Published online Feb 26, 2023. doi: 10.12998/wjcc.v11.i6.1224
Peer-review started: October 10, 2022
First decision: October 28, 2022
Revised: November 28, 2022
Accepted: February 2, 2023
Article in press: February 2, 2023
Published online: February 26, 2023
Processing time: 136 Days and 15.6 Hours
Approximately 1.5 billion chronic liver disease (CLD) cases have been estimated worldwide, encompassing a wide range of liver damage severities. Moreover, liver disease causes approximately 1.75 million deaths per year. CLD is typically characterized by the silent and progressive deterioration of liver parenchyma due to an incessant inflammatory process, cell death, over deposition of extracellular matrix proteins, and dysregulated regeneration. Overall, these processes impair the correct function of this vital organ. Cirrhosis and liver cancer are the main complications of CLD, which accounts for 3.5% of all deaths worldwide. Liver transplantation is the optimal therapeutic option for advanced liver damage. The liver is one of the most common organs transplanted; however, only 10% of liver transplants are successful. In this context, regenerative medicine has made significant progress in the design of biomaterials, such as collagen matrix scaffolds, to address the limitations of organ transplantation (e.g., low donation rates and biocompatibility). Thus, it remains crucial to continue with experimental and clinical studies to validate the use of collagen matrix scaffolds in liver disease.
Core Tip: The relevance of this review-opinion focuses on new strategies of regenerative medicine and the use of collagen matrix scaffolds as an option in the field of chronic liver disease (fibrosis/cirrhosis and hepatocellular carcinoma). Collagen matrix scaffold can be used as a niche for native or stem cells and as a carrier for antineoplastic drugs; these strategies exhibit the potential to restore liver function and address problems associated with the scarcity of organ donors.
- Citation: Martinez-Castillo M, Altamirano-Mendoza I, Zielinski R, Priebe W, Piña-Barba C, Gutierrez-Reyes G. Collagen matrix scaffolds: Future perspectives for the management of chronic liver diseases. World J Clin Cases 2023; 11(6): 1224-1235
- URL: https://www.wjgnet.com/2307-8960/full/v11/i6/1224.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i6.1224
Chronic liver disease (CLD) constitutes a complex health problem, as it can be induced by various factors, including hepatitis B virus (HBV), hepatitis C virus (HCV), alcohol abuse and nonalcoholic fatty liver disease (NAFLD), among other genetic and autoimmune conditions[1,2]. In the last 50 years, several advances have been made in the field of CLD, including the development of vaccines and antiviral regimens against viral hepatitis (HBV and HCV, respectively)[3]. Vaccination and antiviral treatment programs have reduced HBV incidence and its complications[4]. Moreover, patients with chronic HCV can be treated with direct-acting antiviral agents (DAAs)[5]. The World Health Organi
Regenerative medicine represents a promising approach in the field of tissue damage restoration and organ transplantation. Typically, tissue-engineered grafts consist mainly of three elements: scaffolds (or templates), stem cells, and growth-stimulating factors[13]. The scaffolds play a fundamental role as structural support for cell attachment, survival, and proliferation. Thus, its structure and biological and physiochemical properties must be compatible with the organ or tissue without the risk of chronic inflammation and rejection. Additionally, the ideal scaffold needs to be biodegradable and mimic the shape and function of the specific organ[14,15]. At present, several biomaterials have been reported as suitable scaffolds, including natural and synthetic polymers (e.g., polylactic acid, polyglycolic acid, and polycaprolactone)[16]. Natural biomaterial that have been modeled include collagen, fibrin, laminin, and fibronectin, which are components of the extracellular matrix (ECM). Collagen is the protein of choice during the construction of synthetic and natural scaffolds, showing great biocompatibility and minimal immunogenicity. Furthermore, this protein can be degraded by the host[15,17]. It is important to mention that the generation of extracellular matrix scaffolds (EMSs) involves lyophilization and/or electrospinning methods; thus, the associated physical or chemical treatments may affect the native properties of collagen[14]. The decellularization process is another strategy for obtaining an intact EMS. This process has gained great traction; however, it needs an organ source, which requires an allogenic or xenogeneic donor[16,18,19]. After retrieval, the organ is processed by physical (e.g., sonication, pressure gradient), chemical (e.g., detergents, acidic or basic solutions) or biological treatments (e.g., enzymes, such as trypsin and dispase) that may interfere with the compatibility and stability of the biomaterial[16]. Recently, the production of a collagen matrix scaffold (CMS) from the bone matrix after a demineralization process was described in the literature, which does not imply aggressive treatments[15,20,21]. A few studies have been conducted using ECM as a strategy to restore normal organ function in cirrhosis and HCC[18,19,22,23]. It is important to mention that the regenerative capacity of the liver can be inhibited by the excessive accumulation of ECM because it can reduce the area for liver parenchymal cell proliferation[15]. Additionally, studies in animal models have shown that stem cell transplantation promotes hepatocyte proliferation and improves liver function[24,25]. In preclinical studies, the infusion of mesenchymal stem cells (MSCs) is typically achieved by intravenous, intraarterial, intraperitoneal, intraportal, or intrasplenic routes; consequently, the number of cells and doses required are uncertain[25]. It is possible that CMS could be used as a vehicle for the direct administration of MSCs[25,26]. Moreover, the implantation of CMS after the surgical extraction of partial tissue can function as an anchor for hepatocyte proliferation, thus improving organ function[15].
Although therapeutic options largely depend on the underlying cause of liver disease, there are few proven effective treatments for advanced stages[27]. In recent years, DAAs have transformed the treatment of HCV in patients with advanced fibrosis or compensated cirrhosis. For instance, it has been shown that a long-term sustained viral response (SVR) is associated with a significant decrease in liver tissue collagen content and even regression of fibrosis in greater than 60% of patients[28]. However, the impact of such treatment in patients with decompensated cirrhosis is limited, achieving only marginal improvements[29]. The incidence of HCV-related decompensated cirrhosis and HCC are expected to decrease due to the advent of DAAs[30]. Despite these promising results, a large group of patients will still be at risk of developing HCC even after SVR has been achieved. These patients will continue to represent potential candidates for LT. Untreated HCV prior to LT results in universal recurrence of allograft infection, accelerated liver fibrosis, and subsequent graft failure[31]. The recurrence of HCV infection is universal in patients with detectable HCV RNA at the time of LT. Of the total number of recipients with post-transplantation HCV recurrence, one-third will develop cirrhosis within 5 years of LT in the absence of antiviral treatment[32]. Graft survival is lower in HCV patients compared with noninfected recipients due to various factors, such as HCV recurrences, extrahepatic manifestations of HCV infection, management issues, and complications of immunosuppression[33,34]. Complete abstinence from alcohol consumption is the cornerstone in the management of every spectrum of alcoholic liver disease (ALD)[35]. However, there are several factors that make abstinence difficult to achieve, such as lack of social support, psychiatric comorbidities, polysubstance abuse, environmental influences, and family history of alcoholism[36]. On the other hand, dietary and physical approaches are the mainstay of the management of NAFLD[37]. The amount of weight loss considered to be an effective therapeutic option is achievable in trial settings but is challenging in the clinical environment[38]. To date, several new therapeutic targets have been proposed, leading to new pharmacological therapies being tested for ALD and NAFLD; however, the majority have not been approved or evaluated in advanced liver disease[39,40].
LT is the most effective therapeutic option for patients with end-stage liver disease[41]. The procedure is typically justified in liver failure, decompensated cirrhosis (MELD ≥ 15), and/or HCC[31,35,37,41,42]. In cirrhosis, survival after LT is restricted to patients with advanced decompensation, whereas LT does not improve survival of patients with intermediate disease severity[37,43,44]. Recently, an unequivocal survival increment was demonstrated in patients with alcoholic hepatitis not responding to medical therapy compared with patients who received early transplantation[35,41,45]. However, LT is not a formal indication in all transplant centers, especially in the United States[46]. Typically, a 6-mo period of abstinence is required to identify ALD patients who will be able to refrain from alcohol consumption and not relapse after LT. However, this criterion is not mandatory in some organizations, such as the United Network for Organ Sharing, International LT Society, European Association for the Study of the Liver, and American College of Gastroenterology[35,41]. Although the requirements are changing worldwide, the number of donors is not enough to meet the demand for patients waiting for transplant. LT during end-stage liver disease related to NAFLD represents a challenge due to the high incidence of associated comorbid diseases, such as obesity, type 2 diabetes, and hypertension, with 50% of patients with BMI > 35 kg/m2 dying within the 1st year of transplantation[47,48]. However, an upper limit of BMI that contraindicates the procedure has not been identified[49]. The post-transplant survival in NAFLD is significantly higher than that in HCV (5-year survival: NAFLD 77.81% vs HCV 72.15%)[50]. Although quality of life and liver function improve in patients after LT, both decrease with time[51]. For instance, in a meta-analysis, the mean 1-, 3-, and 5-year incidence rates of recurrent and de novo NAFLD after LT were 59%, 57%, and 82% as well as 67%, 40%, and 78%, respectively[52]. Nonetheless, it has been demonstrated that the prevalence of advanced fibrosis is low after LT with values of 2%–5% at 5 years, 5%–10% at 10 years, and up to 24% reported in one of the studies that followed the patients up to 15 years[53-56]. In contrast, the recurrence of alcoholic cirrhosis was responsible for approximately 90% of deaths in recipients who resumed abusive alcohol drinking[41]. Transplant recipients have a higher incidence of cardiovascular events and neoplastic diseases. The risk of de novo malignancies increases from 6% before LT to 55% by 15 years post-LT[57]. The incidence of de novo tumors as a cause of death was at least twofold higher in patients transplanted for ALD compared to other indications[58]. Additionally, tobacco use has been particularly associated with this increased risk[58]. In addition to host factors, immunosuppression is an important contributing factor for developing malignancies. NAFLD carries an increased risk of death from cardiovascular complications and sepsis[59,60]. Screening for neoplastic and cardiovascular diseases during the transplant evaluation process is crucial[35,37,41,49]. Although the number of patients waiting for LT is expected to increase, donor availability is predicted to decrease, highlighting the demand for new therapeutic options for CLD[20,60].
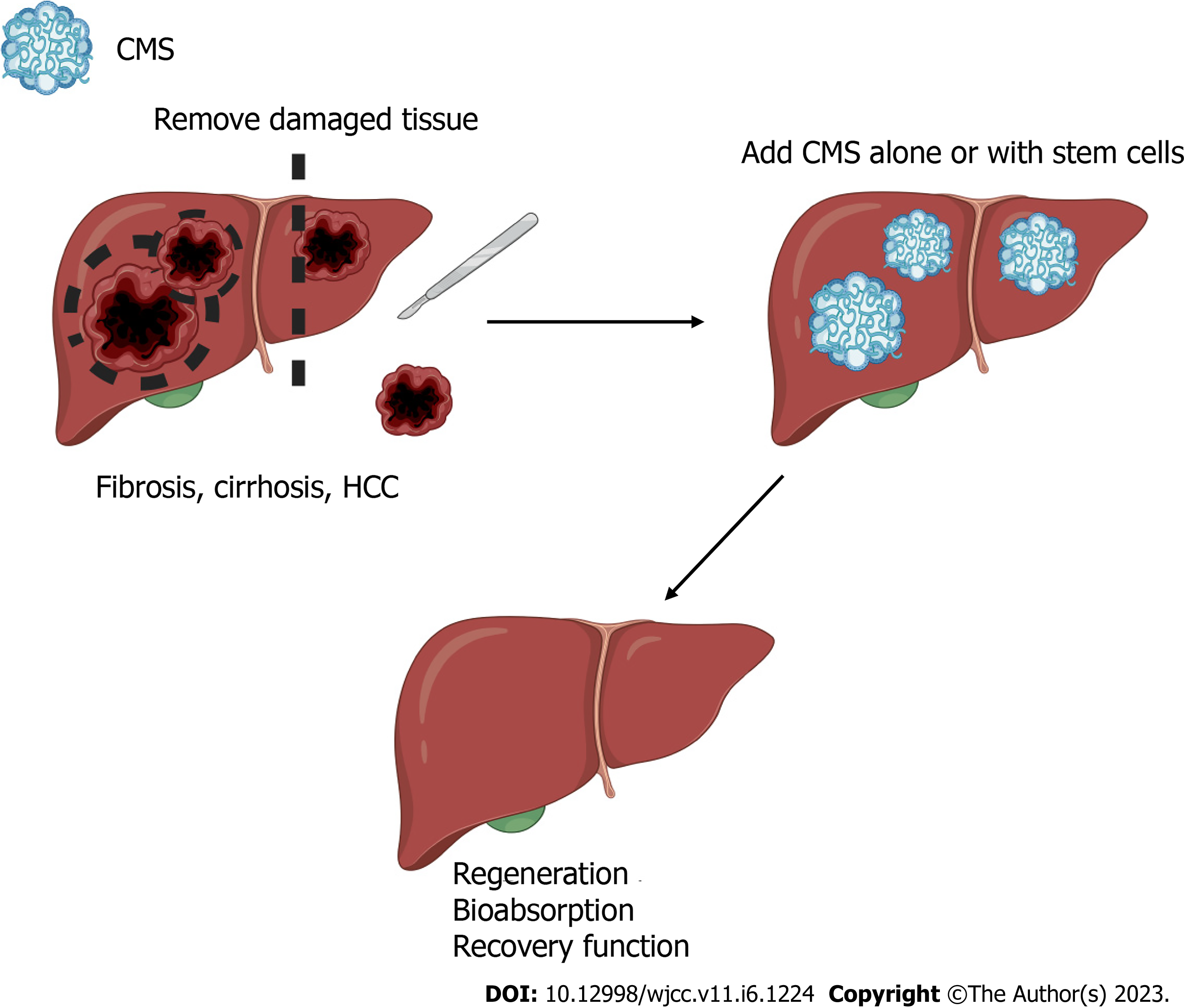
It is well understood that the main sources of fibrillar collagens in the liver are hepatic stellate cells[61]. The use of exogenous collagen during liver disease is an unconventional idea because the liver has the capability to produce and degrade its own ECM compounds[61,62]. However, it is important to highlight that dysregulation of collagen synthesis occurs in patients with liver disease and is especially marked in later stages[61-63]. For this reason, the use of CMS made with the same collagens that are present in the healthy liver could delay the progression of liver disease[15,20]. The use of CMS as a niche for liver cells was recently investigated, and positive results were revealed[15]. The authors demonstrated that CMS from bovine condyles does not cause rejection or exacerbate the inflammatory response; moreover, they demonstrated that cells like-hepatocytes, grew in the CMS. After 21 d, this biomaterial showed natural biodegradation. Nevertheless, the cellular and biological processes were not examined[15,20]. Liver regeneration has been associated with the presence of hepatic progenitor cells and a plethora of other signaling mechanisms[64,65]; however, control over regeneration is lost in CLD[65]. The allosteric effect of excessive production of ECM has been suggested as a possible mechanism that inhibits proliferation; moreover, the ratio of proliferation and apoptosis is dysregulated in advanced CLD[15,66]. The use of stem cells for tissue regeneration has been explored in several organs and tissues, but it remains an important challenge in the liver[25]. However, recent in vitro studies of human stem cells seeded in CMS have been published, showing that CMS is a good niche for this type of cells[25]. Furthermore, the use of CMS alone or in combination with MSCs can be used to restart or improve regeneration and organ function (Figure 1).
Liver cirrhosis is among the top 20 causes of disability-adjusted life years and years of life lost worldwide[67]. The incidence of liver cirrhosis is rapidly increasing worldwide, and the currently available treatment is suboptimal[68]. At present, the most effective therapeutic option is LT[41]. However, the lifelong consequences of transplantation and the scarcity of donors limits patient eligibility[69]. This leads to poor quality of life and eventually to death. Patients with cirrhosis have disease-related barriers preventing liver regeneration, but novel strategies, such as scaffolds, have driven progress toward the development of successful therapies for this condition[70,71]. In a CCl4-induced cirrhosis rat model, a comparative evaluation indicated that the group that underwent implantation of scaffolds with cultured hepatocytes displayed a better long-term recovery of liver function than the group with direct infusion of liver cells[72]. Moreover, in the same study, the authors reported better outcomes regarding liver function in the group that underwent implantation of the scaffold cultured in vitro with hepatocytes compared to the cell-free scaffold group[72]. The ECM is an important regulator of liver fibrogenesis[73]. It is well known that ECM proteins have an immunomodulatory role in the liver disease microenvironment, leading to the chemotaxis of leukocytes; modulation of growth factor and cytokine functions, such as TGF-β1 and TNF-α, fibroblast migration; and some anti-inflammatory responses[62,73,74]. Recently, ECM proteins were described as prognostic biomarkers of early-stage cirrhosis[75]. Given the role of the ECM in disease progression, the incorporation of scaffolds as models in vitro is essential in creating the appropriate microenvironment that allows investigation of underlying pathological mechanisms and/or testing new therapies. In this context, cirrhotic human 3D liver scaffolds have been obtained through a decellularization process[76]. The cirrhotic 3D scaffold was used as a novel model to evaluate the inherent features of cirrhotic human liver and the ECM microenvironment, including the efficient homing and targeting of cells to their correct localization[19,76].
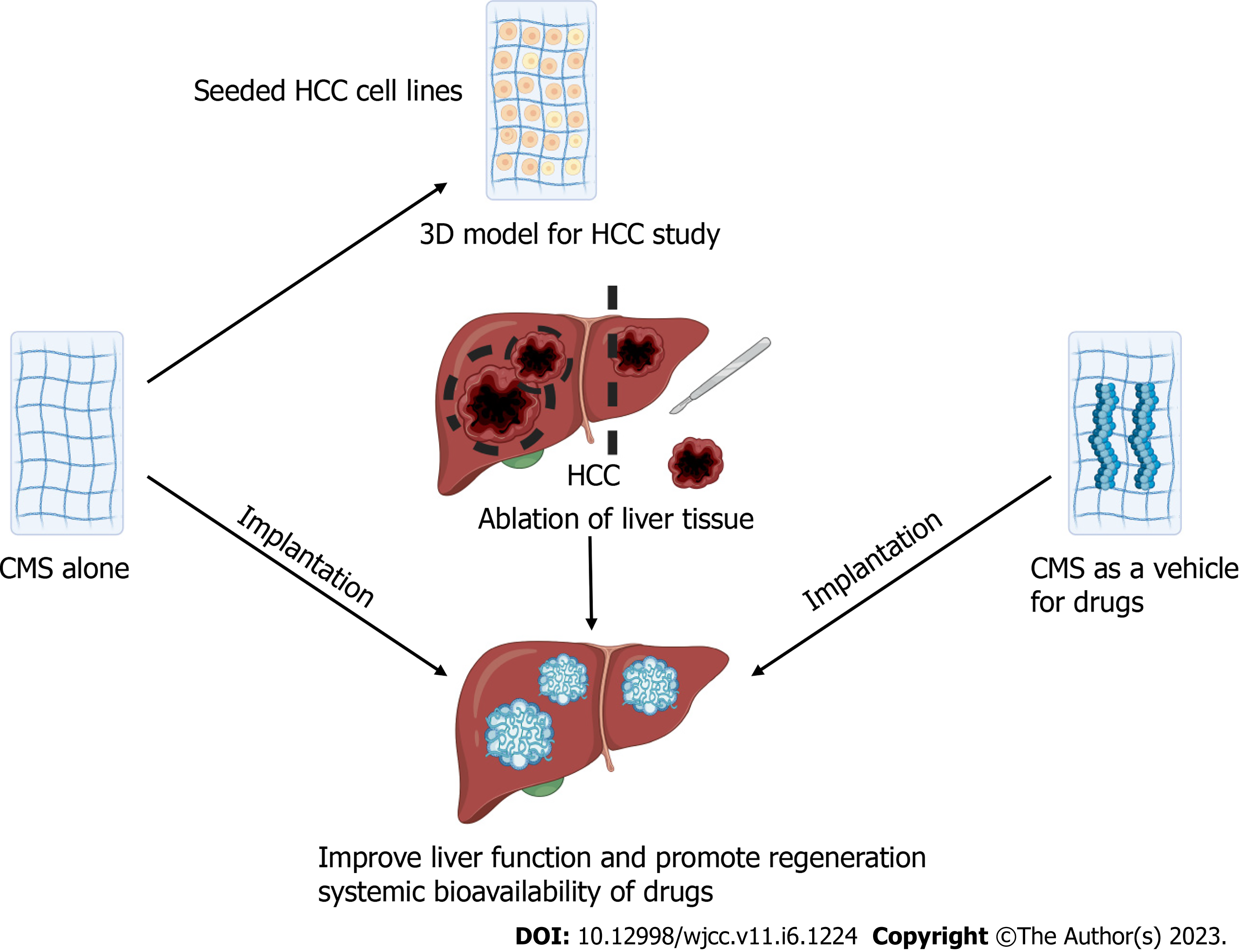
HCC is among the principal causes of cancer deaths worldwide[23]. The liver parenchyma in HCC typically exhibits necrosis, inflammation, oxidative stress, and a dysregulated ECM. These events are related to genetic alterations and deregulation of multiple signaling pathways[66,77-79]. LT, resection, novel thermal and nonthermal techniques for tumor ablation and embolization are the preferred strategies to treat HCC[23]. Moreover, only a few pharmacological options (e.g., bevacizumab, cabozantinib, lenvatinib, ramucirumab, regorafenib, and sorafenib), immunotherapy (e.g., atezolizumab, nivolumab, and pembrolizumab), and radiation therapy (e.g., conformal, stereotactic and proton beam radiation) have been used in the treatment of HCC, and these treatments are typically reserved for the advanced phase of HCC with limited success[23]. The study of HCC includes cell lines (e.g., HEP-G2, HEPA 1-6, HuH7, SK-HEP-1, Hep3B)[80,81] and animal models (e.g., orthotopic and xenotransplantation)[82,83]. Moreover, decellularized tumors have been proposed as a strategy of study[22]. Recently, decellularization of liver explants from human cirrhotic liver tissue (explant primary sclerosing cholangitis; cirrhotic 3D scaffolds) were used for the first time as a model for the evaluation of HCC[76]. Immunohistochemical staining showed that collagen types I, III and IV; fibronectin; and laminin were present after the decellularization process. The authors also compared the expression of different proteins after seeding Hep-G2 cells in cirrhotic 3D scaffolds[76]. Interestingly, their results showed that cell repopulation of cirrhotic scaffolds highlighted a unique upregulation in genes related to epithelial to mesenchymal transition and TGFβ signaling. Moreover, higher concentrations of TGFβ1 and fibronectin were produced by seed cells in cirrhotic scaffolds than in healthy scaffolds. This methodology allowed the authors to evaluate the microenvironment in HCC and healthy ECM from the liver with the possibility of identifying new potential therapeutic targets for drug development[76].
The ECM plays a pivotal role from the beginning of tumorigenesis to metastasis. Collagen, fibronectin and laminin can induce intracellular signaling that participates in apoptosis evasion, metastasis, angiogenesis, and proliferation[62]. Nevertheless, opposing results regarding the role of the ECM in tumor progression have been reported. Specifically, in pancreatic tumors, ECM composition inhibits tumor progression, whereas an increase in the deposition of ECM stimulates tumor progression in breast cancer. Thus, the role of ECM may depend on the cancer type[62]. Collagen synthesis increases in severe liver fibrosis (F3 and F4) compared with a healthy liver (fibrillar collagen types I, III and V)[84,85]. In fact, collagen and other ECM proteins are used as biomarkers to determine the stage of fibrosis and as predictors of cancer development[73,86,87]. It is possible that the use of CMS containing similar collagen to healthy livers from human or other mammal sources (such as ®Nukbone) improves the regeneration process. The use of CMS as support alone or in combination with HCC cell lines represents an excellent strategy for: (1) The evaluation of therapeutic drugs, including assessments of the IC50, stability and the diffusion ratio[26,88,89]; (2) Implantation of CMS plus HCC cells in animals to evaluate the progression of HCC in situ and/or as a metastasis model; and (3) Tumor ablation and implantation of CMS impregnated with antineoplastic drugs to ensure elimination of all malignant cells to reduce recurrence (Figure 2)[88].
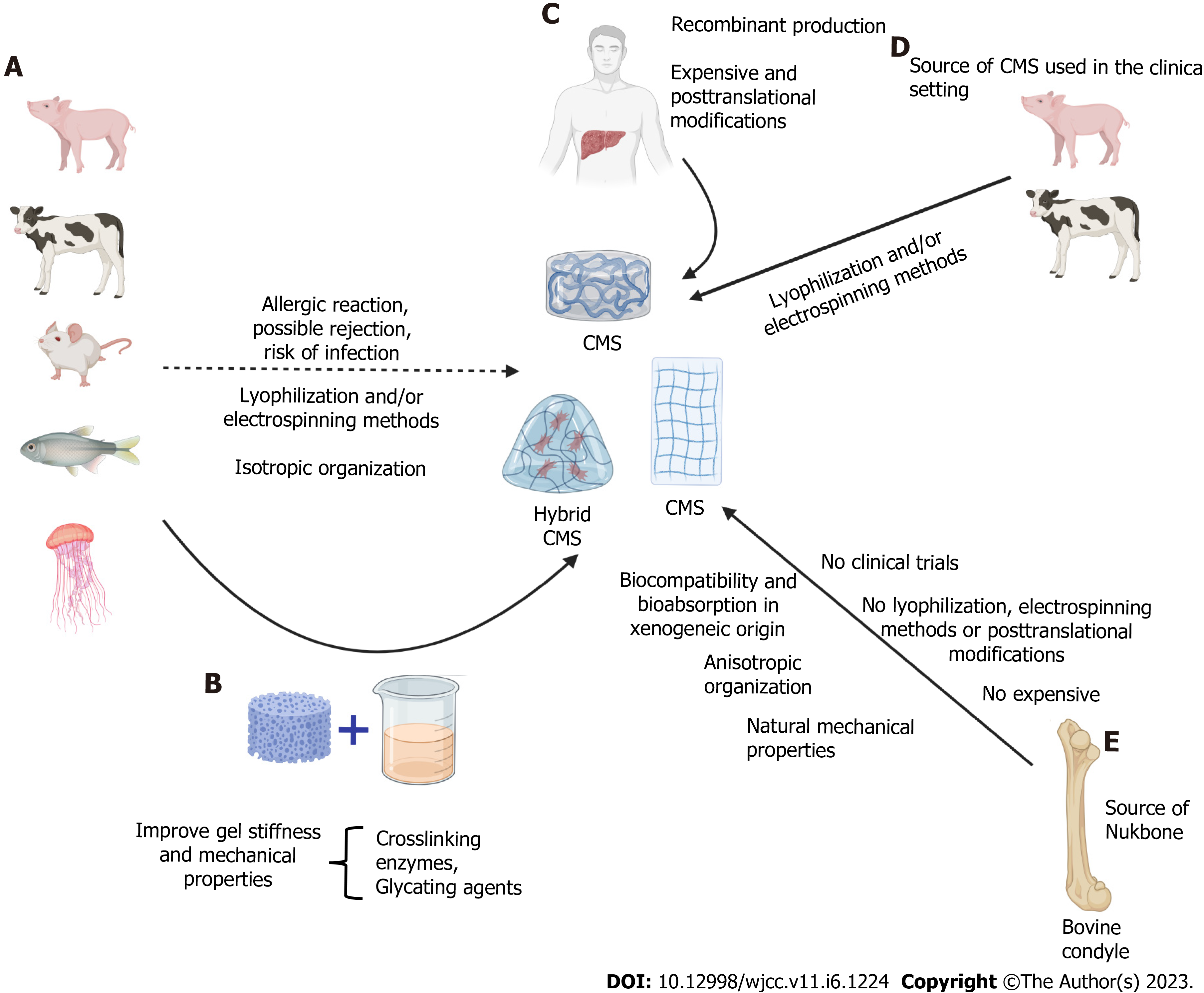
In a general context, the collagen used during CMS design is commonly derived from bovine, porcine, rodent, human, and marine sources, which are commercially available[90,91]. Collagen obtained from these sources exhibits differences in primary amino acid sequence, and it has been estimated that approximately 3% of the population is allergic to bovine collagen I[91-93]. Human collagen is the ideal source of collagen to eliminate certain concerns associated with xenogeneic sources, but the mass production of recombinant collagen is unsustainably expensive. Furthermore, the synthesis of hybrid collagen matrices has been proposed as a method to extract and purify collagen and modify its mechanical properties. Another important consideration is that during the creation of CMSs, the fiber arrangement/alignment is not equal to native disposition (anisotropic)[17]. However, this last concern seems to be eliminated with the incorporation of new strategies, such as CMS from Nukbone®[15,20,94]. Despite the disadvantages mentioned, collagen from bovine and porcine sources is widely and successfully used in clinical settings[95,96] (Figure 3).
It is important to mention that the predominant type of collagen in healthy liver is collagen I, III and V, whereas the dysregulation of several collagens has been reported during liver disease progression induced by different factors[86]. In this sense, it was reported that patients with ALD displayed higher levels of type III collagen in the cirrhosis stage than healthy controls[97]. Moreover, collagen III formation progressively supersedes the degradation of this type of collagen. However, increased collagen VI degradation compared with synthesis (PRO-C3) was noted in the same stages[97]. In a similar manner, PRO-C3 (marker of synthesis) allowed discrimination of F3 and F4 in NAFLD, and the results revealed superior ROCs at this stage compared with the aspartate aminotransferase to platelet ratio index, FIB-4, and NAFLD fibrosis score[98]. In addition, collagens III, IV, V, and VI showed significant increases from early to late fibrosis (F4 or cirrhosis) in hepatitis C. Collagen IV was the most useful discriminator between early and late stages, whereas collagen V and VI showed the strongest expression in early fibrosis stages[99]. Taken together, these studies provide evidence that the synthesis and degradation of collagens is not a static process. The extirpation of liver zones with excessive deposition of atypical collagens followed by the implantation of CMS that mimics normal liver tissue collagen, such as CMS from Nukbone®, could improve and restore normal liver function[15]. However, it is important to research the implications of the use of different types of collagen in the context of CLD and CMS during fibrosis, cirrhosis, and HCC induced by the different etiologies.
The use of CMS in CLD is a promising tissue engineering strategy to recover liver function. It avoids the use of organs from donors and, thus, also sidesteps the transplant waiting list, compatibility issues, pre- and postoperative care (immunosuppression), and other ethical considerations. The use of CMS also represents an exciting and important, novel tool for the development and evaluation of pharmacological options for cirrhosis and HCC. Furthermore, CMS could even be developed in the future as a treatment targeting the early stages of liver disease, including fibrosis.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Mexico
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Corrales FJ, Spain; Tanaka N, Japan S-Editor: Xing YX L-Editor: Filipodia P-Editor: Xing YX
| 1. | Sepanlou SG, Safiri S, Bisignano C, Ikuta KS, Merat S, Saberifiroozi M, Poustchi H, Tsoi D, Colombara DV, Abdoli A, Adedoyin RA, Afarideh M, Agrawal S, Ahmad S, Ahmadian E, Ahmadpour E, Akinyemiju T, Akunna CJ, Alipour V, Almasi-Hashiani A, Almulhim AM, Al-Raddadi RM, Alvis-Guzman N, Anber NH, Angus C, Anoushiravani A, Arabloo J, Araya EM, Asmelash D, Ataeinia B, Ataro Z, Atout MMW, Ausloos F, Awasthi A, Badawi A, Banach M, Ramirez DFB, Bhagavathula AS, Bhala N, Bhattacharyya K, Biondi A, Bolla SR, Boloor A, Borzi AM, Butt ZA, Camera LLAA, Campos-Nonato IR, Carvalho F, Chu DT, Chung SC, Cortesi PA, Costa VM, Cowie BC, Daryani A, de Courten B, Demoz GT, Desai R, Dharmaratne SD, Djalalinia S, Do HT, Dorostkar F, Drake TM, Dubey M, Duncan BB, Effiong A, Eftekhari A, Elsharkawy A, Etemadi A, Farahmand M, Farzadfar F, Fernandes E, Filip I, Fischer F, Gebremedhin KBB, Geta B, Gilani SA, Gill PS, Gutierrez RA, Haile MT, Haj-Mirzaian A, Hamid SS, Hasankhani M, Hasanzadeh A, Hashemian M, Hassen HY, Hay SI, Hayat K, Heidari B, Henok A, Hoang CL, Hostiuc M, Hostiuc S, Hsieh VCR, Igumbor EU, Ilesanmi OS, Irvani SSN, Balalami NJ, James SL, Jeemon P, Jha RP, Jonas JB, Jozwiak JJ, Kabir A, Kasaeian A, Kassaye HG, Kefale AT, Khan RKMA, Khan EA, Khater A, Kim YJ, Koyanagi A, La Vecchia C, Lim LL, Lopez AD, Lorkowski S, Lotufo PA, Lozano R, Abd El Razek MM, Mai HT, Manafi N, Manafi A, Mansournia MA, Mantovani LG, Mazzaglia G, Mehta D, Mendoza W, Menezes RG, Mengesha MM, Meretoja TJ, Mestrovic T, Miazgowski B, Miller TR, Mirrakhimov EM, Mithra P, Moazen B, Moghadaszadeh M, Mohammadian-Hafshejani A, Mohammed S, Mokdad AH, Montero-Zamora PA, Moradi G, Naimzada MD, Nayak V, Negoi I, Nguyen TH, Ofori-Asenso R, Oh IH, Olagunju TO, Padubidri JR, Pakshir K, Pana A, Pathak M, Pourshams A, Rabiee N, Radfar A, Rafiei A, Ramezanzadeh K, Rana SMM, Rawaf S, Rawaf DL, Reiner RC, Roever L, Room R, Roshandel G, Safari S, Samy AM, Sanabria J, Sartorius B, Schmidt MI, Senthilkumaran S, Shaikh MA, Sharif M, Sharifi A, Shigematsu M, Singh JA, Soheili A, Suleria HAR, Teklehaimanot BF, Tesfay BE, Vacante M, Vahedian-Azimi A, Valdez PR, Vasankari TJ, Vu GT, Waheed Y, Weldegwergs KG, Werdecker A, Westerman R, Wondafrash DZ, Wondmieneh AB, Yeshitila YG, Yonemoto N, Yu CH, Zaidi Z, Zarghi A, Zelber-Sagi S, Zewdie KA, Zhang ZJ, Zhao XJ, Naghavi M, Malekzadeh R. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:245-266. [PubMed] [DOI] [Full Text] |
| 2. | Cheemerla S, Balakrishnan M. Global Epidemiology of Chronic Liver Disease. Clin Liver Dis (Hoboken). 2021;17:365-370. [PubMed] [DOI] [Full Text] |
| 3. | Almeida PH, Matielo CEL, Curvelo LA, Rocco RA, Felga G, Della Guardia B, Boteon YL. Update on the management and treatment of viral hepatitis. World J Gastroenterol. 2021;27:3249-3261. [PubMed] [DOI] [Full Text] |
| 4. | Nelson NP, Easterbrook PJ, McMahon BJ. Epidemiology of Hepatitis B Virus Infection and Impact of Vaccination on Disease. Clin Liver Dis. 2016;20:607-628. [PubMed] [DOI] [Full Text] |
| 5. | Das D, Pandya M. Recent Advancement of Direct-acting Antiviral Agents (DAAs) in Hepatitis C Therapy. Mini Rev Med Chem. 2018;18:584-596. [PubMed] [DOI] [Full Text] |
| 6. | Pedrana A, Munari S, Stoove M, Doyle J, Hellard M. The phases of hepatitis C elimination: achieving WHO elimination targets. Lancet Gastroenterol Hepatol. 2021;6:6-8. [PubMed] [DOI] [Full Text] |
| 7. | Dhiman RK, Premkumar M. Hepatitis C Virus Elimination by 2030: Conquering Mount Improbable. Clin Liver Dis (Hoboken). 2021;16:254-261. [PubMed] [DOI] [Full Text] |
| 8. | Beste LA. Concerns About Direct-Acting Antiviral Agents for Hepatitis C-Cause for Reassurance. JAMA Netw Open. 2019;2:1-2. [PubMed] [DOI] [Full Text] |
| 9. | Zeng QL, Li ZQ, Liang HX, Xu GH, Li CX, Zhang DW, Li W, Sun CY, Wang FS, Yu ZJ. Unexpected high incidence of hepatocellular carcinoma in patients with hepatitis C in the era of DAAs: Too alarming? J Hepatol. 2016;65:1068-1069. [PubMed] [DOI] [Full Text] |
| 10. | O'Beirne J. Liver Transplantation for Alcoholic Liver Disease: Absence of Evidence for the Relevance of Abstinence. Dig Dis Sci. 2020;65:1599. [PubMed] [DOI] [Full Text] |
| 11. | Satapathy SK, Bernstein DE, Roth NC. Liver transplantation in patients with non-alcoholic steatohepatitis and alcohol-related liver disease: the dust is yet to settle. Transl Gastroenterol Hepatol. 2022;7-23. [PubMed] [DOI] [Full Text] |
| 12. | Younossi ZM, Stepanova M, Younossi Y, Golabi P, Mishra A, Rafiq N, Henry L. Epidemiology of chronic liver diseases in the USA in the past three decades. Gut. 2020;69:564-568. [PubMed] [DOI] [Full Text] |
| 13. | Matsuzaki Y, John K, Shoji T, Shinoka T. The Evolution of Tissue Engineered Vascular Graft Technologies: From Preclinical Trials to Advancing Patient Care. Appl Sci (Basel). 2019;9:1274. [PubMed] [DOI] [Full Text] |
| 14. | Dong CJ, Lv YG. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers (Basel). 2016;8:42. [PubMed] [DOI] [Full Text] |
| 15. | Martinez-Castillo M, Leon-Mancilla B, Ramirez-Rico G, Alfaro A, Perez-Torres A, Diaz-Infante D, Garcia-Loya J, Medina-Avila Z, Sanchez-Hernandez J, Pina-Barba C, Gutierrez-Reyes G. Xenoimplant of Collagen Matrix Scaffold in Liver Tissue as a Niche for Liver Cells. Front Med (Lausanne). 2022;9:1-13. [PubMed] [DOI] [Full Text] |
| 16. | Gilpin A, Yang Y. Decellularization Strategies for Regenerative Medicine: From Processing Techniques to Applications. Biomed Res Int. 2017;2017:1-13. [PubMed] [DOI] [Full Text] |
| 17. | Patil VA, Masters KS. Engineered Collagen Matrices. Bioengineering (Basel). 2020;7:1-20. [PubMed] [DOI] [Full Text] |
| 18. |
Shimoda H, Yagi H, Higashi H, Tajima K, Kuroda K, Abe Y, Kitago M, Shinoda M, Kitagawa Y.
Decellularized liver scaffolds promote liver regeneration after partial hepatectomy |
| 19. | Mazza G, Rombouts K, Hall AR, Urbani L, Luong TV, Al-Akkad W, Longato L, Brown D, Maghsoudlou P, Dhillon AP, Fuller B, Davidson B, Moore K, Dhar D, De Coppi P, Malago M, Pinzani M. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:1-15. [PubMed] [DOI] [Full Text] |
| 20. | Leon-Mancilla B, Martinez-Castillo M, Medina-Avila Z, Perez-Torres A, Garcia-Loya J, Alfaro-Cruz A, Pina-Barba C, Gutierrez-Reyes G. Three-Dimensional Collagen Matrix Scaffold Implantation as a Liver Regeneration Strategy. J Vis Exp. 2021;1-16. [PubMed] [DOI] [Full Text] |
| 21. | Castillo JFCD, Valdes-Gutierrez GA, Elizondo-Vazquez F, Perez-Ortiz O, Barba MCP, Leon-Mancilla BH. Bone loss treatment, pseudoarthrosis, arthrodesis and benign tumors using xenoimplant: clinical study. Cir Cir. 2009;77:267-271. [PubMed] |
| 22. | Garcia-Gareta E, Perez MA, Garcia-Aznar JM. Decellularization of tumours: A new frontier in tissue engineering. J Tissue Eng. 2022;13:1-16. [PubMed] [DOI] [Full Text] |
| 23. | Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J, Finn RS. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7:1-6. [PubMed] [DOI] [Full Text] |
| 24. | Esrefoglu M. Role of stem cells in repair of liver injury: Experimental and clinical benefit of transferred stem cells on liver failure. World J Gastroenterol. 2013;19:6757-6773. [PubMed] [DOI] [Full Text] |
| 25. | Li S, Bi Y, Duan Z, Chang Y, Hong F, Chen Y. Stem cell transplantation for treating liver diseases: progress and remaining challenges. Am J Transl Res. 2021;13:3954-3966. [PubMed] |
| 26. | Romagnoli C, D'Asta F, Brandi ML. Drug delivery using composite scaffolds in the context of bone tissue engineering. Clin Cases Miner Bone Metab. 2013;10:155-161. [PubMed] |
| 27. | Riazi K, Azhari H, Charette JH, Underwood FE, King JA, Afshar EE, Swain MG, Congly SE, Kaplan GG, Shaheen AA. The prevalence and incidence of NAFLD worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2022;7:851-861. [PubMed] [DOI] [Full Text] |
| 28. | Kronborg TM, Ytting H, Hobolth L, Moller S, Kimer N. Novel Anti-inflammatory Treatments in Cirrhosis. A Literature-Based Study. Front Med (Lausanne). 2021;8:1-19. [PubMed] [DOI] [Full Text] |
| 29. | D'Ambrosio R, Aghemo A, Rumi MG, Ronchi G, Donato MF, Paradis V, Colombo M, Bedossa P. A morphometric and immunohistochemical study to assess the benefit of a sustained virological response in hepatitis C virus patients with cirrhosis. Hepatology. 2012;56:532-543. [PubMed] [DOI] [Full Text] |
| 30. | Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, Zeuzem S, Landis CS, Kwo P, Hassan M, Manns MP, Vainorius M, Akushevich L, Nelson DR, Fried MW, Reddy KR. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020;73:540-548. [PubMed] [DOI] [Full Text] |
| 31. | Durand F, Francoz C. The future of liver transplantation for viral hepatitis. Liver Int. 2017;37 Suppl 1:130-135. [PubMed] [DOI] [Full Text] |
| 32. | Garcia-Retortillo M, Forns X, Feliu A, Moitinho E, Costa J, Navasa M, Rimola A, Rodes J. Hepatitis C virus kinetics during and immediately after liver transplantation. Hepatology. 2002;35:680-687. [PubMed] [DOI] [Full Text] |
| 33. | EASL, Clinical Practice Guidelines Panel C, representative EGB, Panel memebers. EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020;73:1170-1218. [PubMed] [DOI] [Full Text] |
| 34. | Samuel D, Forns X, Berenguer M, Trautwein C, Burroughs A, Rizzetto M, Trepo C. Report of the monothematic EASL conference on liver transplantation for viral hepatitis (Paris, France, January 12-14, 2006). J Hepatol. 2006;45:127-143. [PubMed] [DOI] [Full Text] |
| 35. | Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. 2018;113:175-194. [PubMed] [DOI] [Full Text] |
| 36. | Matthews LA, Lucey MR. Psychosocial Evaluation in Liver Transplantation for Patients with Alcohol-Related Liver Disease. Clin Liver Dis (Hoboken). 2022;19:17-20. [PubMed] [DOI] [Full Text] |
| 37. | Marchesini G, Roden M, Vettor R. Response to: Comment to "EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease". J Hepatol. 2017;66:466-467. [PubMed] [DOI] [Full Text] |
| 38. | Kenneally S, Sier JH, Moore JB. Efficacy of dietary and physical activity intervention in non-alcoholic fatty liver disease: a systematic review. BMJ Open Gastroenterol. 2017;4:1-12. [PubMed] [DOI] [Full Text] |
| 39. | Zhang C, Yang M. Current Options and Future Directions for NAFLD and NASH Treatment. Int J Mol Sci. 2021;22:7571. [PubMed] [DOI] [Full Text] |
| 40. | Singal AK, Shah VH. Current trials and novel therapeutic targets for alcoholic hepatitis. J Hepatol. 2019;70:305-313. [PubMed] [DOI] [Full Text] |
| 41. | EASL. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J Hepatol. 2018;69:154-181. [PubMed] [DOI] [Full Text] |
| 42. | Mahmud N. Selection for Liver Transplantation: Indications and Evaluation. Curr Hepatol Rep. 2020;19:203-212. [PubMed] [DOI] [Full Text] |
| 43. | Poynard T, Naveau S, Doffoel M, Boudjema K, Vanlemmens C, Mantion G, Messner M, Launois B, Samuel D, Cherqui D, Pageaux G, Bernard PH, Calmus Y, Zarski JP, Miguet JP, Chaput JC. Evaluation of efficacy of liver transplantation in alcoholic cirrhosis using matched and simulated controls: 5-year survival. J Hepatol. 1999;30:1130-1137. [PubMed] [DOI] [Full Text] |
| 44. | Vanlemmens C, Di Martino V, Milan C, Messner M, Minello A, Duvoux C, Poynard T, Perarnau JM, Piquet MAA, Pageaux GP, Dharancy S, Silvain C, Hillaire S, Thiefin G, Vinel JP, Hillon P, Collin E, Mantion G, Miguet JP, Grp TS. Immediate Listing for Liver Transplantation Versus Standard Care for Child-Pugh Stage B Alcoholic Cirrhosis A Randomized Trial. Ann Intern Med. 2009;150:153-161. [PubMed] [DOI] [Full Text] |
| 45. | Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, Castel H, Duhamel A, Pageaux GP, Leroy V, Dharancy S, Louvet A, Boleslawski E, Lucidi V, Gustot T, Francoz C, Letoublon C, Castaing D, Belghiti J, Donckier V, Pruvot FR, Duclos-Vallee JC. Early Liver Transplantation for Severe Alcoholic Hepatitis. New Engl J Med. 2011;365:1790-1800. [PubMed] [DOI] [Full Text] |
| 46. | Hasanin M, Dubay DA, McGuire BM, Schiano T, Singal AK. Liver transplantation for alcoholic hepatitis: A survey of liver transplant centers. Liver Transplant. 2015;21:1449-1452. [PubMed] [DOI] [Full Text] |
| 47. | Heuer M, Kaiser GM, Kahraman A, Banysch M, Saner FH, Mathe Z, Gerken G, Paul A, Canbay A, Treckmann JW. Liver Transplantation in Nonalcoholic Steatohepatitis Is Associated with High Mortality and Post-Transplant Complications: A Single-Center Experience. Digestion. 2012;86:107-113. [PubMed] [DOI] [Full Text] |
| 48. | Steggerda JA, Mahendraraj K, Todo T, Noureddin M. Clinical considerations in the management of non-alcoholic steatohepatitis cirrhosis pre- and post-transplant: A multi-system challenge. World J Gastroenterol. 2020;26:4018-4035. [PubMed] [DOI] [Full Text] |
| 49. | Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328-357. [PubMed] [DOI] [Full Text] |
| 50. | Cholankeril G, Wong RJ, Hu M, Perumpail RB, Yoo ER, Puri P, Younossi ZM, Harrison SA, Ahmed A. Liver Transplantation for Nonalcoholic Steatohepatitis in the US: Temporal Trends and Outcomes. Dig Dis Sci. 2017;62:2915-2922. [PubMed] [DOI] [Full Text] |
| 51. | Ruppert K, Kuo S, DiMartini A, Balan V. In a 12-year study, sustainability of quality of life benefits after liver transplantation varies with pretransplantation diagnosis. Gastroenterology. 2010;139:1619-1629, 1629 e1. [PubMed] [DOI] [Full Text] |
| 52. | Saeed N, Glass L, Sharma P, Shannon C, Sonnenday CJ, Tincopa MA. Incidence and Risks for Nonalcoholic Fatty Liver Disease and Steatohepatitis Post-liver Transplant: Systematic Review and Meta-analysis. Transplantation. 2019;103:e345-e354. [PubMed] [DOI] [Full Text] |
| 53. | Shetty A, Giron F, Divatia MK, Ahmad MI, Kodali S, Victor D. Nonalcoholic Fatty Liver Disease after Liver Transplant. J Clin Transl Hepatol. 2021;9:428-435. [PubMed] [DOI] [Full Text] |
| 54. | Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431-439. [PubMed] [DOI] [Full Text] |
| 55. | Bhati C, Idowu MO, Sanyal AJ, Rivera M, Driscoll C, Stravitz RT, Kohli DR, Matherly S, Puri P, Gilles H, Cotterell A, Levy M, Sterling RK, Luketic VA, Lee H, Sharma A, Siddiqui MS. Long-term Outcomes in Patients Undergoing Liver Transplantation for Nonalcoholic Steatohepatitis-Related Cirrhosis. Transplantation. 2017;101:1867-1874. [PubMed] [DOI] [Full Text] |
| 56. | Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684-689. [PubMed] [DOI] [Full Text] |
| 57. | Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, Slooff MJ, Jansen PL. Increased cancer risk after liver transplantation: a population-based study. J Hepatol. 2001;34:84-91. [PubMed] [DOI] [Full Text] |
| 58. | Herrero JI, Pardo F, D'Avola D, Alegre F, Rotellar F, Inarrairaegui M, Marti P, Sangro B, Quiroga J. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl. 2011;17:402-408. [PubMed] [DOI] [Full Text] |
| 59. | Zhou GP, Jiang YZ, Sun LY, Zhu ZJ. Clinical evidence of outcomes following liver transplantation in patients with nonalcoholic steatohepatitis: An updated meta-analysis and systematic review. Int J Surg. 2022;104:1-12. [PubMed] [DOI] [Full Text] |
| 60. | Wang X, Li J, Riaz DR, Shi G, Liu C, Dai Y. Outcomes of liver transplantation for nonalcoholic steatohepatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2014;12:394-402 e391. [PubMed] [DOI] [Full Text] |
| 61. | Wells RG. Cellular sources of extracellular matrix in hepatic fibrosis. Clin Liver Dis. 2008;12:759-768. [PubMed] [DOI] [Full Text] |
| 62. | Pickup MW, Mouw JK, Weaver VM. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep. 2014;15:1243-1253. [PubMed] [DOI] [Full Text] |
| 63. | Martinez-Castillo M, Hernandez-Barragan A, Flores-Vasconcelos I, Galicia-Moreno M, Rosique-Oramas D, Perez-Hernandez JL, Higuera-De la Tijera F, Montalvo-Jave EE, Torre-Delgadillo A, Cordero-Perez P, Munoz-Espinosa L, Kershenobich D, Gutierrez-Reyes G. Production and activity of matrix metalloproteinases during liver fibrosis progression of chronic hepatitis C patients. World J Hepatol. 2021;13:218-232. [PubMed] [DOI] [Full Text] |
| 64. | Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40-55. [PubMed] [DOI] [Full Text] |
| 65. | Gilgenkrantz H, Collin de l'Hortet A. Understanding Liver Regeneration: From Mechanisms to Regenerative Medicine. Am J Pathol. 2018;188:1316-1327. [PubMed] [DOI] [Full Text] |
| 66. | Fabregat I. Dysregulation of apoptosis in hepatocellular carcinoma cells. World J Gastroenterol. 2009;15:513-520. [PubMed] [DOI] [Full Text] |
| 67. | Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019;70:151-171. [PubMed] [DOI] [Full Text] |
| 68. | Zhai M, Liu Z, Long J, Zhou Q, Yang L, Liu S, Dai Y. The incidence trends of liver cirrhosis caused by nonalcoholic steatohepatitis via the GBD study 2017. Sci Rep. 2021;11:5195. [PubMed] [DOI] [Full Text] |
| 69. | Toro-Diaz H, Mayorga ME, Barritt AS, Orman ES, Wheeler SB. Predicting Liver Transplant Capacity Using Discrete Event Simulation. Med Decis Making. 2015;35:784-796. [PubMed] [DOI] [Full Text] |
| 70. | Bizzaro D, Russo FP, Burra P. New Perspectives in Liver Transplantation: From Regeneration to Bioengineering. Bioengineering (Basel). 2019;6:1-19. [PubMed] [DOI] [Full Text] |
| 71. | Ali M, Payne SL. Biomaterial-based cell delivery strategies to promote liver regeneration. Biomater Res. 2021;25:5. [PubMed] [DOI] [Full Text] |
| 72. | Kokorev O, Hodorenko V, Chekalkin T, Gunther V, Kang SB, Chang MJ, Kang JH. Evaluation of allogenic hepato-tissue engineered in porous TiNi-based scaffolds for liver regeneration in a CCl4-induced cirrhosis rat model. Biomed Phys Eng Expr. 2019;5:1-13. |
| 73. | Karsdal MA, Manon-Jensen T, Genovese F, Kristensen JH, Nielsen MJ, Sand JMB, Hansen NUB, Bay-Jensen AC, Bager CL, Krag A, Blanchard A, Krarup H, Leeming DJ, Schuppan D. Novel insights into the function and dynamics of extracellular matrix in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G807-G830. [PubMed] [DOI] [Full Text] |
| 74. | McQuitty CE, Williams R, Chokshi S, Urbani L. Immunomodulatory Role of the Extracellular Matrix Within the Liver Disease Microenvironment. Front Immunol. 2020;11:574276. [PubMed] [DOI] [Full Text] |
| 75. | Wu Y, Cao Y, Xu K, Zhu Y, Qiao Y, Wu Y, Chen J, Li C, Zeng R, Ge G. Dynamically remodeled hepatic extracellular matrix predicts prognosis of early-stage cirrhosis. Cell Death Dis. 2021;12:163. [PubMed] [DOI] [Full Text] |
| 76. | Mazza G, Telese A, Al-Akkad W, Frenguelli L, Levi A, Marrali M, Longato L, Thanapirom K, Vilia MG, Lombardi B, Crowley C, Crawford M, Karsdal MA, Leeming DJ, Marrone G, Bottcher K, Robinson B, Del Rio Hernandez A, Tamburrino D, Spoletini G, Malago M, Hall AR, Godovac-Zimmermann J, Luong TV, De Coppi P, Pinzani M, Rombouts K. Cirrhotic Human Liver Extracellular Matrix 3D Scaffolds Promote Smad-Dependent TGF-beta 1 Epithelial Mesenchymal Transition. Cells. 2019;9:83. [PubMed] [DOI] [Full Text] |
| 77. | Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2:1-10. [PubMed] [DOI] [Full Text] |
| 78. | Wang Z, Li Z, Ye Y, Xie L, Li W. Oxidative Stress and Liver Cancer: Etiology and Therapeutic Targets. Oxid Med Cell Longev. 2016;2016:7891574. [PubMed] [DOI] [Full Text] |
| 79. | Keenan BP, Fong L, Kelley RK. Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer. 2019;7:267. [PubMed] [DOI] [Full Text] |
| 80. | Mu H, Lin KX, Zhao H, Xing S, Li C, Liu F, Lu HZ, Zhang Z, Sun YL, Yan XY, Cai JQ, Zhao XH. Identification of biomarkers for hepatocellular carcinoma by semiquantitative immunocytochemistry. World J Gastroenterol. 2014;20:5826-5838. [PubMed] [DOI] [Full Text] |
| 81. | Arzumanian VA, Kiseleva OI, Poverennaya EV. The Curious Case of the HepG2 Cell Line: 40 Years of Expertise. Int J Mol Sci. 2021;22:13135. [PubMed] [DOI] [Full Text] |
| 82. | Lee TK, Na KS, Kim J, Jeong HJ. Establishment of animal models with orthotopic hepatocellular carcinoma. Nucl Med Mol Imaging. 2014;48:173-179. [PubMed] [DOI] [Full Text] |
| 83. | Wu T, Heuillard E, Lindner V, Bou About G, Ignat M, Dillenseger JP, Anton N, Dalimier E, Gosse F, Foure G, Blindauer F, Giraudeau C, El-Saghire H, Bouhadjar M, Calligaro C, Sorg T, Choquet P, Vandamme T, Ferrand C, Marescaux J, Baumert TF, Diana M, Pessaux P, Robinet E. Multimodal imaging of a humanized orthotopic model of hepatocellular carcinoma in immunodeficient mice. Sci Rep. 2016;6:35230. [PubMed] [DOI] [Full Text] |
| 84. | Yamamoto M, Sumiyoshi H, Nakagami K, Tahara E. Distribution of collagen types I, III, and V in fibrotic and neoplastic human liver. Acta Pathol Jpn. 1984;34:77-86. [PubMed] [DOI] [Full Text] |
| 85. | Acharya P, Chouhan K, Weiskirchen S, Weiskirchen R. Cellular Mechanisms of Liver Fibrosis. Front Pharmacol. 2021;12:671640. [PubMed] [DOI] [Full Text] |
| 86. | Karsdal MA, Daniels SJ, Holm Nielsen S, Bager C, Rasmussen DGK, Loomba R, Surabattula R, Villesen IF, Luo Y, Shevell D, Gudmann NS, Nielsen MJ, George J, Christian R, Leeming DJ, Schuppan D. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020;40:736-750. [PubMed] [DOI] [Full Text] |
| 87. | Hong WS, Hong SI, Park SY, Son Y, Lee YS, Chung YH, Yang SK, Suh DJ, Min YI. Elevation of serum type IV collagen in liver cancer as well as liver cirrhosis. Anticancer Res. 1995;15:2777-2780. [PubMed] |
| 88. | Hwang J, Sullivan MO, Kiick KL. Targeted Drug Delivery via the Use of ECM-Mimetic Materials. Front Bioeng Biotechnol. 2020;8:69. [PubMed] [DOI] [Full Text] |
| 89. | Akcora BO, Gabriel AV, Ortiz-Perez A, Bansal R. Pharmacological inhibition of STAT3 pathway ameliorates acute liver injury in vivo via inactivation of inflammatory macrophages and hepatic stellate cells. FASEB Bioadv. 2020;2:77-89. [PubMed] [DOI] [Full Text] |
| 90. | Felician FF, Xia CL, Qi WY, Xu HM. Collagen from Marine Biological Sources and Medical Applications. Chem Biodivers. 2018;15:1-18. [PubMed] [DOI] [Full Text] |
| 91. | Park SH, Song T, Bae TS, Khang G, Choi BH, Park SR, Min BH. Comparative analysis of collagens extracted from different animal sources for application of cartilage tissue engineering. Int J Precis Eng Man. 2012;13:2059-2066. |
| 92. | Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014-1017. [PubMed] [DOI] [Full Text] |
| 93. | Davison-Kotler E, Marshall WS, Garcia-Gareta E. Sources of Collagen for Biomaterials in Skin Wound Healing. Bioengineering (Basel). 2019;6:56. [PubMed] [DOI] [Full Text] |
| 94. | Rodriguez-Fuentes N, Rodriguez-Hernandez AG, Enriquez-Jimenez J, Alcantara-Quintana LE, Fuentes-Mera L, Pina-Barba MC, Zepeda-Rodriguez A, Ambrosio JR. Nukbone promotes proliferation and osteoblastic differentiation of mesenchymal stem cells from human amniotic membrane. Biochem Biophys Res Commun. 2013;434:676-680. [PubMed] [DOI] [Full Text] |
| 95. | Smith M, McFetridge P, Bodamyali T, Chaudhuri JB, Howell JA, Stevens CR, Horrocks M. Porcine-derived collagen as a scaffold for tissue engineering. Food Bioprod Process. 2000;78:19-24. |
| 96. | Badylak SE. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377-383. [PubMed] [DOI] [Full Text] |
| 97. | Thiele M, Johansen S, Gudmann NS, Madsen B, Kjaergaard M, Nielsen MJ, Leeming DJ, Jacobsen S, Bendtsen F, Moller S, Detlefsen S, Karsdal M, Krag A; Consortium GALAXY. Progressive alcohol-related liver fibrosis is characterised by imbalanced collagen formation and degradation. Aliment Pharmacol Ther. 2021;54:1070-1080. [PubMed] [DOI] [Full Text] |
| 98. | Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, Karsdal MA, Grove JI, Neil Guha I, Kawaguchi T, Torimura T, McLeod D, Akiba J, Kaye P, de Boer B, Aithal GP, Adams LA, George J. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology. 2019;69:1075-1086. [PubMed] [DOI] [Full Text] |
| 99. | Chen W, Rock JB, Yearsley MM, Ferrell LD, Frankel WL. Different collagen types show distinct rates of increase from early to late stages of hepatitis C-related liver fibrosis. Hum Pathol. 2014;45:160-165. [PubMed] [DOI] [Full Text] |