Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1341
Peer-review started: November 26, 2022
First decision: December 19, 2022
Revised: December 28, 2022
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: February 26, 2023
Processing time: 89 Days and 15.8 Hours
Functioning gonadotroph adenomas are extremely rare pituitary tumors that secrete gonadotropins and exhibit distinct clinical manifestations. Here, we report a case of functioning gonadotroph adenoma in a reproductive-aged woman and discuss its diagnosis and management.
A 21-year-old female patient with abdominal pain, irregular menstruation, hyperestrogenemia, and an ovarian mass was included. Brain magnetic resonance imaging (MRI) revealed a pituitary macroadenoma, and transsphenoidal surgery relieved her clinical symptoms. Before transsphenoidal surgery, plasma CA125, estradiol levels were elevated, while prolactin, luteinizing hormone, follicle-stimulating hormone, PROG, cortisol, FT4, thyroid-stimulating hormone, para
Early diagnosis of functioning gonadotroph adenomas should be considered in patients with hyperestrogenism, irregular menstruation, large or recurrent ovarian cysts, and visual field defects. Pituitary MRI should be performed, and transsphenoidal surgery is recommended for the management of this disease.
Core Tip: Functional gonadotroph adenomas (FGAs) are rare pituitary gland tumors. Here, we describe a case of FGAs in a woman of reproductive age with abdominal pain, irregular menstruation, hyperestrogenemia, and ovarian mass. We would like to share our experience of diagnosis and treatment, which will help clinicians make appropriate decisions in the future.
- Citation: He Y, Gao YT, Sun L. Functioning gonadotroph adenoma with hyperestrogenemia and ovarian hyperstimulation in a reproductive-aged woman: A case report and review of literature. World J Clin Cases 2023; 11(6): 1341-1348
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1341.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1341
Gonadotroph adenomas are the most common histological subtype of pituitary adenomas that originate from the adenohypophysis. Functioning gonadotroph adenomas (FGAs) are extremely rare, accounting for less than 1% of all gonadotroph adenomas[1]. Gonadotroph adenomas are classified as functioning and non-functioning gonadotroph adenomas, and the former is distinct from other hormone-secreting pituitary adenomas, which are also easily misdiagnosed due to their low proportion. FGAs secrete one or more biologically active hormones, such as gonadotropins, follicle-stimulating hormone (FSH), and luteinizing hormone (LH)[2], and this leads to different clinical manifestations, such as menstrual irregularity, infertility, ovarian hyperstimulation syndrome in females, testicular hypertrophy, sexual dysfunction, erythrocytosis in males, and isosexual precocious puberty in children[2,3]. Non-functioning gonadotroph adenomas (NFGAs) patients always present with mass effect symptoms, such as visual disorders, headache, and cranial nerve dysfunction, or are discovered as incidental imaging findings, and they often lack hormone hypersecretion symptoms[4]. The most common presenting clinical manifestations of FGAs are menstrual irregularity, including oligomenorrhea, secondary amenorrhea, menorrhagia, and irregular vaginal bleeding[2,5,6]. The pathogenesis of FGAs remains unclear; however, early diagnosis facilitates the selection of proper therapeutic methods by clinicians and would be beneficial to the prognosis of the disease. Here, we describe a case of functioning gonadotroph adenomas in a reproductive-aged woman with abdominal pain, irregular menstruation, hyperestrogenemia, ovarian mass, and fibroadenoma of the breast and discuss the diagnosis and management of the disease.
A 21-year-old female patient with abdominal pain, irregular menstrual cycles, hyperestrogenemia, and recurrence of an ovarian mass was transferred to our hospital in July 2020.
Four months prior, the patient was referred to another hospital because of abdominal pain and irregular menstrual cycles. Ultrasonography revealed a large multilocular cystic mass in the abdominal cavity. As the abdominal pain increased, the patient underwent single-port laparoscopic removal of the bilateral ovarian cysts. During surgery, the bilateral ovaries were enlarged in a multilocular mass with yellow fluid inside, and histopathology revealed multiple luteinized follicular cysts of the ovary.
After one month of clinical treatment, the patient experienced abdominal pain again. Pelvic ultrasound indicated recurrence of enlarged ovaries with multiple large cysts, and its upper edge reached 20 mm above the umbilicus; both sides reached the midclavicular line, while the thickness of the endometrium was 17.4 mm. Biochemical evaluation demonstrated normal serum levels of LH, FSH, progesterone (PROG), cortisol, Free T4 (FT4), thyroid-stimulating hormone (TSH), parathyroid hormone (PTH), calcium, phosphate, and growth hormone (GH), and elevated levels of plasma CA125, estradiol, and prolactin (PRL). Brain magnetic resonance imaging (MRI) revealed the presence of a pituitary macroadenoma (17 mm × 27 mm × 19 mm), and visual field examination after brain MRI revealed bitemporal hemianopsia. Ultrasound showed a 48 mm × 30 mm × 23 mm mass in the right breast, and histopathology revealed breast fibroadenoma. Ultrasound imaging revealed no abnormalities in the thyroid, adrenal, or parathyroid glands. The female patient was then treated with oral bromocriptine (2.5 mg) three times a day.
The patient had no significant history of illness, medical history, drug allergy, transfusion, injury, pregnancy, or other complications.
The patient had smoking history for over four years and had no remarkable personal or family history.
The patient had menarche at age 12 years, and she had a regular menstrual cycle. In the past year, the menstrual cycle of the patient had extended to 2–3 months, accompanied by progressively increased dysmenorrhea.
The patient was 167 cm tall, weighed 68 kg, and had a body mass index of 24.38 kg/m2. temperature was 36.7 °C, heart rate was 96 beats/min, respiratory rate was 20 breaths/min, and blood pressure was 98/68 mmHg.
Biochemical evaluation showed that plasma estradiol and CA125 levels were elevated, while PRL, LH, FSH, PROG, cortisol, FT4, TSH, PTH, and GH levels were maintained at normal levels.
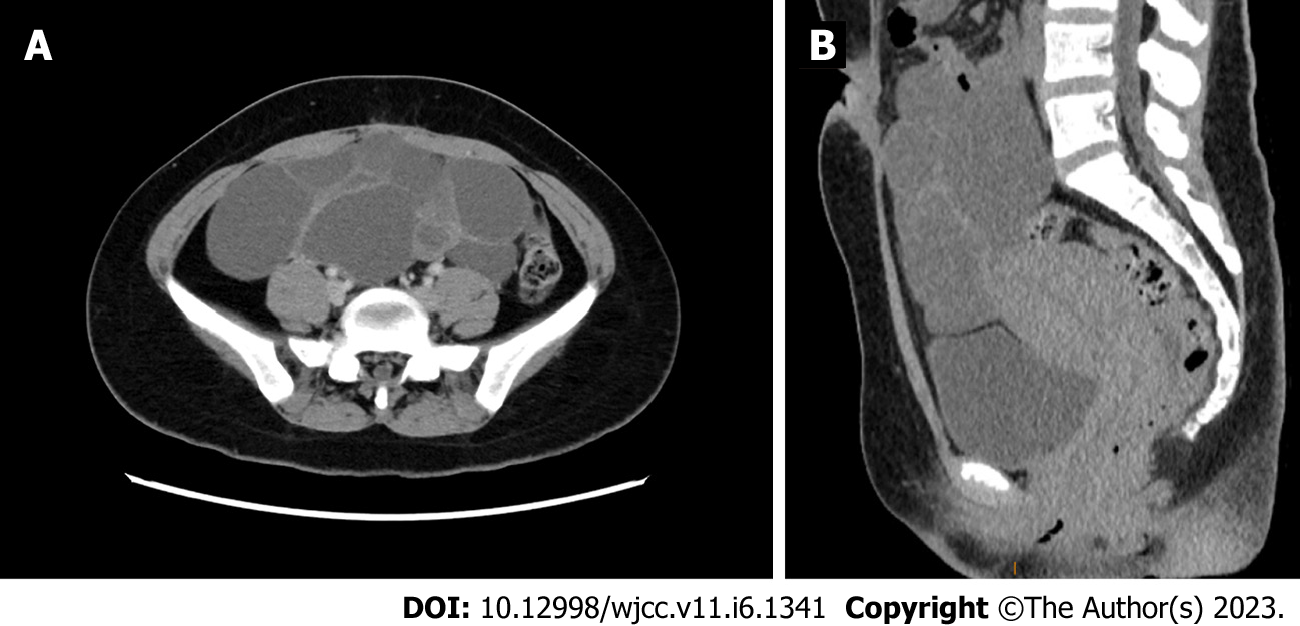
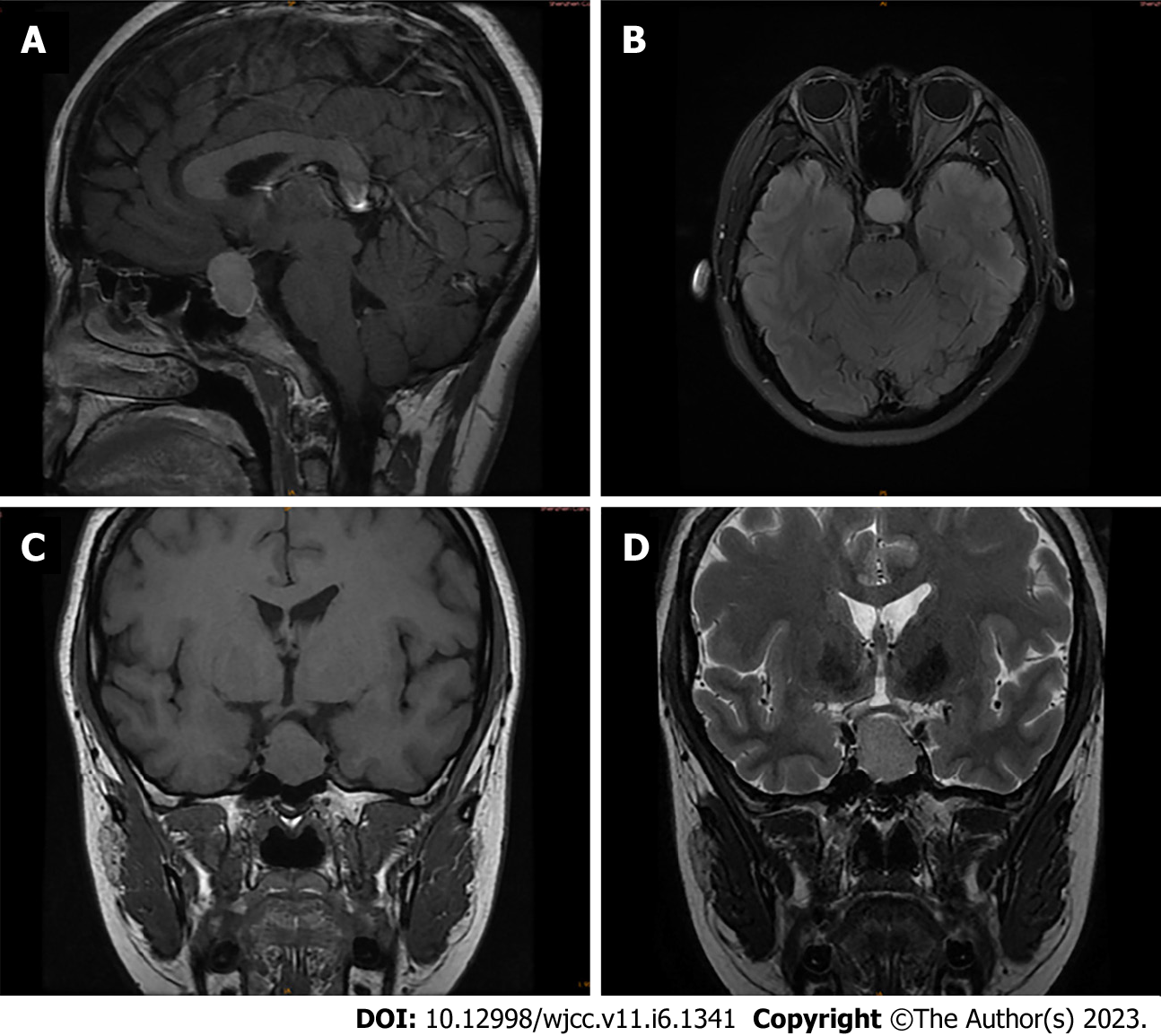
An ultrasonographic study of the pelvis revealed multicystic ovaries, similar to the typical signs of spontaneous ovarian hyperstimulation syndrome (OHSS). Computed tomography confirmed a large cystic mass in the abdominopelvic cavity (Figure 1A and B), with a range of 19.4 cm × 7.9 cm × 15.9 cm. A homogeneously enhancing 21 mm × 16 mm× 28 mm sellar mass imaged by Brain MRI was presented (Figure 2A-D).
In July 2020, the patient presented with abdominal pain and was transferred to our hospital. We invited neurosurgeons, pathologists, and radiologists to form a multidisciplinary team to discuss the diagnosis and treatment of the disease. Since the FSH levels of the patient were within normal ranges, one group of physicians suggested that ovarian cystectomy should be performed first, and then determined whether the histopathology is an estrogen-secreting tumor. However, different viewpoints expressed that pituitary adenomas should be treated first, and changes in the size of the ovarian cyst tumor should be observed after surgery.
Functioning gonadotroph adenoma.
The patient underwent transsphenoidal surgery.
Tumor specimens demonstrated gonadotroph adenoma immunopositive for PIT-1(+), AE1/AE3 (+), SYN(+), and CgA(+), weakly positive for LH, FSH, PRL, TSH, and KI67, and absolute negativity for ACTH, GH, and P53. Immediate postoperative biochemical evaluation revealed a significant reduction in estradiol, and a normal range of PRL, LH, FSH, PROG, and GH levels remained. Postoperative pelvic ultrasound at one month showed an ovarian cyst measuring 2.4 cm × 2.0 cm in size, which was significantly smaller than before. One month after surgery, endocrine investigations were almost within normal laboratory limits. After three and six months, pelvic ultrasound confirmed normal size ovaries in the patient, the menstrual cycle returned to regular, and her endocrine investigations, including estradiol, PRL, LH, FSH, PROG, TSH, PTH, GH, ACTH, and CA125, were maintained within the normal range, but anti-Müllerian hormone (AMH) seemed slightly lower. The patient was then treated with bromocriptine 1.25 mg orally three times a day after surgery. As the prolactin level from one month to six months after surgery was within the normal range, the dosage was reduced gradually until the final stop. There was no evidence of tumor recurrence after two years of follow-up.
All the endocrine investigations are presented in Table 1. Informed consent was obtained from the patient.
| Biochemicals | Normal values | Before surgery | After surgery | After surgery | After surgery |
| 1 d | 1 mo | 3 mo | |||
| Estradiol (pg/mL) | FP:12.5-166, LP:43.8-211 | 6977 | 53.7 | 39.5 | 69.1 |
| FSH (mIU/mL) | FP:3.5-12.5, LP:1.7-7.7 | 2.3 | 1.8 | 8.5 | 7.9 |
| LH (mIU/mL) | FP:2.4-12.6, LP:1.0-11.4 | 3.8 | 0.6 | 3.8 | 3.2 |
| PRL (uIU/mL) | 102-496 | 65.87 | 33.26 | 550.3 | 59.28 |
| PROG (ng/mL) | FP:0.057-0.893, LP:1.83-23.9 | 21.98 | 0.14 | 0.26 | 0.42 |
| GH (pg/mL) | 126-9880 | 463.1 | 438.1 | 158.7 | 336 |
| Testosterone (ng/mL) | 0.69 | 0.06 | 0.11 | 0.05 | |
| ACTH (pmol/L) | 1.6-13.9 | 4.79 | 4.48 | ||
| CA125 (U/mL) | < 35 | 41.6 | 14.2 | ||
| AMH (ng/mL) | 2.06-6.98 | 3.06 | 1.58 | ||
| PTH (pmol/L) | 1.27-9.33 | 3.55 | 3.82 | ||
| FT4 (pmol/L) | 6.44-18.02 | 10.34 | 13.32 | ||
| TSH (µIU/mL) | 0.35-5.1 | 1.46 | 2.126 | ||
| Cortisol (nmol/L) | 6-10 am:133-537 | 404 |
The exact prevalence of clinical FGAs is unknown because of their rarity, non-specific clinical signs and symptoms, and a lack of understanding of their histopathology and pathogenesis. The FGAs can occur in premenopausal and postmenopausal females, males and children. Postmenopausal females may not manifest with a syndromic presentation because the ovaries are insensitive to FSH stimulation[7]. Testicular enlargement, sexual dysfunction, and elevated serum FSH have been reported in male patients with FGAs; most of them are macroadenomas[8]. FGAs rarely occure in children; symptoms caused by central precocious puberty have been reported in both boys and girls[9,10]. While FGAs are typically histologically benign, there are cases of patients with severe comorbidities (via endocrine effects and/or mass effect) that lead to a shortened lifespan, as well as cases of tumor regrowth and metastasis[11,12]. In this context, the female patient had irregular menstrual cycles for one year. Furthermore, the menstrual period was extended, the menstrual cycle was prolonged to every 2-3 months, and dysmenorrhea was aggravated in the past year. After surgery, the patient resumed regular menstruation, abdominal distention disappeared, and pelvic ultrasound confirmed normal-sized ovaries three months later. Due to compression of the macroadenoma, the patient also showed a partial visual field defect, and the visual field recovered gradually after transsphenoidal surgery.
OHSS usually occurs in women undergoing assisted reproductive techniques when applying hormone medications to stimulate ovulation and can develop severe symptoms such as discomfort, abdominal pain, nausea, vomiting, diarrhea, ascites, hypovolemia, hemoconcentration, and thromboembolism[13]. The patient exhibited a typical sign of spontaneous ovarian hyperstimulation syndrome with enlarged ovaries, multiple large cysts, and abdominal pain, but without ascites and hypercoagulability. The secretion of FSH stimulates the recruitment of multiple follicles and promotes the excessive secretion of estradiol, which in turn leads to the downregulation of FSH[14]. The histopathology revealed that FSH and LH were weakly positive, despite the levels of the serum FSH and LH being maintained in the normal range, suggesting that the secretion of FSH may increase the bioactivity of FSH and its isoforms[15]. The negative feedback control by estradiol may disappear, and it could not inhibit the secretion of FSH by the pituitary gland, even if its level was too high. It has also been proposed that the specificity of the intramolecular barrier to activate the FSH receptor would be lower in ovarian hyperstimulation mutants, thus allowing even low-affinity agonists, such as LH, HCG, or TSH, to become effective[16]. High concentrations of estradiol were observed in this case, and the estradiol level returned to the normal range after transsphenoidal surgery, which may indicate that FGAs were the cause of this phenomenon. Prolactin levels are elevated in female patients, probably due to compression of the pituitary stalk and/or excessive estrogen secretion[16]. Anti-Müllerian hormone (AMH) levels may represent the quantity of the ovarian follicle pool and may be a useful marker of ovarian reserve[17]. Serum AMH levels decreased with age and were greatly reduced or undetectable in women with premature ovarian failure or in the postmenopausal period[18]. The decrease in AMH in this patient may have been caused by ovarian hyperstimulation due to the reduction in ovarian function. Preoperative cortisol, FT4, TSH, PTH, ACTH, and GH levels were normal, which helped differentiate them from other diseases and complications. Breast fibroadenoma is a common benign tumor in premenopausal women. Although the pathogenesis remains unclear, high levels of estrogen may promote disease development[19]. We also observed a reduction in the size of the fibroadenoma three months after the operation.
Functioning gonadotroph adenomas must be differentiated from polycystic ovarian syndrome (PCOS) and ovarian tumors, such as granulosa cell tumors. PCOS patients usually show mildly enlarged polycystic ovaries and menstrual irregularity, but they always have normal estradiol levels, increased LH relative to FSH, hyperandrogenism, insulin resistance, and obesity[20], which may help clinicians distinguish them from FGAs. The diameter of ovarian follicles in women with PCOS ranges from 2 to 9 mm, whereas those in women with gonadotroph adenomas are usually larger (> 2 cm). Ovarian granulosa cell tumors are often present in postmenopausal women and are characterized by a unilateral solid pelvic mass, abdominal pain, excessive estradiol secretion, endometrial hyperplasia, abnormal uterine bleeding, and low levels of LH and FSH[21]. Moreover, pathological examination and Brain MRI may help clinicians avoid misdiagnosis. Endocrine investigations, particularly estradiol levels, can provide an important reference for diagnosis. In previous reports of OHSS caused by functioning gonadotroph adenomas, estradiol levels were usually elevated, ranging from mild to markedly elevated[6,15,22,23]. In most cases of FGA, normal to high levels of serum FSH, decreased LH levels, and elevated PRL levels have also been reported[24]. Women with PCOS often have normal estradiol and increased AMH levels[18]. Ovarian granulosa cell tumors not only induced an increase in estradiol levels but also had a significant effect on elevating serum AMH and inhibin B concentrations[25]. Fully understanding the mechanism of the hypothalamic-pituitary-ovarian axis can facilitate correct diagnosis and treatment decisions.
Several studies have reported beneficial results following surgical treatment, adjuvant radiotherapy, and drug treatments. Due to the small number of cases, the optimal management of FGAs is based mainly on case reports or small systematic case series. Transsphenoidal surgery remains the primary choice, which not only relieves the mass effect symptoms and restores gonadotropin secretion, but also reduces the size of the ovaries, helps resume regular menses, resolves ovarian hyperstimulation syndrome, and enables the collection of tissue for histological analysis[5,15,16,23,26,27]. In cases where complete resection is difficult to achieve by surgery alone and tumor recurrence is detected during long-term follow-up, other treatments like adjuvant radiotherapy, radiosurgery, and drug treatments have been used to control the disease. Adjuvant radiotherapy has been reported to be helpful in several cases and is commonly used for managing residual tumors after surgery; nonetheless, the effectiveness of its routine usage remains controversial[6,26]. Gamma knife radiosurgery (GKRS) has recently been proposed for treating of residual or recurrent pituitary adenomas, which effectively and safely controls the tumor in the long term with minimal complications[28,29]. In studies of patients with pituitary adenoma after GKRS, hypopituitarism, visual decline, and tumor regrowth were reported during the follow-up[30,31]. GKRS is often employed in patients with NFGAs; long-term safety and efficacy data are still lacking for using GKRS in FGAs. Medical treatments of FGAs, including dopamine agonists (bromocriptine, cabergoline), somatostatin analogs (octreotide), and GnRH agonists and antagonists, temporarily suppress hormone production in a few cases. However, they are typically ineffective in controlling the growth of tumors or clinical syndromes[6,32,33,34,35].
In conclusion, we report a rare case of ovarian hyperstimulation due to a functional gonadotroph adenoma in a reproductive-aged woman. Early diagnosis of functioning gonadotroph adenomas should be considered in patients with hyperestrogenism, irregular menstruation, large or recurrent ovarian cysts, and visual field defects. Pituitary MRI should be performed, and transsphenoidal surgery is recommended for the management of this disease.
The authors thank the patient for allowing the publication of her case and their colleagues who commented on disease treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Obstetrics and gynecology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Atqiaee K, Iran; Zhang H S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ
| 1. | Aflorei ED, Korbonits M. Epidemiology and etiopathogenesis of pituitary adenomas. J Neurooncol. 2014;117:379-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Ntali G, Capatina C, Grossman A, Karavitaki N. Clinical review: Functioning gonadotroph adenomas. J Clin Endocrinol Metab. 2014;99:4423-4433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Ceccato F, Occhi G, Regazzo D, Randi ML, Cecchin D, Gardiman MP, Manara R, Lombardi G, Denaro L, Mantero F, Scaroni C. Gonadotropin secreting pituitary adenoma associated with erythrocytosis: case report and literature review. Hormones (Athens). 2014;13:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and Pathological Aspects of Silent Pituitary Adenomas. J Clin Endocrinol Metab. 2019;104:2473-2489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 5. | Sicilia V, Earle J, Mezitis SG. Multiple ovarian cysts and oligomenorrhea as the initial manifestations of a gonadotropin-secreting pituitary macroadenoma. Endocr Pract. 2006;12:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Garmes HM, Grassiotto OR, Fernandes YB, Queiroz Lde S, Vassalo J, de Oliveira DM, Benetti-Pinto CL. A pituitary adenoma secreting follicle-stimulating hormone with ovarian hyperstimulation: treatment using a gonadotropin-releasing hormone antagonist. Fertil Steril. 2012;97:231-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Hall JE. Neuroendocrine changes with reproductive aging in women. Semin Reprod Med. 2007;25:344-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Ntali G, Capatina C. Updating the Landscape for Functioning Gonadotroph Tumors. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Ceraudo M, Criminelli Rossi D, Di Iorgi N, Cama A, Piatelli G, Consales A. Pediatric pituitary adenoma with mixed FSH and TSH immunostaining and FSH hypersecretion in a 6 year-old girl with precocious puberty: case report and multidisciplinary management. Int J Neurosci. 2022;132:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Ambrosi B, Bassetti M, Ferrario R, Medri G, Giannattasio G, Faglia G. Precocious puberty in a boy with a PRL-, LH- and FSH-secreting pituitary tumour: hormonal and immunocytochemical studies. Acta Endocrinol (Copenh). 1990;122:569-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Benito M, Asa SL, Livolsi VA, West VA, Snyder PJ. Gonadotroph tumor associated with multiple endocrine neoplasia type 1. J Clin Endocrinol Metab. 2005;90:570-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Mehta GU, Lonser RR. Management of hormone-secreting pituitary adenomas. Neuro Oncol. 2017;19:762-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 13. | Humaidan P, Quartarolo J, Papanikolaou EG. Preventing ovarian hyperstimulation syndrome: guidance for the clinician. Fertil Steril. 2010;94:389-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Nastri CO, Teixeira DM, Moroni RM, Leitão VM, Martins WP. Ovarian hyperstimulation syndrome: pathophysiology, staging, prediction and prevention. Ultrasound Obstet Gynecol. 2015;45:377-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 109] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 15. | Roberts JE, Spandorfer S, Fasouliotis SJ, Lin K, Rosenwaks Z. Spontaneous ovarian hyperstimulation caused by a follicle-stimulating hormone-secreting pituitary adenoma. Fertil Steril. 2005;83:208-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Castelo-Branco C, del Pino M, Valladares E. Ovarian hyperstimulation, hyperprolactinaemia and LH gonadotroph adenoma. Reprod Biomed Online. 2009;19:153-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, Stabile G, Volpe A. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update. 2010;16:113-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 18. | La Marca A, Broekmans FJ, Volpe A, Fauser BC, Macklon NS; ESHRE Special Interest Group for Reproductive Endocrinology--AMH Round Table. Anti-Mullerian hormone (AMH): what do we still need to know? Hum Reprod. 2009;24:2264-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 236] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 19. | Bonney RC, Reed MJ, Davidson K, Beranek PA, James VH. The relationship between 17 beta-hydroxysteroid dehydrogenase activity and oestrogen concentrations in human breast tumours and in normal breast tissue. Clin Endocrinol (Oxf). 1983;19:727-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr Rev. 2016;37:467-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 853] [Article Influence: 94.8] [Reference Citation Analysis (0)] |
| 21. | Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008;34:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Kajitani T, Liu S, Maruyama T, Uchida H, Sakurai R, Masuda H, Nagashima T, Ono M, Arase T, Yoshimura Y. Analysis of serum FSH bioactivity in a patient with an FSH-secreting pituitary microadenoma and multicystic ovaries: A case report. Hum Reprod. 2008;23:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Murakami T, Higashitsuji H, Yoshinaga K, Terada Y, Ito K, Ikeda H. Management of ovarian hyperstimulation due to follicle-stimulating hormone-secreting gonadotroph adenoma. BJOG. 2004;111:1297-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Broughton C, Mears J, Williams A, Lonnen K. A clinically functioning gonadotroph adenoma presenting with abdominal pain, ovarian hyperstimulation and fibromatosis. Endocrinol Diabetes Metab Case Rep. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Färkkilä A, Koskela S, Bryk S, Alfthan H, Bützow R, Leminen A, Puistola U, Tapanainen JS, Heikinheimo M, Anttonen M, Unkila-Kallio L. The clinical utility of serum anti-Müllerian hormone in the follow-up of ovarian adult-type granulosa cell tumors--A comparative study with inhibin B. Int J Cancer. 2015;137:1661-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Tashiro H, Katabuchi H, Ohtake H, Kaku T, Ushio Y, Okamura H. A follicle-stimulating hormone-secreting gonadotroph adenoma with ovarian enlargement in a 10-year-old girl. Fertil Steril. 1999;72:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Castelbaum AJ, Bigdeli H, Post KD, Freedman MF, Snyder PJ. Exacerbation of ovarian hyperstimulation by leuprolide reveals a gonadotroph adenoma. Fertil Steril. 2002;78:1311-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 37] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Fu P, He YS, Cen YC, Huang Q, Guo KT, Zhao HY, Xiang W. Microneurosurgery and subsequent gamma knife radiosurgery for functioning pituitary macroadenomas or giant adenomas: One institution's experience. Clin Neurol Neurosurg. 2016;145:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Albano L, Losa M, Nadin F, Barzaghi LR, Parisi V, Del Vecchio A, Bolognesi A, Mortini P. Safety and efficacy of multisession gamma knife radiosurgery for residual or recurrent pituitary adenomas. Endocrine. 2019;64:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Zibar Tomšić K, Dušek T, Kraljević I, Heinrich Z, Solak M, Vučinović A, Ozretić D, Mihailović Marasanov S, Hršak H, Kaštelan D. Hypopituitarism after gamma knife radiosurgery for pituitary adenoma. Endocr Res. 2017;42:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Gopalan R, Schlesinger D, Vance ML, Laws E, Sheehan J. Long-term outcomes after Gamma Knife radiosurgery for patients with a nonfunctioning pituitary adenoma. Neurosurgery. 2011;69:284-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 32. | Murata Y, Ando H, Nagasaka T, Takahashi I, Saito K, Fukugaki H, Matsuzawa K, Mizutani S. Successful pregnancy after bromocriptine therapy in an anovulatory woman complicated with ovarian hyperstimulation caused by follicle-stimulating hormone-producing plurihormonal pituitary microadenoma. J Clin Endocrinol Metab. 2003;88:1988-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Knoepfelmacher M, Danilovic DL, Rosa Nasser RH, Mendonça BB. Effectiveness of treating ovarian hyperstimulation syndrome with cabergoline in two patients with gonadotropin-producing pituitary adenomas. Fertil Steril. 2006;86:719.e15-719.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Karapanou O, Tzanela M, Tamouridis N, Tsagarakis S. Gonadotroph pituitary macroadenoma inducing ovarian hyperstimulation syndrome: successful response to octreotide therapy. Hormones (Athens). 2012;11:199-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Macchia E, Simoncini T, Raffaelli V, Lombardi M, Iannelli A, Martino E. A functioning FSH-secreting pituitary macroadenoma causing an ovarian hyperstimulation syndrome with multiple cysts resected and relapsed after leuprolide in a reproductive-aged woman. Gynecol Endocrinol. 2012;28:56-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |