Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.989
Peer-review started: December 2, 2022
First decision: January 17, 2023
Revised: January 17, 2023
Accepted: January 28, 2023
Article in press: January 28, 2023
Published online: February 16, 2023
Processing time: 73 Days and 13.4 Hours
Pleural effusion usually causes a diagnostic dilemma with a long list of differential diagnoses. Many studies found a high prevalence of pleural effusions in critically ill and mechanically ventilated patients, with a wide range of variable prevalence rates of up to 50%-60% in some studies. This review emphasizes the importance of pleural effusion diagnosis and management in patients admitted to the intensive care unit (ICU). The original disease that caused pleural effusion can be the exact cause of ICU admission. There is an impairment in the pleural fluid turnover and cycling in critically ill and mechanically ventilated patients. There are also many difficulties in diagnosing pleural effusion in the ICU, including clinical, radiological, and even laboratory difficulties. These difficulties are due to unusual presentation, inability to undergo some diagnostic procedures, and heterogenous results of some of the performed tests. Pleural effusion can affect the patient’s outcome and prognosis due to the hemodynamics and lung mechanics changes in these patients, who usually have frequent comorbidities. Similarly, pleural effusion drainage can modify the ICU-admitted patient’s outcome. Finally, pleural effusion analysis can change the original diagnosis in some cases and redirect the management toward a different way.
Core Tip: Pleural effusion has a high prevalence in critically ill patients. It poses a significant challenge in the intensive care unit with diagnostic and therapeutic difficulties. These difficulties are due to unusual presentation, inability to undergo some diagnostic procedures, and heterogenous results of some of the performed tests. Pleural effusion impacts the outcome in patients who need intensive care unit care. Consequently, proper management of pleural effusion significantly improves the patient’s prognosis.
- Citation: Bediwy AS, Al-Biltagi M, Saeed NK, Bediwy HA, Elbeltagi R. Pleural effusion in critically ill patients and intensive care setting. World J Clin Cases 2023; 11(5): 989-999
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/989.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.989
Pleural effusion is a common finding in patients who need intensive care management. It could occur in more than half of these patients. The presence of pleural effusion could hint at the underlying pathology affecting the patients in the intensive care setting and expect their prognosis. It can also explain some challenges healthcare professionals and intensivists face while managing such patients. For example, difficult weaning of mechanically ventilated patients may be caused by the presence of pleural effusion[1]. Therefore, this review aims to highlight the importance of the presence of pleural effusion in patients in the intensive care setting,
To create an evidence-based visualization of this aim, we conducted a systematic literature review by searching the existing electronic databases, including PubMed, PubMed Central, Cochrane Library, Embase, Scopus, Cumulative Index to Nursing and Allied Health Literature, Library, and Information Science Abstracts, Web of Science, and the National Library of Medicine catalog up until October 31, 2022, using the keywords: Pleural effusion, intensive care unit, mechanical ventilation, diagnosis, drainage, pigtail catheters.
The pleural cavity lies between the parietal pleura, lining the chest wall, and the visceral pleura, covering the lung surface. Mesothelial cells line the pleura with the projection of microvilli into the pleural cavity. Naturally, pleural space contains about 0.1-0.2 mL/kg of pleural fluid, with a potential space that can accommodate up to four litres of fluid (about 50 mL/kg)[2].
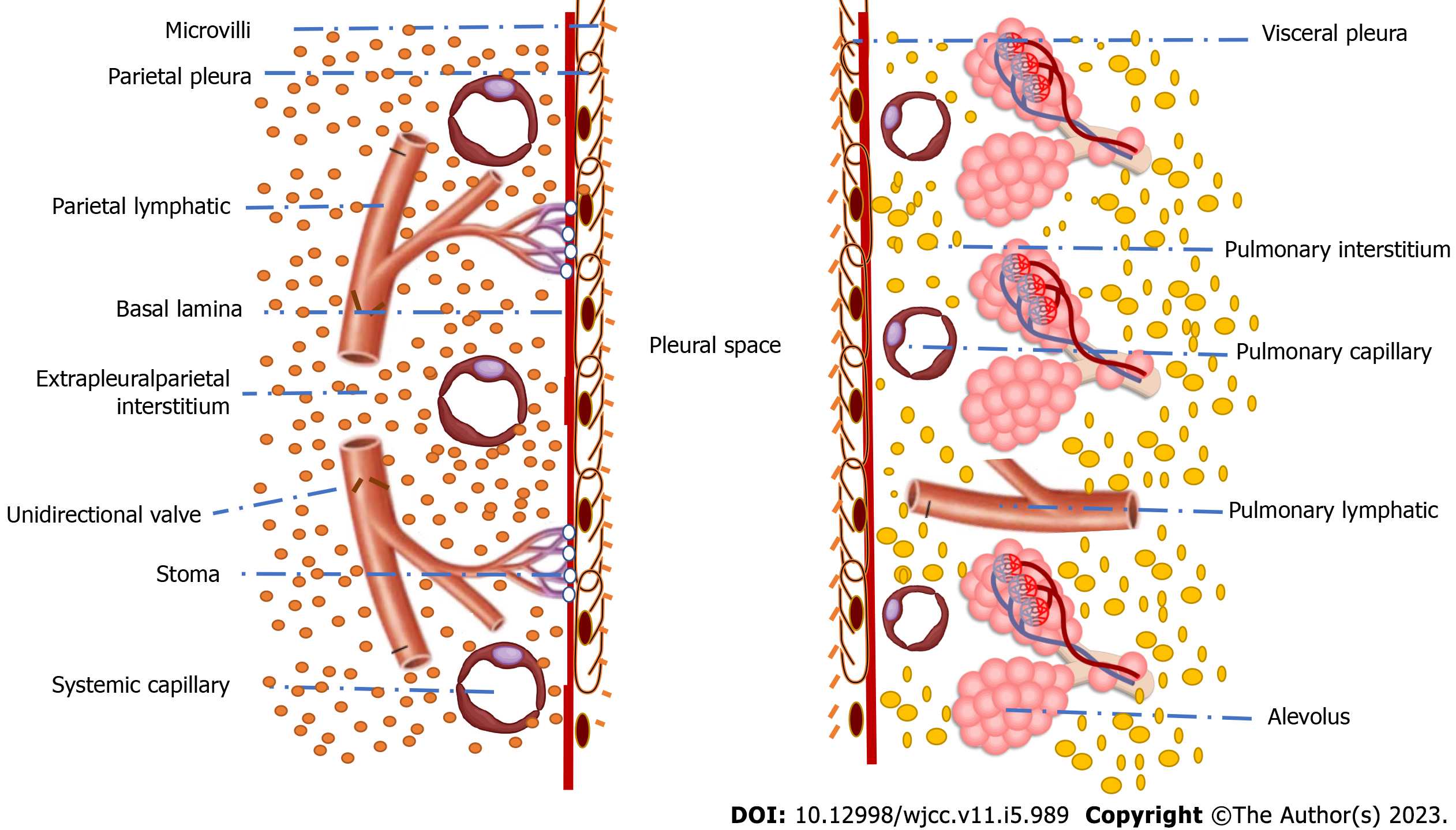
The pleura and pleural cavity facilitate lung inflation and deflation by smoothing the friction against the chest wall. Pleura can transmit the force produced by the respiratory muscles to the lungs. The negative pressure created in the pleural cavity at the functional residual capacity is about 0.66 kPa. This negative pressure helps to suck the capillary fluids and gases from the surrounding tissues into the pleural cavity. There is a hydrostatic pressure gradient of about 2.5 kPa between the parietal pleura capillaries supplied by systemic arterioles (pressure is about four kPa) and visceral pleura capillaries supplied by pulmonary arteries (pressure is about 1.5 kPa). While the oncotic plasma pressure is the same in both capillary types (pressure is about 4.66 kPa), the pleural osmotic pressure is low (only about 0.8 kPa) due to the low protein content and the inability of the protein to escape from adjacent healthy capillaries[3]. Figure 1 shows the microscopic anatomic structures of the visceral and parietal pleura and the pleural cavity through which the pleural fluid has its turnover process (formation and reabsorption). Parietal pleural microcirculation (systemic) and lymphatics, visceral pleural microcirculation (systemic and pulmonary) and lymphatics, and pleural cavity are the spaces through which the process of pleural fluid turnover is done.
Consequently, a net force of 0.8 kPa drives fluid from the parietal capillaries to the pleural cavity (+4.66 - 0.66 - 4 - 0.8 = -0.8 kPa). Simultaneously, a net force of 1.7 kPa drives the pleural fluid towards the visceral capillaries and lymphatics (+4.66 -0.66 - 1.5 - 0.8 = +1.7 kPa). Therefore, low-protein fluid is regularly transferred from the parietal to the pleural space. The pleural fluid pH usually is alkaline (pH 7.6) with low protein contents (less than 1.5 g/dL)[2-4]. Starling's equation described the pleural fluid turnover as follows:
Jv = Kf[( HP1 - HP2) - σ (π1 - π2)]. Jv is the water flux, Kf is the filtration coefficient, HP and π are the hydrostatic pressure and the colloid osmotic pressure, respectively, of the different spaces, and σ is the solute reflection coefficient of the membrane.
This model suggests that pleural fluid is a filtrate produced by the vessels around the parietal pleura and then reabsorbed through the visceral pleura. This theory has many criticisms, as the differential absorption of both fluid and protein across the semipermeable visceral pleura will lead to protein accumulation over time[4,5]. Evidence suggests little fluid reabsorption through the visceral pleura. The pleural fluid's major homeostatic processes are the microcirculatory filtration in the parietal pleura and the lymphatic drainage from the pleural space via parietal lymphatics. Pleural lymphatics are pulsatile under normal physiological conditions because of the intrinsic smooth muscle rhythm and the extrinsic tissue pressure oscillations due to respiratory movements. The pleural lymphatics' pulsatile nature creates negative sub-atmospheric pressure (about -10 cm H2O) within the lymphatics, acting as a vacuum to increase pleural fluid drainage[6].
The pleural fluid has a continuous dynamic state, with 30%-75% of the fluid turning over hourly. This process is accelerated by excess lung movements, as during exercise. The protein and other particles have less rapid turnover as their absorption is through the lymphatic vessels only. The stomata leading into lymphatics, together with the valves of lymphatic vessels, help transport protein and particles from the pleural cavity. With inflammation or neoplasm affecting the parietal pleura, protein reabsorption decreases with subsequent alteration of the fluid hydrodynamics leading to increased effusion size[2].
Under normal conditions, the pressure inside the pleural cavity is negative (sub-atmospheric). When pleural fluid accumulates, the pressure increases and gradually becomes positive depending on the amount of the accumulated fluid and the compliance of the surrounding structures such as the lungs, heart, and chest wall. This effect is more marked on the pleural basal parts due to the additional effect of gravity[1,4].
The presence of pleural effusion induces a restrictive lung function pattern with subnormal carbon monoxide transfer factor and reduced vital capacity, total lung capacity, and functional residual capacity, resulting in hypoxemia in some cases. These changes may result from the pleural effusion-induced positive intrapleural pressure, leading to impaired respiratory mechanics and lung expansion. Experimental studies showed that pleural effusions reduce respiratory system compliance and increase intrapulmonary shunt with subsequent hypoxemia[7-9].
Pleural effusion could also affect haemodynamics, as the effusion-induced increase in intrapleural pressure leads to lung collapse and increased pulmonary vascular resistance. Experimentally induced pleural effusion in animal models could result in right ventricular failure and collapse. Similar consequences of right ventricular diastolic dysfunction and collapse can occur in patients with pleural effusion. The effusion-induced haemodynamics impairment rapidly improves after drainage, as seen in cardiac surgical cases, hepatic hydrothorax, and malignant pleural effusion[10-12].
Pulmonologists usually classify the pleural effusions according to the accumulated fluid type into transudate and exudate. Transudate fluid has low protein due to the impaired balance between the hydrostatic and/or oncotic pressure in both the pleural cavity and blood vessels, leading to an increased fluid entrance rate to the pleural cavity compared to the rate of turnover. On the other hand, the exudate type of pleural effusion has a high protein content due to pleural injury, increased pleural membrane permeability, and/or fluid extravasation from blood vessels to the pleural cavity. Other types of pleural effusion include haemothorax, chylothorax, and pseudo-chylous effusion. With the accumulation of blood in the pleural space, haemothorax can result from chest trauma or surgery. Chylothorax resulting from the accumulation of lymph in the pleural cavity can result from malignancy or trauma involving the lymphatic channels, especially the thoracic duct leading to the leak of lymph toward pleural space. Pseudochylous or chyliform pleural effusion usually occurs because of long-standing pleural effusion, mostly secondary to rheumatoid diseases. In this condition, the fluid contains a large quantity of lipids, and its color is milky, like the chylous effusion. However, it differs from the chylous effusion by the absence of chylomicrons and thoracic duct lesions[13-15]. Table 1 shows the causes of both transudative and exudative pleural effusion.
| Causes of transudative pleural fluid | Causes of exudative pleural fluid |
| Increased hydrostatic pressure: Congestive heart failure; constrictive pericarditis; pericardial effusion; massive pulmonary embolism; constrictive cardiomyopathy; pulmonary veno-occlusive disease | Infections: Parapneumonic effusion; complication of lung abscess; acquired immune deficiency syndrome; tuberculosis; fungal and actinomycotic disease; hantavirus syndrome; subphrenic abscess; hepatic amoebiasis |
| Reduced capillary oncotic pressure: Liver cirrhosis (hepatic hydrothorax); nephrotic syndrome; protein-losing enteropathy; malnutrition; small bowel disease | Neoplasm: Mesothelioma; metastasis; lymphoma; Meigs syndrome; rare tumors such as pleural sarcoma |
| Transmission from peritoneum: All causes of ascites; peritoneal dialysis; liver transplantation; ventriculoperitoneal shunt | Connective tissue diseases and immune disorders: Rheumatoid disease; systemic lupus erythematosus; post-myocardial infarction/cardiotomy syndrome; churg-Strauss syndrome; Wegener’s granulomatosis; rheumatic fever; Behcet syndrome; lymphangioleiomyomatosis |
| Increased capillary permeability: Small pulmonary emboli; myxoedema | |
| Obstructed lung lymphatics: Lung transplantation | Abdominal diseases: Pancreatitis and pancreatic-pleural fistula. uraemia; other causes of peritoneal exudates |
| Others: Urinothorax; cerebrospinal fluid leakage into the pleura; trapped lung; central venous catheter migration | Others: Pulmonary embolism, sarcoidosis, drug reactions, radiation exposure, asbestos exposure, recurrent polyserositis, yellow nails syndrome, oesophageal rupture, superior vena cava syndrome, endometriosis, amyloidosis, extra-medullary hematopoiesis |
Diagnosis of the cause of the pleural effusion is usually challenging. It requires a systematic approach starting with a detailed history and physical examination. History of exposures (environmental or occupational, e.g., asbestos exposure), past infection (e.g., tuberculosis), underlying known disease (e.g., rheumatoid arthritis, heart failure, pneumonia), and symptoms related to a disease not yet diagnosed (e.g., symptoms of myositis or arthritis) may suggest the cause of the pleural effusion. Symptoms usually depend on the amount of fluid, rate of accumulation, and the underlying cause. The patient may be asymptomatic even with large pleural effusions with accidental discovery during regular workups for other conditions. The symptoms include cough (dry or productive according to the causative condition), pleuritic chest pain, and dyspnoea. Physical examination reveals a reduced tactile vocal fremitus, stony dullness on percussion, and diminished or absent breath sounds. During the early stages of the disease, a pleural rub may also be present. A thorough physical examination may be beneficial even with the less common pleural effusion causes. Abnormal nails with lymphedema suggest yellow nail syndrome, and a malar rash indicates lupus pleuritis[1,3,16].
After initial history taking and physical examination, a list of differential diagnoses arises that becomes narrowed by further investigations. Chest X-ray is usually the most crucial technique for initial diagnosis. Obliteration of the costo-phrenic angle and homogeneous opacity rising toward the axilla with no air bronchogram are the classic radiological findings of pleural effusion. Large pleural effusions can cause a complete opacification of the hemithorax with possible mediastinal shift to the opposite side[1,3].
With ultrasonography, pleural effusions appear as an echo-free space between the visceral and parietal pleura. Ultrasonography can assess fluid nature and confirm the presence of loculations. It can also estimate pleural effusion volume by measuring its dimensions in various planes and the distance between the diaphragm and lower lung margin[17]. Ultrasonography also measures the pleural thickness and diagnoses pleural masses. It can also identify the viscosity of the pleural fluid and guide thoracentesis and pleural biopsy[18].
Computerized tomography (CT) scans can visualize interlobar and para-mediastinal pleura that ultrasound may not detect. Like ultrasound, it can guide the pleural biopsy and drain placement. Contrast enhancement during CT scanning helps for better evaluation of the pleural effusion. CT scanning can aid in differentiating benign from malignant pleural thickening. With advanced CT machines, multi-detector CT provides a more accurate visualization of the pleura and thoracic structures and better diagnoses. The advanced CT machine can give multiplanar reconstruction images with coronal and sagittal sections providing more accurate lesion localization. CT angiography can diagnose pulmonary embolism as a cause of pleural effusions[19-21].
Diagnostic pleural fluid aspiration is indicated in the presence of an undiagnosed aetiology of significant pleural effusion (thickness of fluid >1 to 2 cm by imaging studies) unless congestive heart failure (CHF) is the most probable cause. Assessment of the fluid as a transudate or exudate helps to simplify the differential diagnosis. To assess the pleural fluid, we can use Light’s criteria depending upon serum and pleural fluid protein and lactate dehydrogenase (LDH) or use other alternative ways, e.g., using only pleural fluid protein, cholesterol, and/or LDH (Table 2). Pleural fluid values of different substances and/or cells can guide the diagnosis of its cause, e.g., pleural fluid cellular pattern, presence of malignant cells, pleural fluid glucose, amylase, cholesterol, pH, chylomicrons, adenosine deaminase (ADA), and others (Table 3)[3,22,23].
| Light’s criteria | Pleural fluid only dependent criteria |
| Pleural fluid is considered exudate if Pleural fluid/serum protein > 0.5, pleural fluid/serum LDH > 0.6, or pleural fluid LDH > two-thirds of upper limits of the laboratory’s normal serum LDH | Pleural fluid is considered exudate if Pleural fluid protein ≥ 3 gm/dL, or pleural fluid cholesterol > 45 mg/dL, or pleural fluid LDH > 0.45 times the upper limit of the laboratory’s normal serum LDH |
| Test result | Significance |
| Lymphocytes > 85% | Tuberculous pleural effusion, sarcoidosis, chronic rheumatoid pleurisy, yellow nail syndrome, chylothorax |
| Neutrophils > 10000 / µL | Para-pneumonic effusion, lupus pleuritis, acute pancreatitis |
| Neutrophils > 50000 / µL | Empyema |
| Red blood cells: pleural fluid to serum haematocrit value > 0.5 | Haemothorax |
| Protein < 1 gm/dL | Peritoneal dialysis, central venous catheter migration, cerebrospinal fluid leakage into pleura |
| Protein > 4 gm/dL | Tuberculous pleural effusion |
| Eosinophils > 10% | Haemothorax, pulmonary infarction, benign asbestos pleurisy, coccidioidomycosis, drug-induced pleurisy, Churg-Strauss syndrome, polyarteritis nodosa, paragonimiasis and other parasites, Sarcoidosis, Hodgkin’s disease |
| Glucose: Pleural fluid to serum < 0.5 | Complicated para-pneumonic effusion, chronic rheumatoid pleurisy, paragonimiasis, amoebic empyema, oesophageal rupture, tuberculous pleural effusion, lupus pleuritis, urinothorax |
| Glucose: pleural fluid to serum > 1 | Peritoneal dialysis, central venous catheter migration |
| Lactate dehydrogenase > 1000 IU/L | Bacterial empyema, pancreatitis, pancreatic-pleural fistula, amoebic empyema, septic emboli, rheumatoid pleurisy |
| Pleural fluid pH < 7.3 | Oesophageal rupture, chronic rheumatoid pleurisy, complicated para-pneumonic effusion, paragonimiasis, amoebic empyema, tuberculous pleural effusion, lupus pleuritis, urinothorax, pancreatic-pleural fistula |
| Elevated pleural fluid amylase | Oesophageal rupture, acute pancreatitis, pancreatic-pleural fistula |
| Creatinine: pleural fluid to serum > 1 | Urinothorax |
| Cholesterol > 200 mg/dL | Pseudo-chylous effusion |
| Presence of chylomicrons | Chylothorax |
| Triglycerides > 110 mg/dL | Chylothorax, central venous catheter migration with lipid infusion |
| Beta 2 transferrin level elevated | Cerebro-spinal fluid leakage to pleura |
| Adenosine deaminase > 40 IU/dL with lymphocytosis. | Tuberculous pleural effusion |
For exudative pleural effusion cases that are still undiagnosed after thoracentesis, pleural biopsy provides a reasonable diagnostic value (Table 4). The biopsy can be either closed needle biopsy, image-guided (percutaneous pleural biopsy), or thoracoscopic biopsy. Closed needle biopsy, e.g., using an Abrams needle, is very helpful when tuberculous pleuritis is suspected. The image-guided biopsy can use either CT or ultrasound guidance. The thoracoscopic biopsy can be either medical thoracoscopy or video-assisted thoracoscopic surgery[24,25].
| Importance of pleural effusion in the ICU setting | |
| 1 | Sometimes, the cause of ICU admission is the underlying cause of the pleural effusion |
| 2 | Difficult diagnosis of pleural effusion in the ICU: Clinical, Radiological, and Laboratory-related difficulties |
| 3 | Impaired turnover and cycling of pleural fluid in critically ill patients |
| 4 | The presence of pleural effusion affects the outcome and prognosis of ICU patients |
| 5 | Drainage of pleural effusion can modify the outcome and/or alter the diagnosis of patients |
| Factors affecting the success rate of pleural effusion drainage | |
| 1 | Timing of drainage: Early versus late drainage |
| 2 | Patient-related factors: Proper selection of the patients |
| 3 | Etiology of the effusion: Traumatic versus post-operative versus empyema |
| 4 | Technical-related: Image-guided aspiration or not, Proper technique of drainage, Type of catheter used (pigtail catheter versus standard tube) |
Other investigations not involving the pleura could help to reach the diagnosis. For example, we may need to order electrocardiography, echocardiography, and N-terminal-B-type natriuretic peptide in the presence of cardiac disorders such as CHF or pericarditis, abdominal imaging, urine analysis, upper or lower gastrointestinal endoscopies, liver function tests, kidney function tests, serum amylase and lipase in the presence of liver diseases, kidney disorders, or pancreatic diseases. Other tests may include investigations for tuberculosis and other infective causes of pleural effusion, tumor markers and workup for malignancy if suspected, erythrocyte sedimentation rate, collagen diseases, and autoimmune profile, and others as indicated[5,16].
In many situations, the cause of intensive care unit (ICU) admission is the underlying cause of the pleural effusion, e.g., heart failure, liver impairment, chronic kidney disease with acute events, sepsis, acute exacerbation of the autoimmune disease, as well as other causes. However, the size of pleural effusion developed in patients who need ICU care is usually larger than those observed in patients who do not need ICU care for the same underlying aetiological disease[5,26].
Additionally, there is an impaired turnover of pleural fluid in critically ill and mechanically ventilated patients. As described before, in normal conditions, pleural fluid cycling depends mainly on two processes, parietal pleural fluid filtration and parietal lymphatic drainage. There is always a balance between these processes. The parietal pleural lymphatics can increase their share of fluid and protein removal from the pleural cavity up to twenty-fold from the baseline. This increased removal can occur when there is a filtration of excess fluid through the visceral pleura or increased extravascular fluid. So, any disturbance of this balance will result in pleural fluid accumulation. Many factors can disturb this balance in ICU patients, especially those requiring mechanical ventilation. The patient's prolonged recumbent position alters the bulk pleural fluid flow and reduces lymphatic drainage due to lymphatic congestions and loss of adequate negative pressure ventilation[26].
Furthermore, the ventilator-produced positive pressure impairs the balance between the hydrostatic and oncotic pressure gradients, which is responsible, in part, for maintaining pleural fluid cycling. This positive pressure can disturb the normal rhythmogenecity of lymphatics responsible for pleural fluid removal. With excess extravascular lung fluid, there will be more fluid filtration toward the pleural cavity. ICU patients may have elevated intra-abdominal pressures, especially after abdominal surgery, or ileus with gut wall oedema and impaired lymphatic drainage[6,27].
Animal models of mechanical ventilation showed contradictory data. Some authors found a non-proportional relationship between the amount of instilled fluid into the pleural cavity and the reduction in the functional residual capacity due to the high compliance of the lungs and chest wall. Others found that hypoxemia occurs with relatively small volumes of saline instillation inside the pleural space and in a dose-dependent manner with an increased risk of ventilator-associated acute lung injury. Increased intrapulmonary shunting can explain the resulting hypoxia, even in the presence of a very mild effect on cardiac output[9,28]. Also, systemic inflammation, which is common in critically ill patients, leads to capillary leak resulting in a reduction of the reflection coefficient with a subsequent increase of both fluid and solute filtration. The reduced plasma oncotic pressure due to large molecules' extravasation and the administration of crystalloid solutions further activates the filtration process[6]. In one animal study, the authors who induced acute lung injury in sheep (oleic acid-induced lung injury) noticed an accumulation of the pleural fluid within five hours after lung injury, causing moderate pleural effusion. They also observed that the pleural fluid came from the pulmonary interstitium through the visceral pleura[27,29]. This observation contradicts our knowledge about the visceral pleura's little contribution to pleural fluid flow in healthy conditions.
The ICU settings not only increase the chance of developing pleural effusion, as previously mentioned, but could also hinder the diagnosis of the pleural effusion. The presence of other more severe conditions can mask the symptoms of pleural effusion. The clinical examination may not detect effusion by the physical signs, as the patient's position and the chest wall oedema may hinder its detection[6]. Using the diagnostic tool is also not easy. For example, obtaining an erect chest radiogram is impossible in a patient admitted to the ICU and ventilated. Instead, we usually obtain an anteroposterior (supine) chest X-ray film that may not detect small and moderate pleural effusions because the fluid settles posteriorly in the para-vertebral gutter, which can accommodate large amounts of fluid. In such cases, we should suspect a pleural effusion if one hemithorax has increased opacity without obscuring the vascular markings[1,3]. This finding could explain the low rate of pleural effusion detected in the study of Fartoukh et al[27]. They examined 1351 patients admitted to the medical ICU for the presence of pleural effusions, using only clinical examination and chest X-ray. They could identify only 113 cases of pleural effusion (8.4%). However, we cannot rely on this number as there was no confirmatory test, and there is a high possibility of missed cases. On the other hand, when Lichtenstein et al[28] added the chest ultrasound to both clinical examination and chest X-ray in studying patients with acute respiratory distress syndrome to detect pleural effusion, compared to the chest CT scan as a gold standard. They found that clinical examination had a sensitivity of 42% and a diagnostic accuracy of 61%, whereas chest X-ray had a sensitivity and diagnostic accuracy of only 39% and 47%, respectively. In contrast, ultrasound had a sensitivity and diagnostic accuracy of 92% and 93%, respectively.
We should perform chest ultrasonography if pleural effusion is suspected and not detected by the chest X-ray. Bedside and portable ultrasonography are helpful tools for diagnosing pleural effusion in the ICU. Ultrasound has high sensitivity and can detect tiny amounts not detectable by the standard chest X-ray, is easy to use, and has no risk of radiation exposure. Therefore, ultrasonography is, by far, the most reliable method to diagnose and monitor pleural effusion in the ICU setting. Ultrasound can guide the aspiration and pleural drain placement as well[30-32]. In critically ill patients, especially with mechanical ventilation support, the changes in capillary permeability and plasma oncotic pressure may affect the accuracy of Light criteria, making distinguishing between the transudates and exudates difficult. Hypoproteinaemia and fluid overload are common findings in critically ill patients. Therefore, diagnosis of heart failure based on the clinical findings and chest X-ray has a low yield in mechanically ventilated patients[30].
The presence of pleural effusion can significantly affect the prognosis and the outcome, especially in critically ill or mechanically ventilated patients. A prospective multicentre French study examined 249 patients admitted to the ICU using a chest ultrasound to assess the presence of moderate-to-large pleural effusion at the start of weaning and during difficult weaning. They detected moderate-to-large-size pleural effusion in one-third of patients at the time of weaning initiation, which was associated with worse outcomes. Unfortunately, depletive strategies such as diuretics or ultrafiltration did not rapidly alter its evolution[33]. The impact of pleural effusion on the hemodynamic and lung mechanics (mentioned earlier in this review) in these critically ill patients with many comorbidities and under mechanical ventilation explains its effect on the prognosis and outcome of these patients[7-12].
Analysis of the aspirated pleural fluid during thoracentesis helps diagnose the effusion type and the initial condition causing ICU admission. The previously mentioned study by Fartoukh et al[27] found that the thoracentesis results changed the diagnosis in 43% of their patients and modified the treatment strategy in 31%. However, they did not see a significant difference in the outcome or the length of ICU stay in the patients who had management modification according to the thoracocentesis result compared to those who were not. On the same track, Godwin et al[32] reported changing the management guided by the pleural fluid analysis results after thoracentesis in 24 (75%) out of 32 cases. Similarly, a prospective study of ICU patients by Fysh et al[33] found that drainage of pleural effusions improved the diagnostic accuracy in 77% of patients, changing the primary diagnosis in 52% of them, and 19% of them had utterly unsuspected diagnoses. They also reported that 58% of drainage procedures caused significant changes in patient management. On the other hand, an ICU-based prospective study by Dres et al[34] examined 136 mechanically ventilated patients using pleural ultrasound at the first spontaneous breathing trial to detect and quantify pleural effusion. Their primary endpoint was the prevalence of pleural effusion according to the weaning outcome. They detected pleural effusion in 37% of the patients, with a significant amount (moderate or large) in 13%. Prevalence of pleural effusion showed no significant difference between the patients with weaning success or with weaning failure. They also noted the same prevalence for moderate/Large pleural effusion. The duration of mechanical ventilation and the length of stay in the ICU were similar between patients with moderate to large pleural effusions and patients with/or without minimal pleural effusions.
Pleural fluid drainage impacts the outcome of critically ill and mechanically ventilated patients. This impact results from the different risk-to-benefit ratios of the drainage procedure in critically ill patients compared with those in hospital wards or outpatient clinics. A systematic review and meta-analysis by Goligher et al[35] reviewed 19 studies performed on 1690 patients; 1124 patients were on mechanical ventilation. Five of these studies (118 patients) identified thoracentesis's effect on the studied patients' oxygenation status. In these five studies, there were recordings of the timing of gas exchange measurements, the volume of pleural fluid drainage, the ventilator settings, and the changes in oxygenation after pleural drainage[36-42]. This meta-analysis demonstrated an 18% improvement in the partial pressure of arterial oxygen to fraction of inspired oxygen ratio (PaO2:FiO2 ratio) after thoracentesis, corresponding to an increase of 31 mmHg. Among the five studies, Roch et al[37] found a rise in the PaO2:FiO2 ratio correlated with the drained volume of the effusion in the subgroup with pleural effusion sizes greater than 500 mL.
Conversely, Talmor et al[38] found no relationship between oxygenation status changes and the drained volume. Regarding the change in respiratory mechanics after thoracentesis, Talmor et al[38] reported a 30% immediate increase in dynamic compliance after thoracentesis. Doelken et al[39] also reported an increase in dynamic compliance. They also reported a statistically significant reduction in the work of breathing after thoracentesis. Ahmed et al[40] observed a decreased respiratory rate after thoracentesis, but no considerable lung mechanics changed. Another meta-analysis included 2265 patients from 31 studies, which proved the beneficial effects of effusion drainage. After pleural drainage, they improved the PaO2/FiO2 ratio and end-expiratory lung volume. Still, there is no change in the alveolar-arterial oxygen gradient, heart rate, mean arterial pressure, left ventricular end-diastolic volume, stroke volume, cardiac output, or ejection fraction. Fortunately, the risks of pneumothorax and hemothorax after pleural drainage were negligible. They concluded that pleural effusion drainage is a safe procedure that improves oxygenation in critically ill patients[43]. Fysh et al[33] conducted a recent prospective multicentre large-scale study involving 7342 patients admitted to four ICUs to study the factors that could affect pleural effusion drainage efficiency. They found that early pleural effusion drainage and proper patient selection, especially those with clinically significant pleural effusion, improved oxygenation status without apparent severe adverse effects than those with conservative management. However, there were no differences in other clinical outcomes, such as the ability to extubate or wean off the non-invasive ventilation, ICU or hospital length of stay, or mortality rates.
Because of the high incidence of pleural effusion in critically ill patients reaching up to 50-60% in some studies, fluid drainage has been a popular intervention in these cases[44]. Thoracentesis or chest drain placement are the two methods commonly used to drain pleural fluid in the ICU setting. Chest drain can be either a standard intercostal tube or a small-bore drain (e.g., pigtail catheter). Tension pneumothorax and hemothorax are the most severe complications of thoracentesis. However, the complication rate is kept low with the proper procedure and ultrasound guidance. Balik et al[43] had a pneumothorax rate of 4.8% in their study, which included 205 patients on mechanical ventilation with 255 procedures over three years. The requirement for intercostal tube placement was only 0.78%. Mayo et al[44] had a lower pneumothorax rate (1.3% from 232 procedures) without any recorded hemothorax. Lichtenstein et al[45] did not observe any complications over a series of 45 procedures.
Similarly, Vignon et al[46] reported no complication of thoracocentesis in 55 patients, but it had a failure rate of 11%. Another study by Tu et al[47] included 94 cases with parapneumonic effusions on mechanical ventilation and reported pneumothorax in 2% of cases. Petersen et al[48] found a complication rate of 1.2% for each pneumothorax and hemothorax in their study, which included 135 patients with 338 procedures. However, the pooled complication rates for thoracocentesis, as observed in a meta-analysis study of 14 studies with 965 patients done by Goligher et al[35], were 3.4% and 1.6% for pneumothorax and hemothorax, respectively.
Using small-bore pleural drains as pigtail catheters is an effective and safe method for pleural fluid drainage. It can replace the standard intercostal tube in most pleural effusions except for hemothorax and thick empyema. Bediwy et al[49] showed that pigtail catheter use had an 82.35% successful rate in draining various pleural fluid types with minor complications. Sharaf-Eldin et al[50] showed a success rate of 80% in draining pleural effusion caused by hepatic hydrothorax and liver impairment using an ultrasound-guided pigtail catheter, with a few recorded complications (3.3% had a catheter blockade and no reported pneumothorax). A retrospective study by Liang et al[51] studied pigtail catheter drainage for pleural effusion in the ICU setting with various types of pleural effusion (empyema, malignant pleural effusion, massive transudative effusions, postoperative pleural effusions, and traumatic hemothorax). They observed that the highest success rate was in traumatic hemothorax (100% of cases), followed by postoperative pleural effusion (85%), and the lowest success rate was in empyema (42%). They recorded no significant complications.
Pleural effusion is a common finding in the ICU setting. The cause of pleural effusion may be the exact cause of ICU admission. Many factors contribute to the impairment of pleural fluid turnover in such cases as prolonged recumbency, ventilator-produced positive pressure, and reduced plasma oncotic pressure. Proper diagnosis of pleural effusion in the ICU faces many difficulties, such as chest wall edema, patient’s position, masked symptoms due to the severity of the condition, and inability to obtain an erect chest radiogram. The presence of pleural effusions can lead to worse outcomes in critically ill patients due to the impact on hemodynamic and lung mechanics. However, drainage of pleural effusion in such cases can improve oxygenation status, reduce work of breathing, and reduce respiratory rate, which improves the patient’s prognosis. Furthermore, the analysis of pleural fluid can suggest an alternative diagnosis and may help to modify the management.
We thank the anonymous referees and editors for their valuable suggestions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chandna S, United States; Liu C, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Maslove DM, Chen BT, Wang H, Kuschner WG. The diagnosis and management of pleural effusions in the ICU. J Intensive Care Med. 2013;28:24-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Miserocchi G. Physiology and pathophysiology of pleural fluid turnover. Eur Respir J. 1997;10:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 159] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiol Rev. 2004;84:385-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 4. | Miserocchi G, Negrini D. Contribution of Starling and lymphatic flows to pleural liquid exchanges in anesthetized rabbits. J Appl Physiol (1985). 1986;61:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Walden AP, Jones QC, Matsa R, Wise MP. Pleural effusions on the intensive care unit; hidden morbidity with therapeutic potential. Respirology. 2013;18:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Negrini D, Ballard ST, Benoit JN. Contribution of lymphatic myogenic activity and respiratory movements to pleural lymph flow. J Appl Physiol (1985). 1994;76:2267-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 7. | Yoo OH, Ting EY. The effects of pleural effusion on pulmonary function. Am Rev Respir Dis. 1964;89:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Gilmartin JJ, Wright AJ, Gibson GJ. Effects of pneumothorax or pleural effusion on pulmonary function. Thorax. 1985;40:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Nishida O, Arellano R, Cheng DC, DeMajo W, Kavanagh BP. Gas exchange and hemodynamics in experimental pleural effusion. Crit Care Med. 1999;27:583-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Venkatesh G, Tomlinson CW, O'Sullivan T, McKelvie RS. Right ventricular diastolic collapse without hemodynamic compromise in a patient with large, bilateral pleural effusions. J Am Soc Echocardiogr. 1995;8:551-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Thomas R, Jenkins S, Eastwood PR, Lee YC, Singh B. Physiology of breathlessness associated with pleural effusions. Curr Opin Pulm Med. 2015;21:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Alam HB, Levitt A, Molyneaux R, Davidson P, Sample GA. Can pleural effusions cause cardiac tamponade? Chest. 1999;116:1820-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Mallick A, Bodenham AR. Disorders of the lymph circulation: their relevance to anaesthesia and intensive care. Br J Anaesth. 2003;91:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hooper C, Lee YC, Maskell N; BTS Pleural Guideline Group. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii4-i17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 15. | Eibenberger KL, Dock WI, Ammann ME, Dorffner R, Hörmann MF, Grabenwöger F. Quantification of pleural effusions: sonography versus radiography. Radiology. 1994;191:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Koegelenberg CF, von Groote-Bidlingmaier F, Bolliger CT. Transthoracic ultrasonography for the respiratory physician. Respiration. 2012;84:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Helm EJ, Matin TN, Gleeson FV. Imaging of the pleura. J Magn Reson Imaging. 2010;32:1275-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Light RW. Pleural effusion in pulmonary embolism. Semin Respir Crit Care Med. 2010;31:716-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Bediwy AS, Badawy ME, Salama AA, and Zayed HA. The use of multi-detector computed tomography and ultrasonography for evaluation of pleural lesions. Egyptian Journal of Chest Diseases and Tuberculosis. 2015;64:161-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Murphy MJ, Jenkinson F. Categorisation of pleural fluids in routine clinical practice: analysis of pleural fluid protein and lactate dehydrogenase alone compared with modified Light's criteria. J Clin Pathol. 2008;61:684-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Heffner JE, Highland K, Brown LK. A meta-analysis derivation of continuous likelihood ratios for diagnosing pleural fluid exudates. Am J Respir Crit Care Med. 2003;167:1591-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Koegelenberg CF, Diacon AH. Pleural controversy: close needle pleural biopsy or thoracoscopy-which first? Respirology. 2011;16:738-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Maskell NA, Gleeson FV, Davies RJ. Standard pleural biopsy versus CT-guided cutting-needle biopsy for diagnosis of malignant disease in pleural effusions: a randomised controlled trial. Lancet. 2003;361:1326-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 224] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 24. | Azoulay E. Pleural effusions in the intensive care unit. Curr Opin Pulm Med. 2003;9:291-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 25. | Wiener-Kronish JP, Broaddus VC, Albertine KH, Gropper MA, Matthay MA, Staub NC. Relationship of pleural effusions to increased permeability pulmonary edema in anesthetized sheep. J Clin Invest. 1988;82:1422-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Graf J, Formenti P, Santos A, Gard K, Adams A, Tashjian J, Dries D, Marini JJ. Pleural effusion complicates monitoring of respiratory mechanics. Crit Care Med. 2011;39:2294-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Fartoukh M, Azoulay E, Galliot R, Le Gall JR, Baud F, Chevret S, Schlemmer B. Clinically documented pleural effusions in medical ICU patients: how useful is routine thoracentesis? Chest. 2002;121:178-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 53] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 663] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 29. | Brogi E, Gargani L, Bignami E, Barbariol F, Marra A, Forfori F, Vetrugno L. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit Care. 2017;21:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Soni NJ, Franco R, Velez MI, Schnobrich D, Dancel R, Restrepo MI, Mayo PH. Ultrasound in the diagnosis and management of pleural effusions. J Hosp Med. 2015;10:811-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 31. | Razazi K, Boissier F, Neuville M, Jochmans S, Tchir M, May F, de Prost N, Brun-Buisson C, Carteaux G, Mekontso Dessap A. Pleural effusion during weaning from mechanical ventilation: a prospective observational multicenter study. Ann Intensive Care. 2018;8:103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Godwin JE, Sahn SA. Thoracentesis: a safe procedure in mechanically ventilated patients. Ann Intern Med. 1990;113:800-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Fysh ETH, Smallbone P, Mattock N, McCloskey C, Litton E, Wibrow B, Ho KM, Lee YCG. Clinically Significant Pleural Effusion in Intensive Care: A Prospective Multicenter Cohort Study. Crit Care Explor. 2020;2:e0070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Dres M, Roux D, Pham T, Beurton A, Ricard JD, Fartoukh M, Demoule A. Prevalence and Impact on Weaning of Pleural Effusion at the Time of Liberation from Mechanical Ventilation: A Multicenter Prospective Observational Study. Anesthesiology. 2017;126:1107-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Goligher EC, Leis JA, Fowler RA, Pinto R, Adhikari NK, Ferguson ND. Utility and safety of draining pleural effusions in mechanically ventilated patients: a systematic review and meta-analysis. Crit Care. 2011;15:R46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | De Waele JJ, Hoste E, Benoit D, Vandewoude K, Delaere S, Berrevoet F, Colardyn F. The effect of tube thoracostomy on oxygenation in ICU patients. J Intensive Care Med. 2003;18:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Roch A, Bojan M, Michelet P, Romain F, Bregeon F, Papazian L, Auffray JP. Usefulness of ultrasonography in predicting pleural effusions > 500 mL in patients receiving mechanical ventilation. Chest. 2005;127:224-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 104] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 38. | Talmor M, Hydo L, Gershenwald JG, Barie PS. Beneficial effects of chest tube drainage of pleural effusion in acute respiratory failure refractory to positive end-expiratory pressure ventilation. Surgery. 1998;123:137-143. [PubMed] |
| 39. | Doelken P, Abreu R, Sahn SA, Mayo PH. Effect of thoracentesis on respiratory mechanics and gas exchange in the patient receiving mechanical ventilation. Chest. 2006;130:1354-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Ahmed SH, Ouzounian SP, Dirusso S, Sullivan T, Savino J, Del Guercio L. Hemodynamic and pulmonary changes after drainage of significant pleural effusions in critically ill, mechanically ventilated surgical patients. J Trauma. 2004;57:1184-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 41. | Vetrugno L, Bignami E, Orso D, Vargas M, Guadagnin GM, Saglietti F, Servillo G, Volpicelli G, Navalesi P, Bove T. Utility of pleural effusion drainage in the ICU: An updated systematic review and META-analysis. J Crit Care. 2019;52:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Walden AP, Garrard CS, Salmon J. Sustained effects of thoracocentesis on oxygenation in mechanically ventilated patients. Respirology. 2010;15:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Balik M, Plasil P, Waldauf P, Pazout J, Fric M, Otahal M, Pachl J. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Med. 2006;32:318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 44. | Mayo PH, Goltz HR, Tafreshi M, Doelken P. Safety of ultrasound-guided thoracentesis in patients receiving mechanical ventilation. Chest. 2004;125:1059-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 132] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Lichtenstein D, Hulot JS, Rabiller A, Tostivint I, Mezière G. Feasibility and safety of ultrasound-aided thoracentesis in mechanically ventilated patients. Intensive Care Med. 1999;25:955-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 171] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 46. | Vignon P, Chastagner C, Berkane V, Chardac E, François B, Normand S, Bonnivard M, Clavel M, Pichon N, Preux PM, Maubon A, Gastinne H. Quantitative assessment of pleural effusion in critically ill patients by means of ultrasonography. Crit Care Med. 2005;33:1757-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 138] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 47. | Tu CY, Hsu WH, Hsia TC, Chen HJ, Tsai KD, Hung CW, Shih CM. Pleural effusions in febrile medical ICU patients: chest ultrasound study. Chest. 2004;126:1274-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 48. | Petersen S, Freitag M, Albert W, Tempel S, Ludwig K. Ultrasound-guided thoracentesis in surgical intensive care patients. Intensive Care Med. 1999;25:1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Bediwy AS, and Amer HG. Pigtail catheter use for draining pleural effusions of various etiologies. ISRN Pulmonol. 2012;143295. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Sharaf-Eldin M, Bediwy AS, Kobtan A, Abd-Elsalam S, El-Kalla F, Mansour L, Elkhalawany W, Elhendawy M, Soliman S. Pigtail Catheter: A Less Invasive Option for Pleural Drainage in Egyptian Patients with Recurrent Hepatic Hydrothorax. Gastroenterol Res Pract. 2016;2016:4013052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Liang SJ, Tu CY, Chen HJ, Chen CH, Chen W, Shih CM, Hsu WH. Application of ultrasound-guided pigtail catheter for drainage of pleural effusions in the ICU. Intensive Care Med. 2009;35:350-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |