Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1318
Peer-review started: November 30, 2022
First decision: January 19, 2023
Revised: January 19, 2023
Accepted: January 31, 2023
Article in press: January 31, 2023
Published online: February 26, 2023
Processing time: 85 Days and 12 Hours
Insulin resistance (IR) was reported in most polycystic ovarian syndrome (PCOS) cases. Metformin, a biguanide drug, successfully reduced IR. Homeostatic Model Assessment for IR (HOMA-IR) and Doppler parameters assessed metformin's effectiveness.
To verify whether the area under the curve of the internal carotid artery (AUC-ICA) Doppler wave can be a useful marker for assessing IR among PCOS cases who presented with menstrual irregularity and were treated with metformin over 6 mo.
An observational, cross-sectional study recruited 54 eligible PCOS women; the anthropometrics were as follows: age, body mass index (BMI), menstrual cycle days, biochemical serum cholesterol, low and high-density lipoprotein, sex hormone-binding globulin, fasting blood glucose, and HOMA-IR, hormonal testosterone, luteinizing hormone over follicle-stimulating hormone ratio, and ultrasonic pulsatility index (PI) and resistance index (RI), carotid artery intima-media thickness (CIMT) and (AUC-ICA) parameters were initially recorded and repeated 3 mo and 6 mo later with metformin tab 500 mg; three times/day for 6 mo. In addition, AUC-ICA was assessed by taking repeated systolic and diastolic wave height measurements.
Metformin caused a progressive reduction in BMI, menstrual cycle days, biochemical hormonal, and Doppler parameters (CIMT, PI, RI, and AUC-ICA). AUC-ICA correlated strongly to all PCOS parameters. AUC-ICA correlated inversely with treatment time (r = -0.98, P < 0.001) and positively with HOMA-IR (r = 0.98, P < 0.0001). Via the best subset regression model, the AUC-ICA had the highest predictive value for HOMA-IR.
AUC-ICA preceded PI, RI, and CIMT with a strong, meaningful correlation to all PCOS parame
Core Tip: Women with polycystic ovarian syndrome (PCOS) suffer from insulin resistance and an atherogenic state evidenced by increased carotid artery intima-media thickness (CIMT). Fortunately, this increase in CIMT is reversible with metformin therapy, an insulin-sensitizing drug. Moreover, treated cases have restored ovulatory cycles and exhibited reduced androgen levels. First, we primarily aimed to examine whether the area under the curve of the internal carotid artery (AUC-ICA) Doppler is related to insulin resistance among PCOS women who presented with menstrual disturbances. Second, is to examine if AUC-ICA can be a useful marker in assessing changes in insulin resistance among treated cases with metformin for follow-up.
- Citation: Akram W, Nori W, Abdul Ghani Zghair M. Metformin effect on internal carotid artery blood flow assessed by area under the curve of carotid artery Doppler in women with polycystic ovarian syndrome. World J Clin Cases 2023; 11(6): 1318-1329
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1318.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1318
Polycystic ovarian syndrome (PCOS) is still one of the most frequently challenging problems facing gynecologists worldwide. Despite much work conducted to understand its nature, its long-term complications from cardiovascular, infertility, and obesity-related problems are still major issues facing all affected women[1-3]. PCOS women are characterized by a state of life-long insulin resistance (IR) with permanently elevated serum insulin[4].
This hyperinsulinemia disturbs ovarian steroidogenesis (estrogens and progesterone) and the pituitary secretion of gonadotropins, represented by chronically elevated serum luteinizing hormones (LH) and serum testosterone[5].
Elevated serum testosterone mainly affects lipid metabolism with an elevation of low-density lipoprotein (LDL) and serum cholesterol and a reduction of serum high-density lipoprotein (HDL)[6]. This disturbed lipid metabolism; leads to acute atherosclerotic changes affecting virtually every artery in the body but mostly in the medium-sized artery like the internal carotid artery, which can be scanned by B-mode ultrasound device. The atherogenic state is evidenced by increased carotid artery intima-media thickness (CIMT); fortunately, this increase in CIMT is usually reversible with the use of insulin-sensitizing drugs among young women below 35 years of age, which makes this reduction one of the most important prognostic variables for IR complications[7].
According to Bernoulli's rule, the increased CIMT per se is associated with a reduction in the arterial diameter, which consequently increases the artery's blood flow speed[8].
According to this rule, the narrower the artery lumen is, the more blood speed per unit area in its section (inverse relationship). Modern Doppler devices allow arterial blood speed to be measured and graphed on paper strips directly from scanned arteries. With ultrasound waves beamed at 60 degrees, we can measure the difference in phase shift of the ultrasound wave[9].
The most commonly used Doppler indices to assess arterial blood flow are pulsatility and resistant index (PI and RI), respectively[10].
The PI is an ultrasonic blood flow parameter calculated from the highest, lowest, and mean Doppler frequency shifts during a given cardiac cycle. As for the RI, it estimates the resistance in a pulsatile vascular tree[11].
However, a new parameter can be easily conducted with current Doppler devices: Measuring the area under the curve (AUC) of one complete heartbeat-associated blood flow graph in an artery represented by a systolic and diastolic wave.
Multiple readings of the heights of a systolic and diastolic wave of a single Doppler wave measure AUC under one Doppler wave. In physical terms, measuring AUC means measuring the amount of blood passing per unit area of the artery cross-section area[12]. For that, the speed of blood flow should increase while the amount of blood passed per unit sectional area of the internal carotid artery should reduce or remain the same according to the resistance distal to the vessel[12].
Metformin is an insulin sensitizer used for type 2 diabetes. Since many PCOS women are IR, metformin administration proved its efficacy in restoring ovulatory cycles and reducing androgen levels. This biguanide inhibits hepatic glycogenesis, increases insulin sensitivity in the periphery, increases glucose uptake, and decreases insulin secretion[1].
Earlier studies in the field have shown reduced atherogenic indicators by insulin sensitizers like metformin and inositol for 3-6 mo, including dyslipidemia, IR, and improved endothelial function and coronary flow. Furthermore, there was a reduction in CIMT among treated women[13]. The aim of the study is to verify whether the AUC of the internal carotid artery (AUC-ICA) Doppler is related to IR among women with PCOS who presented with menstrual disturbances. Second, to examine if AUC-ICA can be a useful marker for the assessment of IR among women treated with metformin for follow-up.
The current study was a cross-sectional study conducted in AL Yarmouk Teaching hospital between April 2019 till December 2021. The ethical committee of Mustansiriyah University approved the study dated February/21/2019 (IRB No. 115). Participants were briefed about the study's aim and methodology; all gave their consent before enrollment, and the Helsinki declaration was followed.
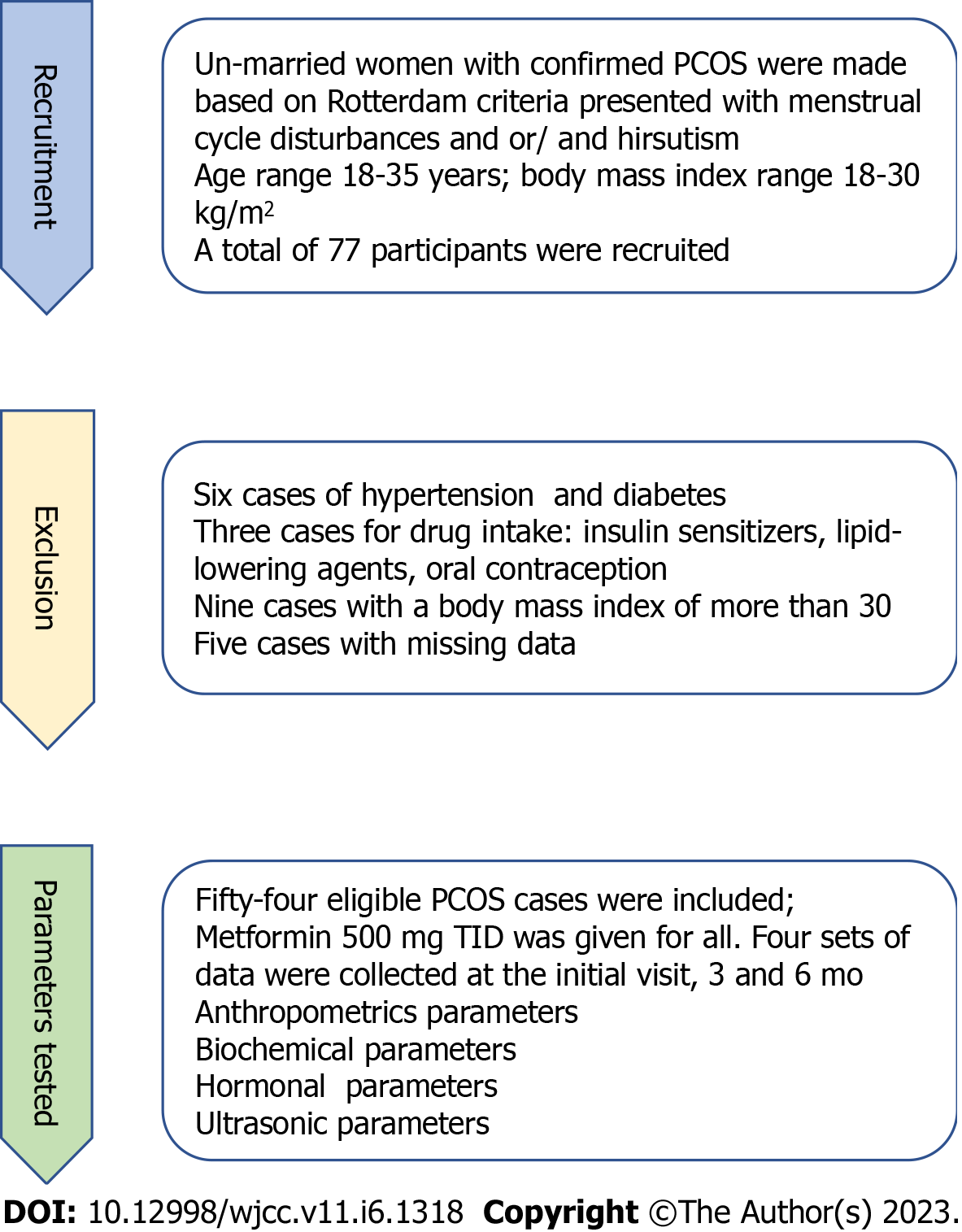
From the outpatient clinic, unmarried women with PCOS who presented with menstrual cycle abnormality and/or hirsutism, the age range of 18-35 years, and a body mass index (BMI) range of 18 to and 30 were invited to participate in the study. During this period, all patients had been prescribed a metformin tab. (Merck Santé/France) 500 mg TID times /d.
The diagnosis of PCOS was made based on Rotterdam criteria, where 2 out of 3 criteria confirm PCOS diagnosis[14]. (1) Oligomenorrhea is defined as < than six cycles/ 12 mo, or amenorrhea is defined as a complete absence of the menstrual cycle (more than 90 d); (2) Clinical hyperandrogenism, with or without acne; and (3) Ultrasonic features of polycystic ovaries[15,16].
The following groups were excluded from this study: (1) Women with hypertension, diabetes mellitus, and thyroid diseases; (2) Drug intake of insulin sensitizers, lipid-lowering medicines, anti-androgenic therapies, oral contraceptives, and steroids; (3) Participants with a BMI of more than 30; and (4) Those with missing data were also excluded.
Fifty-four participants satisfied our criteria and the patients in this study were all subjected to meticulous clinical examination and all had an initial pelvic ultrasound scan to confirm the appearance of the PCOS ovaries. For each participant, four sets of data were collected; demographic criteria, biochemical and hormonal data, and biophysical data. These data were collected at the initial visit, 3 mo, and then 6 mo later, and the patient was supplied with a data collection sheet for the dates of further scheduled visits. During this period, all patients had been prescribed a metformin tab (Merck Santé/France) 500 mg TID times /d. At the end of the study, a total of 54 women with complete data of biophysical, biochemical, and hormonal data were assessed at 0, 3, and 6 mo; all were described in the study flowchart Figure 1.
The initial uptake was based on the clinical criteria of a menstrual cycle (MC) abnormality (oligomenorrhea or amenorrhea). The patients' age was recorded and only BMI was calculated in the outpatient clinic according to the formula: Weight in kg/square meter of height. After exclusion, eligible cases were planned to be further investigated for hormonal, biochemical, and biophysical criteria.
On the 2nd day of the menstrual cycle and after one night's fast, participants in the study were initially sent to the Teaching labs in Yarmouk Hospital for the following biochemical tests: HDL, LDL, serum cholesterol, homeostatic model assessment for insulin resistance (HOMA-IR), fasting blood glucose (FBS), and sex hormone-binding globulin (SHBG). In addition to the hormones: Follicle-stimulating hormone (FSH), LH, and serum testosterone. The HOMA-IR tested insulin sensitivity based on the formula: HOMA-IR = fasting insulin (micro U/L) × fasting glucose (nmol/L)/22.5[17].
All patients were given subsequent appointments based on the initial visit to repeat all of the above investigations 3 mo and 6 mo later while on metformin therapy, in addition to recording the days of the MC.
On the same day of biochemical data uptake, patients were sent for a Doppler assessment of the internal carotid artery.
In the radiology unit, an ultrasound machine TOSHIBA, Logic p5 with a linear array probe, and a frequency range of 5-10 MHZ was used. To measure carotid media intima thickness (CIMT), a special software computer option was used to adjust angle measurements for CIMT with a two–dimensional 2D grayscale maneuver. The assessment was made for both the right and left internal carotid artery (ICA), and the mean of both readings was taken.
CIMT was defined as the distance between the lumen intima to the adventitia media layer line of interference on the far wall in the longitudinal axis[18]. In order to decrease intra- and inter-observer variability for CIMT measurements, all readings were made by the same radiologist in our department after a period of practice to master the technique.
As for blood flow parameters, PI, and RI, a Doppler study of the ICA was performed, and measurements were made to PI and RI.
The patient required no special preparation, and the examination was held while the patient lay in a supine position with their head turned to the other side and did not last more than ten minutes. As mentioned above, patients were provided with scheduled visits for further re-scan 3 mo and 6 mo later.
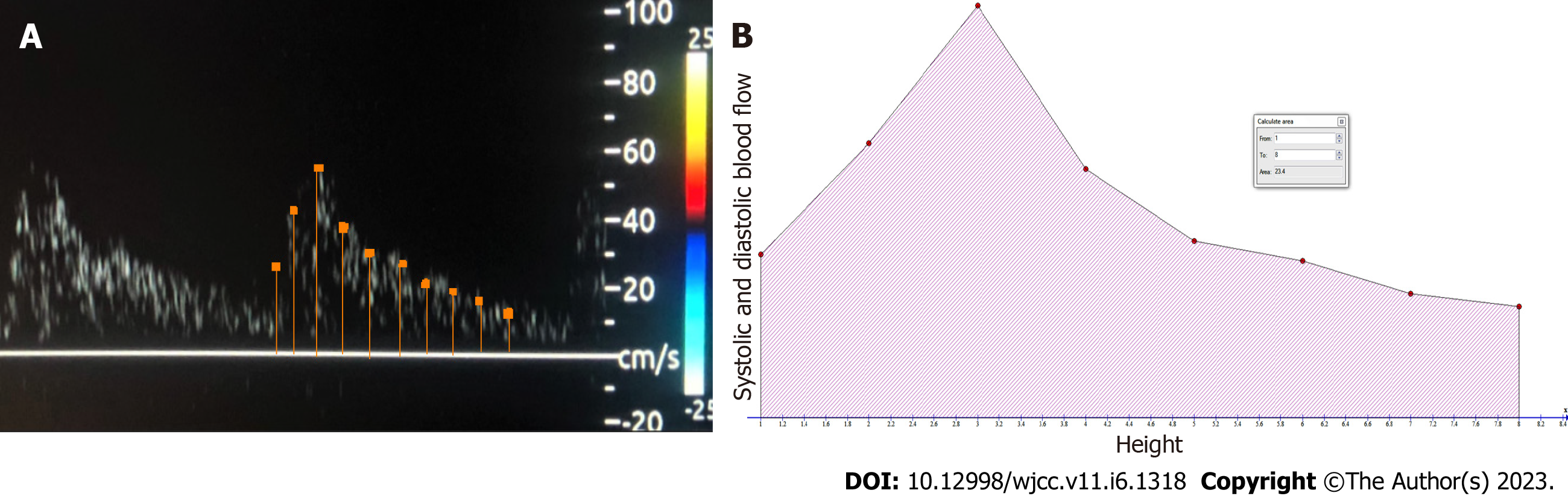
To explain how we calculated the AUC, we will give an example. A sample of ICA Doppler is shown in Figure 2. In this picture, we measured the different heights of the Doppler wave (demarcated by dots in Figure 2A) as it went up and down with a facility supplied by the ultrasound device. Depending on the width of the wave, these measurements averaged between 8 and 10 for each wave; see the horizontal orange line in Figure 2A). The measurement of the heights represents the speed of blood in the systolic and diastolic velocities. The calculated heights were put in an Excel table to analyze later with simple, free software called GRAPH, which can be downloaded from: https://www.padowan.dk/download/[19].
In Figure 2B, a graphical simulation of the internal Carotid Artery Doppler wave in Graph software is shown, with the different heights (demarcated in red dots) used to measure the systolic and diastolic blood flow for the calculation of AUC.
The AUC-ICA was calculated according to the following formula: AUC = ∫ Blood velocity measured at systolic and diastolic velocity for a single Doppler wave[11].
AUC physically means the amount of blood passed per unit area of the internal carotid artery per single heartbeat. It was measured in unit 3. Following the initial measurement of AUC-ICA, a re-assessment was made 3 mo and 6 mo later. All data with associated biochemical and biophysical data were stored in an excel sheet for further data analysis at the end of the study (Figure 2).
The sample size was calculated according to the following equation for a cross-sectional study with quantitative variables[20]:
Sample size = (Z1-α/2) 2 SD2/d2.
Z1-α/2 = is standard normal variate = 1.96.
SD = standard deviation of the variable taken from already published studies.
d = absolute error or precision level as an operator decides.
Sample size = (1.96)2 (0.35)2 / (0.1)2 = (3.84 × 0.1225)/ 0.01 = 43 patients.
So the sample size is 43 patients, and our study involved 54 patients.
Continuous data were expressed as mean and standard deviation. Data normality was checked with the Shapiro-Wilk test, and the data were normally distributed. One-way ANOVA test was used to assess the statistical data differences at the initial visit, at 3 mo, and 6 mo later for all the above biochemical, hormonal, and biophysical study variables. Linear regression was used to evaluate the decline of the AUC-ICA within 6 mo of metformin treatment with the calculation of the correlation coefficient and associated P value. In addition, further linear regression was constructed between AUC as the main dependent variable vs all significant biochemical, hormonal and biophysical variables taken in this study with the calculation of the correlation coefficient and associated value to assess the effect of metformin treatment on those correlations for the 6 mo treatment. AUC-ICA was assessed with a freely downloadable software GRAPH and further checked with MedCalc and NCSS software. P values less than 0.05 were considered significant.
Fifty-four young, unmarried, PCOS women were collected with full hormonal, biochemical, and biophysical profiles at the initial visit, 3 mo, and 6 mo after starting the metformin tablet 500 mg/TID day. Regarding the anthropometric criteria, the mean age of the participants was 24.81 ± 3.49 years. The days of the MC showed a significant decrease (P < 0.034) from 57.6 ± 5.8 in the initial visit to 43 ± 5.6 and 31.97 ± 4.9 d in the second and 3rd visits, respectively. Likewise, BMI showed a significant reduction (P < 0.04) from 28.22 ± 0.75 in the initial visit to 25.88 ± 0.64 and 23.81 ± 0.74 kg/m2, respectively.
The main demographic criteria of these women are given at the three sampling and scanning times expressed as mean and standard deviation and were described in Table 1, while the three columns were compared with a one-way ANOVA test. The results highlight a progressive increase in serum HDL and SHBG throughout the treatment period over 6 mo. FBG showed a trend decrease; however, it fails to have a statistical value. On the other hand, a significant reduction was found in serum cholesterol, LDL, HOMA- IR, testosterone, LH/FSH ratio, PI, RI, and CIMT. As for AUC-ICA, it showed a progressive decrease with the treatment period.
| Parameter | First visit value, n = 54 | 2nd visit at 3 mo, n = 54 | 3rd visit at 6 mo, n = 54 | P value |
| Cholesterol in mg/dL | 384.19 ± 13.64 | 337.25 ± 18.40 | 276.82 ± 12.69 | < 0.030 |
| HDL in mg/dL | 35.70 ± 3.78 | 48.70 ± 4.31 | 62.52 ± 4.09 | < 0.001 |
| LDL in mg/dL | 203.22 ± 8.74 | 167.41 ± 12.26 | 132.33 ± 12.62 | < 0.040 |
| Fasting blood sugar in mg/dL | 92.22 ± 1.80 | 90.93 ± 2.42 | 92.07 ± 2.43 | 0.071 |
| HOMA-IR | 2.26 ± 0.07 | 2.02 ± 0.10 | 1.74 ± 0.07 | < 0.001 |
| SHBG in nmol/L | 29.52 ± 7.11 | 58.29 ± 8.25 | 81.74 ± 7.49 | < 0.001 |
| Testosterone in ng/dL | 108.30 ± 8.29 | 79.74 ± 9.45 | 51.56 ± 7.96 | < 0.010 |
| LH / FSH_Ratio | 2.62 ± 0.12 | 2.23 ± 0.15 | 1.76 ± 0.13 | < 0.001 |
| PI | 1.41 ± 0.08 | 1.13 ± 0.07 | 0.89 ± 0.07 | < 0.034 |
| RI | 0.89 ± 0.04 | 0.77 ± 0.05 | 0.62 ± 0.04 | < 0.040 |
| CIMT in mm | 0.92 ± 0.03 | 0.79 ± 0.03 | 0.66 ± 0.03 | < 0.001 |
| AUC- ICA Doppler | 56.44 ± 4.49 | 42.11 ± 4.06 | 31.29 ± 3.93 | < 0.001 |
In order to shed more light on the trend of AUC-ICA correlation with various study parameters, a linear regression was constructed in Table 2.
| AUC- ICA vs parameter | Coefficient of correlation | P value |
| Cholesterol in mg/dL | 0.81 | 0.03 |
| HDL in mg/dL | -0.92 | 0.002 |
| LDL in mg/dL | 0.88 | 0.04 |
| HOMA_IR | 0.98 | 0.0001 |
| SHBG in nmole/L | -0.90 | 0.01 |
| Testosterone in ng/dL | 0.86 | 0.001 |
| LH/ FSH_ratio | 0.91 | 0.007 |
| PI | 0.87 | 0.002 |
| RI | 0.8 | 0.009 |
| CIMT in mm | 0.95 | 0.0001 |
| 24 wk of metformin therapy | -0.99 | 0.001 |
All biochemical markers; cholesterol, HDL, LDL, SHBG, and HOMA_IR; the hormonal markers of testosterone, and LH/FSH_Ratio; and ultrasonic parameters, RI, and CIMT used in PCOS women evaluation were correlated strongly to AUC-ICA, P < 0.05.
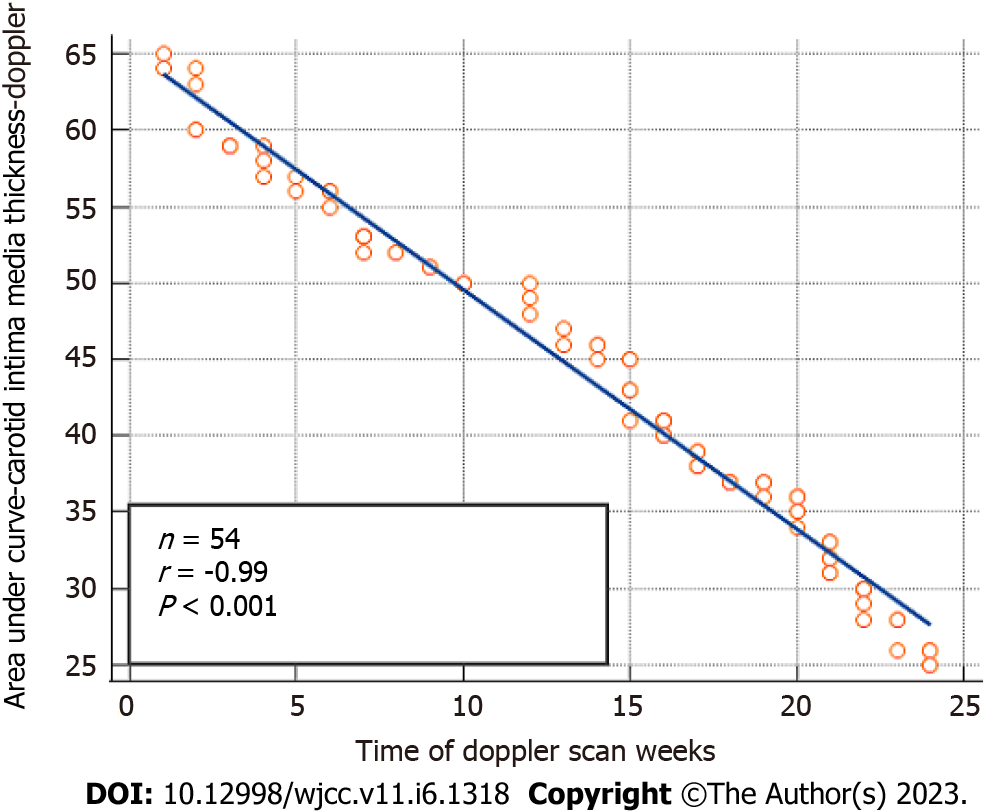
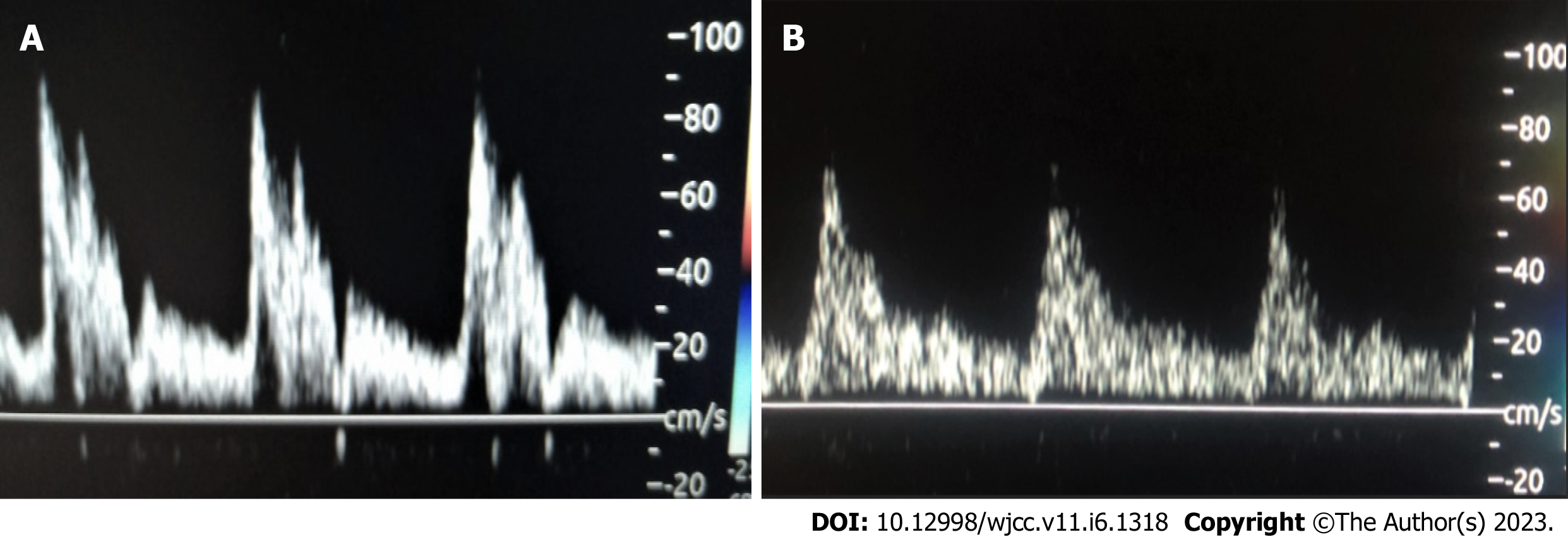
A highly significant negative correlation exists between AUC-ICA Doppler vs HOMA-IR. An inverse correlation was confirmed between the 6 mo time (24 wk) of metformin therapy vs AUC-ICA with r = -0.99 and P value less than 0.001, which was shown in Figure 3 and highlighted by a real-time Doppler scan in Figure 4, where the wave peak decreased after treatment from 80 to 60 and accordingly; the AUC-ICA was reduced.
To assess the strength of association between HOMA-IR as the primary dependent variable vs the predictive biophysical profiles related to the ICA, namely PI, RI, CIMT, and AUC-ICA Doppler; the best subset regression model was constructed with a calculation of Mallow's coefficient described in Table 3. The lowest values are shown on the AUC-ICA Doppler, which means it has the highest predictive value for HOMA-IR. While RI has the highest Mallow's coefficient value, which means it has the lowest predictive value. Both CIMT and PI lie in between the two.
| Variable | (Cp) Mallow’s coefficient |
| AUC-ICA | 49.6 |
| CIMT | 155.5 |
| PI | 326.8 |
| R1 | 637.0 |
Our results showed that metformin therapy for 6 mo caused a progressive improvement in BMI, menstrual cycle days, metabolic markers, hormonal parameters, and Doppler parameters (PI, RI, and CIMT). AUC-ICA exhibits a meaningful correlation to all PCOS parameters. Furthermore, it showed a progressive reduction throughout the treatment period. AUC-ICA correlated inversely with treatment time (r = -0.98, P < 0.001)and positively with HOMA-IR (r = 0.98, P < 0.0001). With the best subset regression model, the AUC-ICA had the highest predictive value for HOMA-IR.
Women with PCOS present with multiple anthropometric, metabolic, and hormonal abnormalities which are successfully reversed by metformin therapy; our results were in accordance with published studies[21].
The reduced BMI in our result was in line with a recently published meta-analysis study. Their result discussed that metformin as a monotherapy or in combination with other drugs can improve all anthropometric parameters (weight, waist-to-hip ratio, and BMI) among PCOS women. This was made irrespective of the dose and the duration of metformin use[22].
The Abdalla meta-analysis declared a meaningful reduction of serum testosterone with metformin, P < 0.0001 with moderate grade evidence and our result was in good agreement with their results[23].
The role of IR in hyperandrogenemia is not well understood. Some authors suggest that hyperinsulinemia plays a dual role in triggering hyperandrogenemia, first via direct stimulation of ovarian androgens due to the insulin receptors on the theca cells, and second via indirect stimulation of LH secretion and suppression of SHBG production by the liver with a net increase of free androgen levels[24].
Metformin's beneficial effects on PCOS include; antihyperglycemic and reduction of IR by increasing the peripheral uptake of glucose in addition to its indirect effect on insulin levels. Our data showed a progressive reduction of FBS and HOMA-IR with the treatment, as other studies pointed out[20]. It has an anti-androgenic effect via reducing CYP17 cytochrome activity involved in androgen production, not to mention increasing SHBG, which consequently reduces free androgens[20].
The favorable effect of metformin in cardiovascular disease (CVD) protection has several benefits. First, it protects endothelial integrity, which is a key player in the triggering and progression of CVD, consequently reducing future CVD risk. Second, metformin may inhibit hepatic de novo lipogenesis paths to lower plasma cholesterol levels and improves the atherogenic milieu, although the exact mechanism is still unknown. Third, metformin has anti-inflammatory and anti-oxidative effects; both are accredited as an etiological factor in PCOS pathogenesis. Indirect action of metformin; via reducing IR and androgen, both were proposed to act independently and synergistically in the progression of atherogenic dyslipidemia and CVD risk among PCOS women[22-25].
Regarding the menstrual cycle, Yilmaz et al[26] studied menstrual irregularities and hirsutism in women treated with two insulin-sensitizing medications, Rosiglitazone and metformin. Their data showed an improvement in cycle regularity and hirsutism score; however, they advised Rosiglitazone over metformin for greater patient acceptance and hirsutism improvement. In line with their result, our data showed significant improvement in MC days and regularity[26].
Androgen excess tends to associate with hyperinsulinemia and IR. This alliance seems to worsen menstrual cycle abnormalities. Several studies showed that many women with metformin therapy have ovulated and had a pregnancy. In contrast, patients with higher levels of serum testosterone were more likely to be infertile[27,28].
There was a debate on which contributes more to improving MC by metformin therapy, reduced IR, reduced androgen, or reduced BMI caused by metformin. The Ezeh et al[29] study results highlighted that menstrual cycle abnormality correlated positively with IR severity and not with hyperandrogenemia with adjustment of IR confounders, including BMI. In fact, amenorrheic PCOS women had the worst IR. Our results showed an improved IR and menstrual cycle pattern with metformin use, which suggests a clinical implication for daily practice; tracking down MC changes is easy and is free of charge, and it can reflect an improvement in the IR that underlies PCOS syndrome[29]. Prior studies demonstrated that MC abnormalities among adolescents would not impact future reproductive performance[30]. However, that was not the case for older women (> 30 years) who presented with oligomenorrheic and hirsute; they had lower reproductive performance than their age-matched controls[31].
IR lies at the heart of PCOS pathogenesis and severity and is a prognostic marker for response to treatment[32]. IR is universally assessed by HOMA-IR, calculated from the fasting serum insulin levels and blood sugar. Nevertheless, the assessment of HOMA-IR is costly and time-consuming, and this has pushed many researchers to find alternatives to assess IR[33,34].
Since 2010 many papers have reported that women with PCOS have universally increased intima-media thickness IMT in all medium-sized arteries like the carotid and ovarian artery[35]. Since then, many researchers have declared an excellent correlation between CIMT and HOMA-IR, and the current study results are in good agreement with the aforementioned studies[36].
Blood flow parameters were used to monitor HOMA-IR among PCOS cases during metformin use. In this study, both PI and RI had a progressive reduction, consistent with results obtained by Kaya et al[37]. Their study highlighted the beneficial role of adding metformin to combined contraceptive pills among PCOS women compared to those treated with combined contraceptive pills alone. CIMT and flow-mediated dilatation (FMD) was improved with the metformin group. The author attributes this to the effect of metformin on endothelial integrity, which is responsible for nitric oxide release, reducing oxidative stress, and the correction of altered cellular signaling pathways[27,37,38].
The foundation of this study is a simple physical fact. Bernoulli's rule states that fluid velocity has an inverse correlation to the diameter of a vessel[8]. The increased ICA-IMT will cause the passing of blood to be faster. Since the area under the curve physically means the amount of blood passed per one sectional area in the vessel wall, consequently, AUC might represent a sensitive marker to HOMA-IR[11].
Scientists have discussed that measuring insulin sensitivity through surrogate markers of insulin sensitivity (HOMA-IR and QUICKI) is no longer enough and raised the necessity for more acute ways[37-40]. Although IMT and FMD were investigated in PCOS, we believe that estimating the change of blood speed mirrored by AUC is more accurate, as described in earlier work[38,39].
Indeed, the strong correlations of AUC-ICA with all biochemical, hormonal, and Doppler parameters (PI, RI, and CIMT) support our hypothesis; furthermore, the progressive reduction of AUC-ICA represents the initial positive predictive ability of AUC-ICA as a possible novel marker for predicting IR. The best subset regression added more strength to our result, which confirmed; that AUC-ICA had the highest predictive value; it preceded PI, RI, and CIMT. The parameters mentioned above are already validated in IR, and thus AUC-ICA use needs no external validation.
The treatment period is relatively short, so we cannot be sure of the future implications of metformin treatment in the long run. The study type is another limitation, as in any cross-sectional study, the exact cause-and-effect link cannot be determined because the IR and AUC-ICA were simultaneously measured[40]. Intra- and inter-observer variability for AUC-ICA measurements is another limitation. Finally, the COVID-19 pandemic has seriously affected the duration of the study due to the repeated lockdown conducted in Iraq at that time[40,41].
Study strengths
AUC is a novel marker proposed by the current study, calculated by serial readings of Doppler wave height using freely downloadable software from the web, namely GRAPH software. It was easily calculated, free of extra charge, as it can be integrated into pelvic scan sessions. Its strong association with HOMA-IR and the duration of the treatment added to its cost make it superior to HOMA-IR in terms of cost-benefit analysis. For that, AUC-ICA is recommended as a reliable predictive marker for IR, follow-up, and prognostic value, especially during metformin therapy. Further studies are warranted for AUC-ICA application in clinical practice.
Measurement of the area under the curve of ICA is a promising marker for assessing IR in polycystic ovarian syndrome cases during the metformin therapy period. AUC-ICA showed strong significant correlations to PCOS parameters and had a superior cost-benefit analysis over HOMA-IR. Further studies are recommended to explore future applications in practice.
Insulin resistance (IR) is implicated in many aspects of polycystic ovarian syndrome (PCOS) pathogenesis. Metformin effectively decreased IR. Improved IR was evaluated via homeostatic model assessment for IR (HOMA-IR) and Doppler parameters; mainly carotid artery intima-media thickness (ICA-IMT). The area under the curve of the internal carotid artery (AUC-ICA) Doppler wave was examined as a helpful marker for determining IR among PCOS cases presented with menstrual irregularity and treated by metformin over 6 mo.
Much research has shown that surrogate measures of insulin sensitivity are no longer sufficient for evaluating insulin sensitivity, which has increased the need for new direct methods. Demonstrating changes in blood flow mirrored by AUC appeared to be more dependable; as indicated in earlier work, ICA-IMT was already examined in PCOS.
To ascertain if IR is related to the AUC-ICA Doppler in PCOS-affected women with menstrual irregularities. The second goal is to analyze the reliability of AUC-ICA as a helpful marker for monitoring IR in women who have received metformin treatment.
The study enrolled 54 PCOS women in a cross-sectional study. Anthropometric data included patient age, body mass index (BMI), menstrual cycle days, biochemical parameters: serum cholesterol, low and high-density lipoprotein, sex hormone-binding globulin, fasting blood glucose, and HOMA-IR, hormonal parameters: testosterone, luteinizing hormone over follicle-stimulating hormone ratio, and ultrasonic parameters: (CIMT, PI, RI, and AUC-ICA). Measurements of the systolic and diastolic wave height were repeated in order to evaluate the AUC-ICA following metformin tab-500 mg; three times/d for 6 mo. Metformin caused a progressive reduction in BMI, menstrual cycle days, biochemical hormonal, and Doppler parameters (CIMT, PI, RI, and AUC-ICA). AUC-ICA correlated strongly to all PCOS parameters. AUC-ICA correlated inversely with treatment time (r = -0.98, P < 0.001) and positively with HOMA-IR (r = 0.98, P < 0.0001). Via the best subset regression model, the AUC-ICA had the highest predictive value for HOMA-IR.
BMI, menstrual cycle days, biochemical hormonal, and Doppler markers (CIMT, PI, RI, and AUC-ICA were all gradually reduced by metformin treatment). All PCOS indicators and AUC-ICA had significant correlations. AUC-ICA had a negative correlation (r = -0.98, P < 0.001) with treatment time and a positive correlation (r = 0.98, P0.0001) with HOMA-IR. The AUC-ICA demonstrated the best subset regression model's maximum predictive value for HOMA-IR.
AUC-ICA was a reliable marker for the assessment of IR, especially during metformin medication. AUC-ICA preceded PI, RI, and CIMT and showed a high, meaningful correlation to other PCOS markers. For further use in practice, more research is suggested.
The area under the curve of the internal carotid artery had a significant correlation with HOMA-IR and the length of metformin therapy, not to mention it has a superior cost-benefit analysis over HOMA-IR. AUC-ICA is a reliable indicator of IR, follow-up, and prognostic value, particularly while using metformin.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Iraq
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He XM, China; Spinelli L, Italy; Zeng Y, China S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Reyes-Muñoz E, Sathyapalan T, Rossetti P, Shah M, Long M, Buscema M, Valenti G, La Rosa VL, Cianci S, Vitale SG. Polycystic Ovary Syndrome: Implication for Drug Metabolism on Assisted Reproductive Techniques-A Literature Review. Adv Ther. 2018;35:1805-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Ali AI, Hassan WNM, Alrawi S. A Copeptin as a Predictor Marker for Insulin Resistance Among Women with Polycystic Ovary Syndrome. CWHR. 2022;18:e081221198670. [RCA] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Zhang J, Xu JH, Qu QQ, Zhong GQ. Risk of Cardiovascular and Cerebrovascular Events in Polycystic Ovarian Syndrome Women: A Meta-Analysis of Cohort Studies. Front Cardiovasc Med. 2020;7:552421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Pani A, Gironi I, Di Vieste G, Mion E, Bertuzzi F, Pintaudi B. From Prediabetes to Type 2 Diabetes Mellitus in Women with Polycystic Ovary Syndrome: Lifestyle and Pharmacological Management. Int J Endocrinol. 2020;2020:6276187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Puttabyatappa M, Padmanabhan V. Ovarian and Extra-Ovarian Mediators in the Development of Polycystic Ovary Syndrome. J Mol Endocrinol. 2018;61:R161-R184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Kakoly NS, Moran LJ, Teede HJ, Joham AE. Cardiometabolic risks in PCOS: a review of the current state of knowledge. Expert Rev Endocrinol Metab. 2019;14:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Song P, Fang Z, Wang H, Cai Y, Rahimi K, Zhu Y, Fowkes FGR, Fowkes FJI, Rudan I. Global and regional prevalence, burden, and risk factors for carotid atherosclerosis: a systematic review, meta-analysis, and modelling study. Lancet Glob Health. 2020;8:e721-e729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 478] [Article Influence: 95.6] [Reference Citation Analysis (0)] |
| 8. | Pisano A. Continuity Equation and Bernoulli’s Theorem: Airplanes, Venturi Masks, and Other Interesting Things (for Anesthesiologists and Intensivists). Physics for Anesthesiologists and Intensivists, 2021. [DOI] [Full Text] |
| 9. | Panayides AS, Amini A, Filipovic ND, Sharma A, Tsaftaris SA, Young A, Foran D, Do N, Golemati S, Kurc T, Huang K, Nikita KS, Veasey BP, Zervakis M, Saltz JH, Pattichis CS. AI in Medical Imaging Informatics: Current Challenges and Future Directions. IEEE J Biomed Health Inform. 2020;24:1837-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Manzoor I, Bacha R, Gilani SA. Sonographic association of polycystic ovaries with intraovarian arterial pulsatility and resistive index. Gynecol Endocrinol. 2019;35:851-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Wang F, Jin P, Feng Y, Fu J, Wang P, Liu X, Zhang Y, Ma Y, Yang Y, Yang A, Feng X. Flexible Doppler ultrasound device for the monitoring of blood flow velocity. Sci Adv. 2021;7:eabi9283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Chirinos JA, Segers P, Hughes T, Townsend R. Large-Artery Stiffness in Health and Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;74:1237-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 617] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 13. | Paul C, Laganà AS, Maniglio P, Triolo O, Brady DM. Inositol's and other nutraceuticals' synergistic actions counteract insulin resistance in polycystic ovarian syndrome and metabolic syndrome: state-of-the-art and future perspectives. Gynecol Endocrinol. 2016;32:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3363] [Cited by in RCA: 4016] [Article Influence: 191.2] [Reference Citation Analysis (0)] |
| 15. | Verhoef SJ, Wielink MC, Achterberg EA, Bongers MY, Goossens SMTA. Absence of menstruation in female athletes: why they do not seek help. BMC Sports Sci Med Rehabil. 2021;13:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Hickey M, Doherty DA, Atkinson H, Sloboda DM, Franks S, Norman RJ, Hart R. Clinical, ultrasound and biochemical features of polycystic ovary syndrome in adolescents: implications for diagnosis. Hum Reprod. 2011;26:1469-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Invest. 2012;35:905-910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 18. | Jabbour R, Ott J, Eppel W, Frigo P. Carotid intima-media thickness in polycystic ovary syndrome and its association with hormone and lipid profiles. PLoS One. 2020;15:e0232299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Meun C, Gunning MN, Louwers YV, Peters H, Roos-Hesselink J, Roeters van Lennep J, Rueda Ochoa OL, Appelman Y, Lambalk N, Boersma E, Kavousi M, Fauser BC, Laven JS; CREW consortium. The cardiovascular risk profile of middle-aged women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2020;92:150-158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 20. | Chow S, Shao J, Wang H, Lokhnygina Y. Sample Size Calculations in Clinical Research: Third Edition, 2017. [DOI] [Full Text] |
| 21. | Costello M, Shrestha B, Eden J, Sjoblom P, Johnson N. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;CD005552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Guan Y, Wang D, Bu H, Zhao T, Wang H. The Effect of Metformin on Polycystic Ovary Syndrome in Overweight Women: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int J Endocrinol. 2020;2020:5150684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Abdalla MA, Shah N, Deshmukh H, Sahebkar A, Östlundh L, Al-Rifai RH, Atkin SL, Sathyapalan T. Impact of pharmacological interventions on anthropometric indices in women with polycystic ovary syndrome: A systematic review and meta-analysis of randomized controlled trials. Clin Endocrinol (Oxf). 2022;96:758-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly Cystic Ovarian Syndrome: An Updated Overview. Front Physiol. 2016;7:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Nafisa A, Gray SG, Cao Y, Wang T, Xu S, Wattoo FH, Barras M, Cohen N, Kamato D, Little PJ. Endothelial function and dysfunction: Impact of metformin. Pharmacol Ther. 2018;192:150-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Yilmaz M, Karakoç A, Törüner FB, Cakir N, Tiras B, Ayvaz G, Arslan M. The effects of rosiglitazone and metformin on menstrual cyclicity and hirsutism in polycystic ovary syndrome. Gynecol Endocrinol. 2005;21:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Xu Y, Qiao J. Association of Insulin Resistance and Elevated Androgen Levels with Polycystic Ovarian Syndrome (PCOS): A Review of Literature. J Healthc Eng. 2022;2022:9240569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 28. | Fornes R, Simin J, Nguyen MH, Cruz G, Crisosto N, van der Schaaf M, Engstrand L, Brusselaers N. Pregnancy, perinatal and childhood outcomes in women with and without polycystic ovary syndrome and metformin during pregnancy: a nationwide population-based study. Reprod Biol Endocrinol. 2022;20:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Ezeh U, Ezeh C, Pisarska MD, Azziz R. Menstrual dysfunction in polycystic ovary syndrome: association with dynamic state insulin resistance rather than hyperandrogenism. Fertil Steril. 2021;115:1557-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 30. | Hudecova M, Holte J, Olovsson M, Sundström Poromaa I. Long-term follow-up of patients with polycystic ovary syndrome: reproductive outcome and ovarian reserve. Hum Reprod. 2009;24:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 31. | West S, Lashen H, Bloigu A, Franks S, Puukka K, Ruokonen A, Järvelin MR, Tapanainen JS, Morin-Papunen L. Irregular menstruation and hyperandrogenaemia in adolescence are associated with polycystic ovary syndrome and infertility in later life: Northern Finland Birth Cohort 1986 study. Hum Reprod. 2014;29:2339-2351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Shorakae S, Ranasinha S, Abell S, Lambert G, Lambert E, de Courten B, Teede H. Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf). 2018;89:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | Rudvik A, Månsson M. Evaluation of surrogate measures of insulin sensitivity - correlation with gold standard is not enough. BMC Med Res Methodol. 2018;18:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 34. | Pantoja-Torres B, Toro-Huamanchumo CJ, Urrunaga-Pastor D, Guarnizo-Poma M, Lazaro-Alcantara H, Paico-Palacios S, Del Carmen Ranilla-Seguin V, Benites-Zapata VA; Insulin Resistance and Metabolic Syndrome Research Group. High triglycerides to HDL-cholesterol ratio is associated with insulin resistance in normal-weight healthy adults. Diabetes Metab Syndr. 2019;13:382-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 35. | Kaya MG, Gunebakmaz O, Zencir C, Yilmazsoy A, Karadag M, Topsakal R, Ergin A, Kelestimur F. An assessment of the elastic properties of the aorta in nonobese women with polycystic ovary syndrome. Fertil Steril. 2010;94:2402-2405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Kupreeva M, Diane A, Lehner R, Watts R, Ghosh M, Proctor S, Vine D. Effect of metformin and flutamide on insulin, lipogenic and androgen-estrogen signaling, and cardiometabolic risk in a PCOS-prone metabolic syndrome rodent model. Am J Physiol Endocrinol Metab. 2019;316:E16-E33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Kaya MG, Yildirim S, Calapkorur B, Akpek M, Unluhizarci K, Kelestimur F. Metformin improves endothelial function and carotid intima media thickness in patients with PCOS. Gynecol Endocrinol. 2015;31:401-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 38. | Nori W, Fleeh NH, Akram W. Will the area under the curve of the umbilical artery Doppler predict fetal growth restriction at 34 weeks of gestation among pre-eclamptic women? 2nd International Conference On Engineering & Science, 2021. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Zhu S, Cheng C, Wang LL, Zhao DJ, Zhao YL, Liu XZ. Prognostic values of optic nerve sheath diameter for comatose patients with acute stroke: An observational study. World J Clin Cases. 2022;10:12175-12183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 40. | Solem RC. Limitation of a cross-sectional study. Am J Orthod Dentofacial Orthop. 2015;148:205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 41. | Nori W, Akram W. Effect of gender on the reliability of COVID-19 rapid antigen test among elderly. World J Clin Cases. 2022;10:10820-10822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |