INTRODUCTION
The hepatorenal syndrome (HRS) occurs in patients with advanced liver disease, cirrhosis and ascites[1-4]. It is characterized by renal failure, which can be rapidly progressive [HRS type I or HRS-acute kidney injury (HRS-AKI)]. More rarely, HRS can present with a more mild course of renal dysfunction [HRS type II or HRS-chronic kidney disease (HRS-CKD)][1-4]. The definitions by the “International Club of Ascites” were revised in 2015; HRS-AKI is now defined in analogy to the definitions of acute kidney injury published by the “Kidney Disease: Improving Global Outcomes”[1-4]. Accordingly, there is no fixed threshold for serum creatinine anymore; rather, the dynamics of renal function reflected by serum creatinine have to be considered. Thus, an increase in serum creatinine by ≥ 0.3 mg/dL from baseline within 48 h or an increase in serum creatinine by ≥ 50% from baseline is considered as AKI in the context of HRS. In addition, some clinical criteria need to be met: (1) No clinical response to withdrawal of diuretics and volume expansion with albumin over a period of 48 h; (2) Absence of shock, and (3) No current or previous administration of other, potential nephrotoxic drugs[1-4]. Furthermore, an underlying, primary renal disease needs to be excluded. Renal ultrasound should be with normal findings. Moreover, urinary analysis should reveal no major pathology, i.e., proteinuria should be less than 0.5 g/d and hematuria should be absent. However, more recently it has been recognized that patients with liver disease may also develop HRS without fulfilling the criteria for AKI, i.e., non-AKI, (HRS-non-acute kidney injury, formerly HRS- type II)[1]. These patients may present with progressive, slow decline of renal function over weeks. Patients without full recovery after an episode of AKI may also fit this category. If renal impairment is present for less than 90 d, this condition shall be termed HRS-acute kidney disease (AKD); in case it persists for more than 90 d with an eGFR < 60 mL/min per 1.73 m², then it is termed HRS-CKD. There are no conclusive data available on the prognostic impact of HRS-AKD or HRS-CKD. Recently, Patidar et al[5] investigated the incidence and outcome of AKD in patients with cirrhosis and AKI; AKD was found in 2004 (31.6%) out of 6250 patients. Mortality was significantly higher in patients with AKD. Although this study did not investigate HRS-AKD, it suggests that AKD per se is a predictor for poor prognosis in cirrhosis patients. For HRS-AKI, there is indeed a considerable amount of evidence suggesting a negative impact on patient survival[6]. Thus, this Minireview focuses on HRS-AKI, formerly known as HRS-type 1.
DIAGNOSIS
The diagnosis of HRS-AKI can be challenging and other etiologies of AKI have to be considered[3,7]. The reduced effective plasma volume can result in pre-renal AKI and non-responsiveness to volume expansion is therefore an important clinical flag to distinguish HRS-AKI from pre-renal AKI. Furthermore, acute tubulus necrosis (ATN) may also cause AKI and distinction from HRS-AKI can be challenging. In the past, fractional excretion of sodium (FeS) has been used to distinguish both entities[3,7]. In HRS-AKI, FeS is below 0.2% and the urinary sodium concentration is lower than 10 mEq Na/L. In contrast, ATN-AKI is characterized by an FeS ≥ 1% and a high urinary sodium concentration ≥ 30 mEq Na/L. Newer biomarkers, e.g., Neutrophil Gelatinase-Associated Lipocalin (NGAL), have the potential to identify tubular damage and ATN but have not yet been used as routine biomarkers in the clinic[8]. Gambino et al[9] studied the value of urinary NGAL (uNGAL) to differentiate between ATN-AKI and HRS-AKI. In general, uNGAL levels were higher in ATN-AKI and significantly lower in HRS-AKI as well was in prerenal AKI. Interestingly, uNGAL levels were also significantly lower in patients with HRS-AKI responding to terlipressin/albumin treatment as compared to non-responders. Thus, uNGAL may not only serve as diagnostic biomarker but also as prognostic tool. In addition, structural renal diseases, i.e., glomerulonephritis (GN), need to be excluded, too[10,11]. IgA nephropathy (IgAN) is a common GN and a specific type with distinct histopathologic findings was described in patients with liver cirrhosis[10,11]. In most patients with IgAN, supportive therapy is the treatment of choice and immunosuppressive therapy is only needed in specific clinical settings. In patients with hepatitis B or C induced liver disease, membranous nephropathy or membranoproliferative GN may cause AKI[7,12]. In these cases, antiviral therapy has to be combined with immunosuppressive therapy. GN should be suspected if urinary abnormalities are present, such as proteinuria and/or acanthocytes. Although certainly not possible for all patients, a renal biopsy should be pursued if GN is suspected to establish the exact diagnosis.
PATHOPHYSIOLOGY
Currently, HRS-AKI is regarded as a functional and not a structural disorder of the kidney mainly mediated by reduced perfusion[13,14]. Splanchnic vasodilatation seems to be of major importance for the development of HRS-AKI; this condition is leading to vascular underfilling compensated by vasoconstrictive mechanisms and salt retention[13,14]. As a result, renal blood supply is sharply reduced. A study by Epstein et al[15] provided evidence in patients with advanced cirrhotic liver disease. Renal arteriograms were performed in five patients twice: once at recruitment and then again post-mortem. Renal blood flow was sharply diminished at recruitment and cortical blood flow of the kidney was virtually absent. Post-mortem, renal blood was normalized with a physiological perfusion pattern. These findings indicate that HRS-AKI is a transient, functional disorder. In another study by Koppel et al[16], seven renal grafts from cadaveric donors with hepatic failure and HRS were transplanted to seven recipients. In four out of seven recipients, the renal allograft was still functional at six months after renal transplantation providing further evidence for the transient nature of HRS. In a case series from the early 70s, full renal recovery was reported after orthotopic liver transplantation (OLT) of three patients with liver failure and AKI[17]. In addition, a recent clinical study investigated the recovery of native kidney function after patients underwent simultaneous liver-kidney transplantation. 28 out of 31 patients recruited suffered from HRS. After transplantation, in 26 patients with HRS a significant recovery of native kidney function was observed with a native-only estimated mean glomerular filtration rate of 49.9 ± 9.4 mL/min/1.73 m2[18].
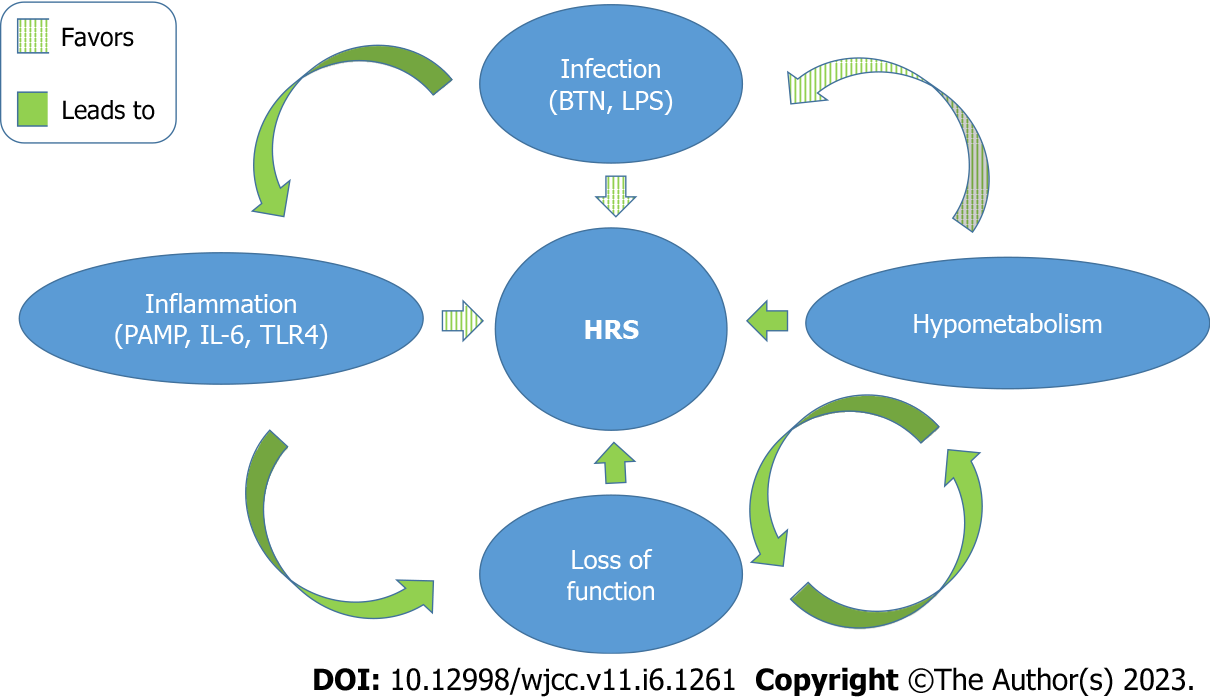
Recently, an immunologic component has been added to the puzzle[19]. Data of two prospective cohort studies introduced the hypothesis that ongoing systemic inflammation contributes to decompensation of liver cirrhosis promoting organ failure[19,20]. Especially IL-6 has been associated with increased severity of organ failure and higher mortality in decompensated liver cirrhosis. Data from the PREDICT and CANONIC trials revealed that patients with severe failure of multiple organs show the highest levels of circulating IL-6[19]. In another study by Solé et al[21], patients with HRS-AKI had significantly elevated serum levels of IL-6 and vascular cell adhesion molecule 1 (VCAM-1)[21]. Notably, patients who achieved resolution of HRS-AKI had markedly lower serum levels of VCAM-1 compared to those patients with persistent HRS-AKI. VCAM-1 serum levels predicted mortality in patients with HRS-AKI. Nevertheless, mechanisms by which systemic inflammation is induced and sustained remain unclear; in this context, bacterial translocation (BTN) was proposed as one of the drivers of inflammation[19,22]. BTN occurs at gastrointestinal sites and is defined as migration of bacteria or bacterial products to extraintestinal sites[23]. Indeed, patients with cirrhosis are susceptible to infections that stem from the intestine; one common infectious complication is spontaneous bacterial peritonitis, most probably facilitated by increased BTN. Data from human studies have confirmed that BTN is promoted by increased intestinal permeability and alterations of the gut microbial flora in patients with liver cirrhosis. BTN may also trigger the release of pathogen-associated-molecular-patterns (PAMP) such as lipopolysaccharide and thereby cause and/or exaggerate a systemic inflammatory response[19]. Some evidence also indicate that AKI might be (at least co-) facilitated by PAMP and systemic inflammation. Shah et al[24] could demonstrate that urinary Toll-like receptor 4 (TLR4) was increased in patients with liver cirrhosis and AKI as compared to patients with stable, uncomplicated cirrhosis. Moreover, the authors found increased TLR4 expression in renal tubular cells. However, there were only few patients with HRS-AKI included and TLR4 expression seemed lower in HRS-AKI when compared to non-HRS-AKI[24]. In an animal model of HRS, the role of TLR4 was studied further. Mice were subjected to bile duct ligation to induce HRS. Additional renal injury was caused by unilateral ureter obstruction. Renal function as measured by blood urea nitrogen and serum creatinine was significantly better in animals with TLR4 deficiency indicating the potential key role in development of HRS[25]. Most importantly, PAMP may induce cell hypometabolism causing a persistent metabolic disorder in peripheral organs. This pathway may be a causal driving force towards functional organ failure as in the case of HRS-AKI in association with liver cirrhosis. The close interplay between infection, inflammation, hypometabolism, loss of function and HRS is summarized in Figure 1.
Figure 1 Pathophysiology of hepatorenal syndrome.
The interplay between infection, inflammation, hypometabolism, loss of function and hepatorenal syndrome is depicted. HRS: Hepatorenal syndrome; IL-6: Interleukin 6; BTN: Bacterial translocation; PAMP: Pathogen associated molecular patterns; TLR4: Toll-like receptor 4; LPS: Lipopolysaccharide.
TREATMENT
The outcome of HRS-AKI is fatal if not treated[2,3,26]. As mainstay of pharmacologic therapy in Europe, the vasoconstrictor terlipressin is widely used in combination with albumin[2,3,26]. A recent placebo-controlled, randomized phase 3 trial investigated the efficacy of terlipressin in patients with HRS-AKI[27]. Recovery rate from HRS was significantly higher in the terlipressin group vs the placebo group (32% vs 17%, P = 0.006). However, survival was not significantly improved in patients treated with terlipressin as compared to placebo (49% vs 55%). In fact, death due to respiratory failure occurred more frequently in patients who received terlipressin (11% vs 2%). This trial showed that treatment with terlipressin is efficacious in reversal of HRS-AKI. However, the patients in this trial showed advanced renal dysfunction as indicated by the baseline serum creatinine of 3.5 mg/dL at recruitment[27]. This may have biased the study outcome and explain the lack of survival benefit in the terlipressin group despite reversal of HRS-AKI. Another recent, randomized, controlled trial compared the administration albumin vs placebo in patients with liver cirrhosis and HRS-AKI[28]. Interestingly, treatment with albumin alone did not show any beneficial effect on HRS-AKI or survival further underscoring the potential clinical value of terlipressin. Renal replacement therapy (RRT) should be considered in patients who are unresponsive to pharmacologic therapy. The application of RRT is limited by the hemodynamic status of the patient. Tatum et al[29] assessed the survival of 55 Liver transplant candidates who received RRT before transplantation[29]. In-hospital mortality was highest in patients on RRT for at least four days reaching 63.5% (4-6 d of RRT) and 59.1% (at least 7 d of RRT). Allegretti et al[30] studied the outcome of 341 non-transplant-listed patients with liver cirrhosis who became RRT-dependent during the hospital-stay. The 6-mo-survival of 56/341 patients with HRS was 16% with 4% being RRT-free. There is currently no evidence that extracorporeal liver support systems offer a lasting beneficial effect with respect to HRS[31]. Thus, RRT in the context of HRS is especially useful in patients awaiting liver transplant.
TRANSPLANTATION
Liver transplantation is the treatment of choice for HRS-AKI. Takahashi et al[32] studied a cohort of 324 patients who underwent living-related liver transplantation (LrLTX). Patients (285/324) were stratified into three groups: patients without HRS (56%), patients with HRS and treatment response (HRSr, 19%), and patients with HRS lacking treatment response (HRSn, 25%). 29/70 patients in the latter group were dialysis-dependent prior to LrLTX, whereas only 9/55 patients in the HRSr group received dialysis at any time-point prior to LrLTX. When patients with RRT were compared to the patients without RRT prior to LrLTX, the 1- year and 10-year survival was significantly decreased (79.0% vs 93.5% and 61.5% vs 80.1%, P = 0.035). Interestingly, 1-, 3- and 5-year survival was comparable between patients without HRS, HRSr and HRSn. Piano et al[4] reported similar findings in patients undergoing cadaveric OLT, i.e., 82 patients with AKI-HRS vs 259 patients without AKI-HRS. However, survival probability at year 1 after LT was not different when the AKI-HRS group was divided into responders and non-responders vs controls (80% vs 86% vs 90%). Finally, the incidence of CKD during the first-year post-transplantation was significantly higher in non-responders as compared to responders or controls. Liver transplantation is considered as potent and efficacious treatment modality in patients with HRS-AKI. However, patients with severe, refractory HRS-AKI may be at higher risk for CKD after liver transplantation.
CONCLUSION
HRS is a multifactorial syndrome and the importance of immunological processes driving the pathology of HRS has been recently noted. The therapeutic options are limited and prognosis remains poor in patients who are not eligible for transplantation. Further studies are needed to unravel the pathophysiology of HRS and to develop new therapeutic strategies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El-Gendy HA, Egypt; Silva LD, Brazil S-Editor: Liu GL L-Editor: A P-Editor: Liu GL