corrons J, Martinez PA, Kattamis A, Gulbis B, Cappellini MD, Roberts I, Tamary H; Working Study Group on Red Cells and Iron of the EHA. Recommendations regarding splenectomy in hereditary hemolytic anemias. Haematologica. 2017;102:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Pincez T, Guitton C, Gauthier F, de Lambert G, Picard V, Fénéant-Thibault M, Turhan A, Mohandas N, Tchernia G, Garçon L. Long-term follow-up of subtotal splenectomy for hereditary spherocytosis: a single-center study. Blood. 2016;127:1616-1618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |