Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1252
Peer-review started: October 15, 2022
First decision: January 5, 2023
Revised: January 17, 2023
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: February 26, 2023
Processing time: 132 Days and 8.6 Hours
Monkeypox (mpox), is a disease from the Poxviridae family that can cause several serious medical issues. This mini-review sought to analyze the existing literature regarding the current mpox outbreak with a focus on the prevalence, diagnostics, and containment measures. Mpox cases have been reported to World Health Organization (WHO) from 85 Member States in all six WHO regions during the period of January 1, 2022, through August 3, 2022. Standardized or optimized guidelines for the clinical care of patients with mpox are limited, particularly in low-resource settings. In an effort to achieve guidance and meet standards, special attention should be paid to this outbreak in order to eradicate such a rare infectious disease by analyzing prevention and control measures. Patient outcomes may also be poor, and their illnesses may last for a long time. The spectrum of clinical symptoms, including complications and sequelae, as well as aspects of the illness may be indicators of sickness severity and complications; therefore, its clinical presentation must be better understood to improve containment measures. In addition, it is important to create and evaluate a standard of care that takes a variety of parameters into account, including antiviral, immune therapies, and clinical metrics that are particular to mpox. The global emergence of mpox has presented new challenges for public health and has called for further investigation into its epidemiological profile across international contexts.
Core Tip: Monkeypox (mpox) cases have been reported to WHO from 85 Member States in all six World Health Organization (WHO) regions from January 1, 2022, through August 3, 2022. Over 25000 laboratory-confirmed cases and 122 suspected cases, including 11 fatalities, had been reported to WHO as of August 3, 2022. Most of these cases since May 13, 2022, have come from nations lacking evidence of mpox transmission. Standardized or optimized guidelines are limited for the clinical care of patients with mpox, especially in low-resource settings. This paper aims to review the prevalence, diagnostics, and containment of mpox.
- Citation: Sanyaolu A, Marinkovic A, Okorie C, Prakash S, Haider N, Dixon Y, Izurieta R, Badaru O, Smith S. Review of the prevalence, diagnostics, and containment measures of the current mpox outbreak. World J Clin Cases 2023; 11(6): 1252-1260
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1252.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1252
The illness known as monkeypox (mpox) is brought on by a mpox virus infection. The mpox virus is a DNA virus of the Orthopoxvirus genus in the Poxviridae family, of which smallpox is also a member[1]. As a result of smallpox eradication in 1980 and the ceasing of smallpox vaccination, mpox is presently the major Orthopoxvirus to affect public health[2]. Mpox, which primarily affects Central and Western Africa regions, has recently started to spread globally[2]. Microscopically, the mpox virus presents as a brick-like virion in its structure. Two distinct strains of mpox have been found in various regions of Africa. Clade 1 has been responsible for spreading illness in the Congo Basin, whereas Clade 2 was isolated in West Africa[3]. The 2022 European/North American outbreak may have revealed a third strain that is related to Clade 2 and has been labeled Clade 3[3] and is comprised of the hMPXV-1A clade, as well as the following lineages: A1, A1.1, A.2, and B.1[4]. Initial sequence data from 15 isolates show that the DNA genome has more mutations than expected, suggesting that the circulating virus may be rapidly adapting to humans[3]. It needs to be considered that the orthopoxviral genome is plastic and that it has experience with the deletion of large regions which may allow the virus to spread faster and become more virulent[5].
The majority of mpox patients during the global outbreak in 2022 were symptomatic. Infections without symptoms seem to be uncommon. Traditional cases of mpox have a systemic sickness that includes fevers, chills, myalgias, and lymphadenopathy, a key differentiating sign, as well as a distinctive rash that must be distinguished from other vesicular eruptions (e.g., herpes simplex, varicella, and smallpox)[3]. However, some individuals have presented with vaginal, rectal, and/or oral lesions without the initial prodrome during the mpox epidemic outbreak that began in May 2022[3].
Several studies of mpox have reported complications, including secondary infections, broncho
In endemic countries of Africa, only wild animals including rodents and primates have been identified as animal reservoirs[6,8]. Nonetheless, prairie dogs in the USA and captive primates in Europe have been identified as new animal reservoirs[6]. The virus that causes mpox is primarily spread through a bite, scratch, or contact with the bodily fluids of an infected animal[3]. Additionally, it can be obtained by preparing bush meat[3]. Mpox virus transmission from person-to-person can happen in several ways: direct contact with infectious sores, scabs, or bodily fluids; indirect contact through objects that have picked up the infection, such as clothing or linens that have come into touch with contaminated body fluids or sores[3]. Though prolonged face-to-face contact might be necessary for transmission to occur via this method, the mpox virus is also thought to be spread by respiratory secretions[3]. Because the mpox virus can pass via the placenta from the mother to the fetus, it is also believed to be transmitted through vertical transmission[3]. This paper aims to review the prevalence, diagnostics, and containment of mpox.
A mini-review was conducted to narrate the epidemiological aspects associated with the current monkeypox outbreak. An electronic literature review was carried out primarily using Med Line Plus, Google Scholar, and PubMed. For the compiled data, the search was not only restricted to peer-reviewed articles released between January 1, 2014, and August 4, 2022. Grey literature sources were also visited to learn more about monkeypox cases. Keywords like the monkeypox virus, its prevalence globally, diagnostic approaches, and containment, respectively were taken into consideration when choosing articles or manuscripts. The topic’s relevance was then considered while deciding which articles to include (Figure 1).
The mpox epidemiological prevalence as reported to the World Health Organization (WHO) on August 3, 2022, is analyzed in this paper[9]. The study's focus is depicted from laboratory-confirmed cases[7]. These data points are from patients who had both confirmed and probable cases that were reported to the WHO European region[9]. Furthermore, mpox cases have been reported to WHO from 85 Member States in all six WHO regions from January 1, 2022, through August 3, 2022[9]. Over 25000 Laboratory-confirmed cases and 122 suspected cases, including 11 fatalities, had been reported to WHO as of August 3, 2022[9]. Most of these cases since May 13, 2022, have come from nations where there has never been evidence of mpox transmission[9]. Alternatively put, this is the first time that sustained chains of transmission and cases have occurred in countries without any immediate or direct epidemiological connections to West or Central Africa[9].
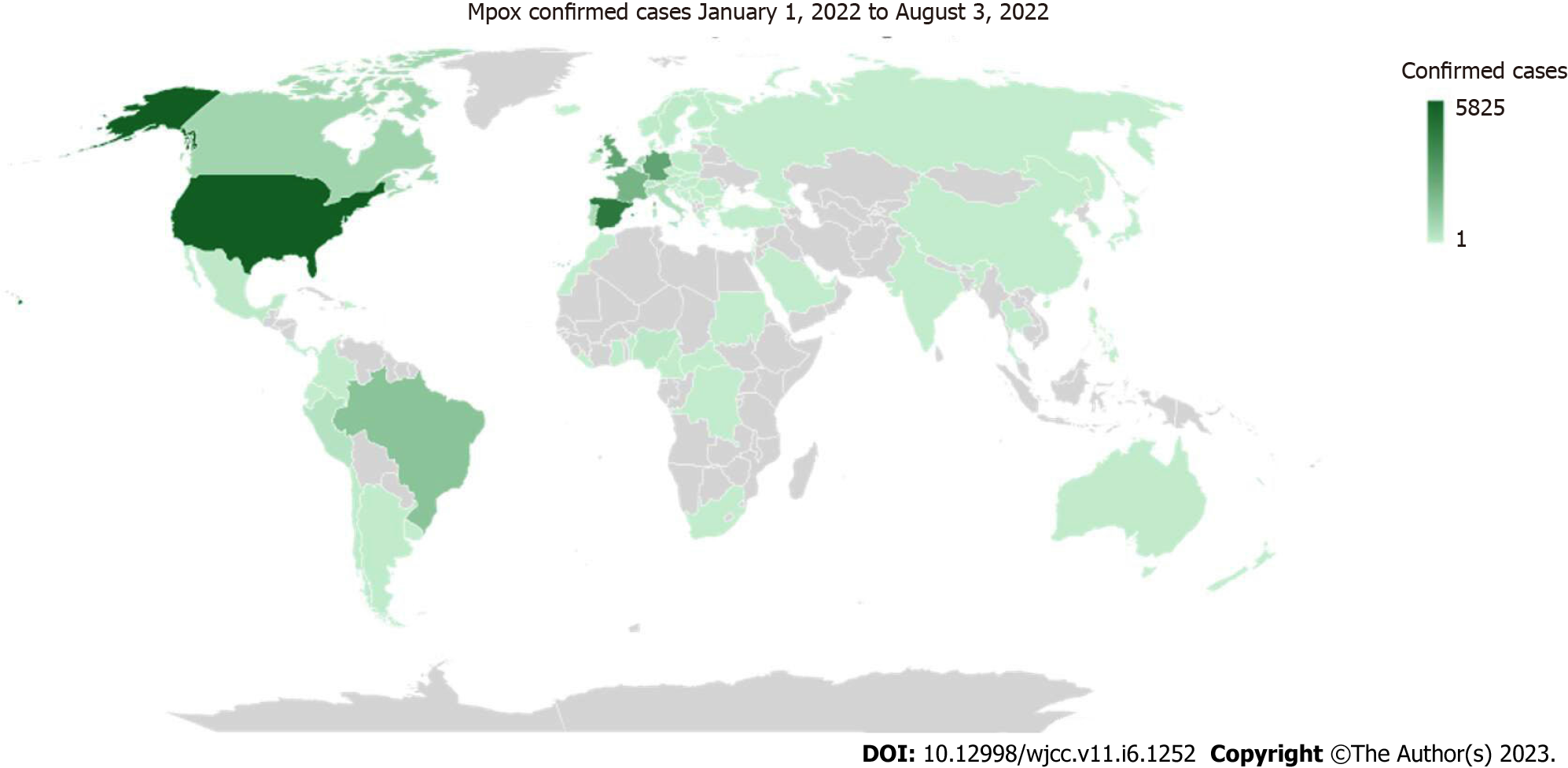
As depicted in Figure 2, the United States of America (USA) leads with 5825 or 23.2% of the confirmed cases of mpox, followed by Spain which accounted for 18.3% (4577), and the United Kingdom (UK) with 11.0% (2759)[9]. Additionally, 88.9% of all cases recorded globally are from the top ten impacted nations which also include France with 8.2% (2054), Brazil with 5.9% (1474), the Netherlands with 3.7% (927), Canada with 3.2% (803), Portugal with 2.5% (633), and Italy with 2.0% (505)[9]. Twenty nations have noted an increase in the weekly number of cases over the last week of July, with the USA reporting the largest increase[9]. As of August 3, 2022, 14 countries have reported no new cases in the past 21 d. In contrast, seven new nations (e.g., the Philippines, Uruguay, Montenegro, Sudan, Liberia, Cyprus, and Bolivia) have reported their first case within the last week[9].
The signaling pathway is a series of chemical processes in which a collection of components in a cell cooperate to regulate a cell's function. Host protection against mpox depends on type I and type II interferon signaling, natural killer cell activity, and serologic immunity[10]. Mpox can suppress interferon signaling and elude host viral detection, which can result in case fatality rates of up to 11.0%[10]. Intriguingly, analytical findings also showed that the mpox-infected rhesus monkey (Macaca mulatta) kidney epithelial (MK2) cell line model was primarily regulated by a cluster of differentiation 40 (CD40), plasmin, and histamine, whereas the mpox-infected human HeLa cell line model was primarily regulated by interferons, macrophages, and neutrophil-related signaling pathways[11]. It was also seen to have several highly significant expressed genes that were essential for the development of mpox infection in both monkey and human models, including CXCL1, TNFAIP3, BIRC3, IL6, CCL2, ZC3H12A, IL11, CSF2, LIF, PTX3, IER3, ADORA2A, and DUOX1[11]. These genes include several epigenetic regulators, including members of the histone cluster family, HIST1H3D, and HIST1H[11].
Comprehensive diagnostic approaches for mpox may help to reduce the outbreak[12-14]. Each lesion is assumed to be clonal in generalized rashes brought on by Orthopoxvirus species[15]. As a result, a genome sequence obtained from a single lesion may not accurately represent the patient's population, albeit this is not always the case for lesions that are the main sites of infection[15]. It is speculated that finding more of these parental sequences will be recovered from secondary rash lesions and a larger proportion of adaptive changes in genomes recovered from initial rash lesions of viruses closer to the zoonotic parent are more suited for disseminated infection[15]. Sequencing genomes from several lesions from both the primary and secondary rash of specific individuals is necessary to fully understand the evolution and adaptation of the mpox virus in this global outbreak[15]. By amplifying DNA fragments, a polymerase chain reaction (PCR) test of skin lesions or fluid can verify a diagnosis[16]. A patient's mpox virus status can be determined by a positive Orthopoxvirus PCR in the case of an individual who is suspected of having mpox[16]. Additionally, seminal fluid, upper respiratory fluid, blood, and urine may contain the virus nucleic acid[17-19]. Table 1 further depicts the possible diagnostic tests for identifying mpox or the Orthopoxvirus from clinical samples[20].
| Test | Definition | Pros | Cons |
| RT-PCR | Checks for the existence of DNA markers unique to mpox | Can provide a diagnosis of an active case utilizing a patient's lesion material. Viral DNA is used in the procedure and can remain stable if the material is stored in a cold, dark environment. Specifically made to target the mpox virus | Extremely sensitive tests where contamination risks are considered high. The tools and materials needed for these tests are costly. Must be carried out by trained professionals at a reputable laboratory |
| Viral culture/isolation | Live virus is grown from a patient specimen | Can produce a pure, live viral culture that will allow for accurate species categorization. Since viremia is not always present during sickness, patient samples from lesions are the most accurate for this approach | It takes many days to finish the test. Attempts to cultivate patient specimens may be hampered by the presence of bacteria. For viral identification, more classification is required. Must be carried out by trained professionals at a reputable laboratory |
| Tetracore Orthopox BioThreat Alert | Tests to see whether Orthopoxvirus antigens are present | A point-of-care diagnostic tool that may quickly diagnose an active case utilizing patient-provided lesion material. Can be done with minimal experience at room temperature | The mpox virus cannot be detected with this technique. Tests must be conducted in endemic areas. Less accurate than PCR |
| Electron microscopy | A distinct picture of a brick-shaped particle is produced by negative staining, enabling visual identification of a poxvirus and other particles | Can be used to locate viral components in a biopsy specimen, scab material, vesicular fluid, or viral culture. Can distinguish between a herpesvirus and an Orthopoxvirus | Orthopoxviruses have morphological similarities to one another. Must be carried out in a reputable laboratory with qualified personnel and an electron microscope |
| Immunohisto - chemistry | Tests to see whether Orthopoxvirus-specific antigens are present | Antigens in biopsy specimens can be found with this method. This method can be applied to eliminate or locate more suspicious agents | Not unique to the mpox virus. Must be carried out by trained professionals at a notable laboratory |
| Anti-Orthopoxvirus IgM | Tests for the presence of Orthopoxvirus antibodies | Can be used to evaluate recent Orthopoxvirus exposure, either from a disease or a smallpox vaccine. Patients with a history of smallpox vaccination who are suspected of having the Orthopoxvirus may utilize this assay as a diagnosis | Utilizes a cold chain and blood (serum) collection. The mpox virus cannot be detected with this technique. Must be carried out by trained professionals at a reputable laboratory |
| Anti-Orthopoxvirus IgG | Tests for the presence of Orthopoxvirus antibodies | Can be used to determine whether a disease or smallpox vaccine has previously exposed a person to an Orthopoxvirus | Necessitates a cold chain and the collection of blood (serum). The mpox virus cannot be detected with this technique. Previous smallpox immunization will have an impact on the results. Variable response times apply. Must be carried out by trained professionals at a reputable laboratory |
Comprehensive measures to combat the mpox virus, including increasing vaccine production and distribution, development and testing of new antiviral drugs, making testing more accessible and convenient, and collaborating with local health departments and trusted messengers in communities may mitigate the spread of the virus[12-14]. To alleviate symptoms, manage complications, and prevent long-term sequelae, clinical care for mpox should be fully optimized[2,21]. Treatment mostly focuses on reducing the effects because there are no specific drugs available for the management of mpox[22,23]. Although there are presently no therapies specifically approved against human mpox, two orally bioavailable medications, brincidofovir, and tecovirimat, have been shown to be relatively effective against Orthopoxviruses (including mpox) in animal models[17]. These two antivirals are currently recommended therapeutics in humans and may be beneficial against mpox[21]. The European Medical Association has authorized the antiviral tecovirimat, marketed under the name TPOXX, which was initially created for smallpox treatment in both children and adults[22]. Tecovirimat interacts with the F13L gene product, which codes for a phospholipase responsible to produce a protein complex that facilitates the envelopment of intracellular mature viral particles, to prevent the generation of extracellular viruses[17,24].
Despite the limitation of antiviral medication for mpox, smallpox vaccination can prevent mpox epidemics (approximately 85.0% efficient in eradicating mpox)[25]. Given worries of severe side effects in a population with an unclear immunocompromised profile, smallpox vaccinations, which are made of completely replicative vaccinia virus, are not currently used in monkeypox-endemic locations[2,20]. In 2019, a newer vaccine based on the Ankara strain of the modified attenuated vaccinia virus was authorized for the prophylaxis of mpox[2]. Moreover, JYNNEOS is the live vaccination created from the attenuated, non-replicating Orthopoxvirus strain Modified Vaccinia Ankara-Bavarian Nordic (MVA-BN)[26]. There is still a limited supply of this two-dose vaccination, despite its approval and mainstay vaccine usage for mpox from many governmental agencies[2]. Due to the cross-protection provided for the immune response to Orthopoxviruses, smallpox and mpox vaccines are created based on the vaccinia virus[2]. Lastly, under the Expanded Access Investigational New Drug (EA-IND) procedure, the ACAM2000 vaccine is also made available in some countries for use against mpox in addition to smallpox[2].
Special attention should be given to this outbreak to accomplish guidance, satisfy standards, and eradicate such a rare infectious illness by examining preventative and control techniques[2]. Transmission by respiratory droplet particles usually necessitates lengthy face-to-face contact, putting health workers, household members, and other close contacts of active cases at greater risk[27]. Due to the infectious nature of mpox, the Centers for Disease Control and Prevention (CDC) recommends that healthcare providers take the necessary steps to reduce transmission and protect themselves by using standard personal protective equipment (PPE) such as gloves, eye guards, gowns, and an N95 filter when treating patients[16]. Close contact with respiratory secretions, skin lesions of an infected individual, or contaminated things such as garments and beddings can result in human-to-human transmission[27].
In an outpatient setting, patients who have mpox or are showing signs of infection need to be isolated and have their lesions covered up. Patients, two years of age and older, need to wear face masks, while those who do not require hospitalization, particularly children and adolescents, are advised to self-quarantine at home[16]. In-patient care should include the considerations mentioned above and arrange a designated room and bathroom for the patient[16]. Infected patients must avoid contact with healthy individuals and pets until the scabs have fallen, a new layer of skin has formed, and the wounds are no longer open[16]. Healthcare providers should use a negative pressure room for situations that could cause the patient to generate oral secretions, such as intubation and extubation, with aerosol production requiring special air handling[16].
Additional guidelines also recommend bypassing actions such as sweeping, dusting, vacuuming, and fans as they can all recirculate dried particles from mpox lesions into the air[16]. There are a limited number of disinfectants approved and designated for use against specific pathogens; to counter this, the United States Environmental Protection Agency (EPA) developed EVP guidance to evaluate the efficacy of disinfectants based on data submitted by manufacturers[28]. In the case of viral disease outbreaks, the CDC recommends sterilization and using high-grade disinfectants with emerging viral pathogens (EVP) claims, a list of which can be found in the EPA’s List Q[16]. Medical waste contaminated with mpox virus, including PPE, needles, and bandages that need to be changed is considered Category A waste and should be treated as regulated medical waste[16]. The CDC, along with the EPA, the Department of Labor, the Department of Transportation, the Department of Defense, and the Assistant Secretary for Preparedness and Response classify Category A waste as any substance that can cause permanent injury or a life-threatening illness in healthy individuals after exposure[29].
Currently, there are no vaccination mandates for health care providers; however, CDC recommends those 18 years and older with a risk of direct exposure to mpox get the JYNNEOS, ACAM2000, or LC16m8 vaccine[16,30]. Post-exposure prophylaxis for those with unprotected direct contact with mpox is available with vaccinia immune globulin or tecovirimat if the vaccine is contraindicated[16]. Vaccinia immune globulin can also be used for post-exposure prophylaxis in children less than 6 mo old[16]. The effectiveness of tecovirimat as post-exposure prophylaxis and of vaccinia immune globulin to prevent mpox are still being researched[16]. There are presently no effective treatments available for mpox; although the condition is self-limiting, persons with immune system disorders, inflammatory or exfoliative skin problems, pregnancy, and children under the age of eight are at greater risk[16]. The Strategic National Stockpile has made available smallpox antivirals such as tecovirimat and brincidofovir as they may be effective for treating mpox. Tecovirimat is a first-line mpox treatment; however, its effectiveness is currently based on animal models, and brincidofovir efficacy is still being studied[16]. Vaccinia immune globulin is also a possible treatment; however, its effectiveness has yet to be determined[16]. Cidofovir, another antiviral used for Cytomegalovirus retinitis, is also being allowed access for treatment; however, both cidofovir and brincidofovir also have high toxicity profiles[16,31].
The coronavirus disease of the 2019 (COVID-19) pandemic effects is still being felt today, particularly in Latin America. The pandemic has made it essential to rapidly respond to and examine viral outbreaks in patients by taking multiple samples and various body fluids to determine where the virus resides and identify the virus and its genome[32]. The COVID-19 pandemic has also demonstrated the need to differentiate the characteristics of a viral outbreak such as mpox from other disease processes of the region in Latin America, including smallpox, cowpox, chickenpox, measles, syphilis, and the Peruvian wart[32]. Furthermore, the COVID-19 pandemic also forced healthcare providers to reconsider the potential for co-infection with other infections or conditions local to Latin America, such as carrying the human immunodeficiency virus[32].
The smallpox vaccine is approximately 85.0% effective in its protection against mpox; however, the original vaccine has not been readily available since smallpox was eliminated in 1980[22]. Therefore, another approach implemented to reduce transmission and contain the outbreak is to vaccinate close contacts of infected patients[22]. While a single source of infection has yet to be determined, there have been cases worldwide indicating a trend in sexually transmitting mpox to the community[22]. Even though mpox is not considered a sexually transmitted disease, recent cases reported from England to the UK Health Security Agency revealed an increase in prevalence among those who deny traveling to endemic areas, are gay and bisexual, and men who have sex with other men[22].
Concerns regarding the geographic distribution and continued return of mpox are growing[33,34]. Mpox outbreaks have been documented during the past 50 years in over 10 African nations and 4 non-African nations[33]. In the African region where mpox is endemic, both the weakness of surveillance and laboratories in African countries have been identified as major challenges. Many suspected cases presently exist in Africa. Democratic Republic of Congo (DRC) and Nigeria have the greatest burden of mpox in the African Region accounting for 92.0% of all cases with the DRC having 80.0% of cases. Although the report of mpox cases is lower in Africa compared to the current global outbreak, it is still a public health concern due to the limited supply of vaccines. The most prevalent explanation for the rise in mpox cases has been diminishing immunity[34], while deforestation may also be a contributing factor or possibly operate as a potentiator[33].
The characteristics of the current outbreak must be defined to determine how to best use the available tools to contain mpox[35]. Implementing screening tools in healthcare settings and maintaining a high level of suspicion with emerging clinical case definitions will aid in the identification of cases and the delineation of the outbreak’s scope[35,36]. Thus, promoting epidemiology, integrated surveillance programs, and laboratory diagnosis is crucial in affected African countries[37].
Following the eradication of smallpox, ongoing human-to-human transmission in many nations exposes the mpox virus as an emergent Orthopoxvirus infection. Moreover, the waning population immunity caused by the discontinuation of smallpox vaccination may have created a favorable environment for the resurgence of monkeypox. This study highlights the global prevalence, diagnostics, and containment measures of mpox considering the current re-emergence of cases. Scientific findings underline the significance of unusual clinical manifestations of human mpox and the demand for additional and ongoing clinico-epidemiological studies. As human-to-non-human transmission has been evidenced, the isolation of pets from mpox virus-infected individuals should be included in the control measures.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: Nigeria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang CY, Taiwan; Welter J, Switzerland S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Moore MJ, Rathish B, Zahra F. Monkeypox. Treasure Island, FL: Stat Pearls; July 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK574519/. |
| 2. | WHO. Monkeypox. Washington, DC: World Health Organization; May 19, 2022. Available from: https://www.who.int/news-room/fact-sheets/detail/monkeypox. |
| 3. | Issacs SN, Shenoy ES. Monkeypox. UpToDate. July 29, 2022. Available from: https://www.uptodate.com/contents/monkeypox. |
| 4. | eBioMedicine. Monkeypox virus outbreak: can evolution guide us to new treatments or vaccines? EBioMedicine. 2022;82:104221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Pfaff F, Hoffmann D, Beer M. Monkeypox genomic surveillance will challenge lessons learned from SARS-CoV-2. Lancet. 2022;400:22-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Seang S, Burrel S, Todesco E, Leducq V, Monsel G, Le Pluart D, Cordevant C, Pourcher V, Palich R. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 115] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 7. | MacNeill AL. Comparative Pathology of Zoonotic Orthopoxviruses. Pathogens. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 8. | Sharma A, Priyanka, Fahrni ML, Choudhary OP. Monkeypox outbreak: New zoonotic alert after the COVID-19 pandemic. Int J Surg. 2022;104:106812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | WHO. 2022 Monkeypox outbreak: Global trends. Washington, DC: World Health Organization; August 3, 2022. Available from: https://worldhealthorg.shinyapps.io/mpx_global/. |
| 10. | Al-Musa A, Chou J, LaBere B. The resurgence of a neglected orthopoxvirus: Immunologic and clinical aspects of monkeypox virus infections over the past six decades. Clin Immunol. 2022;243:109108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 11. | Xuan DTM, Yeh IJ, Wu CC, Su CY, Liu HL, Chiao CC, Ku SC, Jiang JZ, Sun Z, Ta HDK, Anuraga G, Wang CY, Yen MC. Comparison of Transcriptomic Signatures between Monkeypox-Infected Monkey and Human Cell Lines. J Immunol Res. 2022;2022:3883822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 32] [Reference Citation Analysis (0)] |
| 12. | The Lancet Regional Health-Europe. Lessons from COVID-19 are shaping the response to monkeypox outbreak. Lancet Reg Health Eur. 2022;18:100463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | The Lancet. Monkeypox: a global wake-up call. Lancet. 2022;400:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 14. | Rojek A, Dunning J, Olliaro P. Monkeypox: how will we know if the treatments work? Lancet Infect Dis. 2022;22:1269-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 15. | Ulaeto DO, Dunning J, Carroll MW. Evolutionary implications of human transmission of monkeypox: the importance of sequencing multiple lesions. Lancet Microbe. 2022;3:e639-e640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Red Book Online. Monkeypox virus outbreak. AAP. August 3, 2022. Available from: https://publications.aap.org/redbook/resources/20705?autologincheck=redirected. |
| 17. | Mileto D, Riva A, Cutrera M, Moschese D, Mancon A, Meroni L, Giacomelli A, Bestetti G, Rizzardini G, Gismondo MR, Antinori S. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med Infect Dis. 2022;49:102386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 18. | Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L, Schmid ML, Semple MG, Tunbridge AJ, Wingfield T, Price NM; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 739] [Article Influence: 246.3] [Reference Citation Analysis (0)] |
| 19. | Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos MÁ, Vilella A, Navarro M, Rodriguez-Elena L, Riera J, Català A, Martínez MJ, Blanco JL; Hospital Clinic de Barcelona Monkeypox Study Group. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 209] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 20. | McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 827] [Cited by in RCA: 638] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 21. | Evans A, AlShurman BA, Sehar H, Butt ZA. Monkeypox: A Mini-Review on the Globally Emerging Orthopoxvirus. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Thakur V, Thakur P, Srivastava S, Kumar P. Monkeypox virus (MPX) in humans a concern: Trespassing the global boundaries - Correspondence. Int J Surg. 2022;104:106703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Reynolds MG, McCollum AM, Nguete B, Shongo Lushima R, Petersen BW. Improving the Care and Treatment of Monkeypox Patients in Low-Resource Settings: Applying Evidence from Contemporary Biomedical and Smallpox Biodefense Research. Viruses. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 24. | Frenois-Veyrat G, Gallardo F, Gorgé O, Marcheteau E, Ferraris O, Baidaliuk A, Favier AL, Enfroy C, Holy X, Lourenco J, Khoury R, Nolent F, Grosenbach DW, Hruby D, Ferrier A, Iseni F, Simon-Loriere E, Tournier JN. Tecovirimat is highly efficient on the monkeypox virus lineage responsible for the international 2022 outbreak. bioRxiv. 2022.. [DOI] [Full Text] |
| 25. | Dhawan M, Emran TB, Islam F. The resurgence of monkeypox cases: Reasons, threat assessment, and possible preventive measures. Travel Med Infect Dis. 2022;49:102367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | FDA. JYNNEOS. Silver Spring, MD: Food and Drug Administration; June 21, 2021. Available from: https://www.fda.gov/media/131078/download. |
| 27. | Cheema AY, Ogedegbe OJ, Munir M, Alugba G, Ojo TK. Monkeypox: A Review of Clinical Features, Diagnosis, and Treatment. Cureus. 2022;14:e26756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 35] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 28. | EPA. Pesticide registration - Disinfectants for emerging viral pathogens (EVPs): List Q. Washington, DC: United States Environmental Protection Agency; August 2, 2022. Available from: https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q. |
| 29. | CDC. Managing solid waste contaminated with a Category A infectious substance. Atlanta, GA: Centers for Disease Control and Prevention; June 2022. Available from: https://www.phmsa.dot.gov/sites/phmsa.dot.gov/files/2022-06/Cat%20A%20Waste%20Planning%20Guidance%20-%20Final%20-%202022-06.pdf. |
| 30. | Poland GA, Kennedy RB, Tosh PK. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022;22:e349-e358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 137] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 31. | Harapan H, Ophinni Y, Megawati D, Frediansyah A, Mamada SS, Salampe M, Bin Emran T, Winardi W, Fathima R, Sirinam S, Sittikul P, Stoian AM, Nainu F, Sallam M. Monkeypox: A Comprehensive Review. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 89] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 32. | Cimerman S, Chebabo A, Cunha CAD, Barbosa AN, Rodríguez-Morales AJ. Human monkeypox preparedness in Latin America - Are we ready for the next viral zoonotic disease outbreak after COVID-19? Braz J Infect Dis. 2022;26:102372. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 33. | Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox-A potential threat? PLoS Negl Trop Dis. 2022;16:e0010141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1039] [Article Influence: 346.3] [Reference Citation Analysis (0)] |
| 34. | Kaler J, Hussain A, Flores G, Kheiri S, Desrosiers D. Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus. 2022;14:e26531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 129] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 35. | Titanji BK, Tegomoh B, Nematollahi S, Konomos M, Kulkarni PA. Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infect Dis. 2022;9:ofac310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 36. | Singhal T, Kabra SK, Lodha R. Monkeypox: A Review. Indian J Pediatr. 2022;89:955-960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 37. | Okonji OC, Okonji EF. Monkeypox during COVID-19 era in Africa: Current challenges and recommendations. Ann Med Surg (Lond). 2022;81:104381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |