Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1206
Peer-review started: November 16, 2022
First decision: November 30, 2022
Revised: November 30, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: February 16, 2023
Processing time: 89 Days and 13.1 Hours
The incidental detection of a right atrial mass during routine cardioncological workup is a rare condition. The correct differential diagnosis between cancer and thrombi is challenging. A biopsy may not be feasible while diagnostic techniques and tools may not be available.
We report the case of a 59-year-old female patient with a history of breast cancer and current secondary metastatic pancreatic cancer. She developed deep vein thrombosis and pulmonary embolism and was admitted to the Outpatient Clinic of our Cardio-Oncology Unit for follow-up. Transthoracic echocardiogram incidentally found a right atrial mass. Clinical management was difficult due to the abrupt worsening of the patient’s clinical condition and the progressive severe thrombocytopenia. We suspected a thrombus, according to its echocardiographic appearance, the patient’s cancer history and recent venous thromboembolism. The patient was unable to adhere to low molecular weight heparin treatment. Due to worsening prognosis, palliative care was recommended. We also highlighted the distinguishing features between thrombi and tumors. We proposed a diagnostic flowchart to aid diagnostic decision making in the case of an incidental atrial mass.
This case report highlights the importance of cardioncological surveillance during anticancer treatments to detect cardiac masses.
Core Tip: Cardiac masses are rare occurrences. In this case report, the diagnosis of a right atrial mass during cardioncological follow-up of a patient with secondary metastatic pancreatic cancer and a recent diagnosis of cancer-associated thrombosis was challenging. Severe thrombocytopenia and the rapid worsening of the patient’s condition hindered a complete clinical workup. However, echocardiographic differentiation between thrombi and tumors lead to the diagnosis of a new venous thromboembolism event. We also proposed a diagnostic flowchart to aid diagnostic decision making in the case of an incidental atrial mass.
- Citation: Fioretti AM, Leopizzi T, La Forgia D, Scicchitano P, Oreste D, Fanizzi A, Massafra R, Oliva S. Incidental right atrial mass in a patient with secondary pancreatic cancer: A case report and review of literature. World J Clin Cases 2023; 11(5): 1206-1216
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1206
Cardiac masses are unique entities. Primary cardiac tumors are very uncommon, whereas metastases and “pseudotumors,” such as intracardiac thrombi, are more frequent. They are typically discovered incidentally[1]. The differential diagnosis between cardiac tumors and cancer-associated thrombosis (CAT) is challenging because the symptoms are similar to other cardiac conditions[2]. Herein, we report the case of a 59-year-old Caucasian female with a history of breast cancer and secondary metastatic pancreatic cancer who was assessed by cardioncological follow-up, which revealed a large (2 cm × 3 cm) right atrial mass.
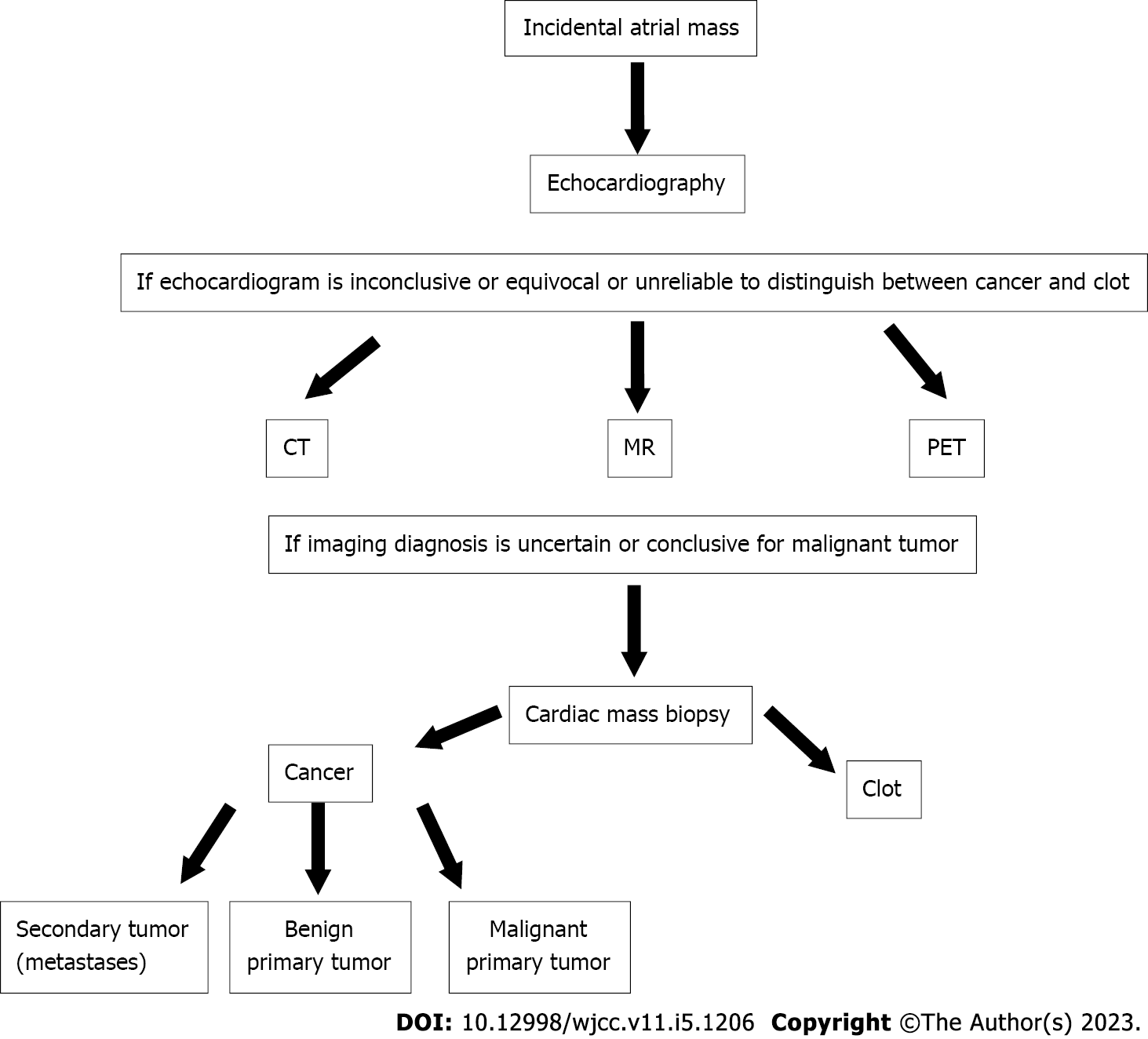
Currently, there is no standardized guideline for the differential diagnosis of cancer and thrombus. Accordingly, the clinical management of patients diagnosed with an incidental atrial mass is challenging, and the diagnosis is rarely recognized by physicians. Thus, a diagnostic algorithm was created as a guide for a practical evidence-based decision making approach for the differential diagnosis between cancer and thrombus.
A 59-year-old Caucasian female (height: 160 cm, weight: 60 kg) was referred to the Cardio-Oncology Unit for surveillance. She reported having shortness of breath at rest and after mild exercise in the prior 2 mo. She had experienced severe chest pain (without any anginal characteristic) in the prior 2 wk. The patient denied any history of syncope. Occasional cough and dizziness with no headache occurred.
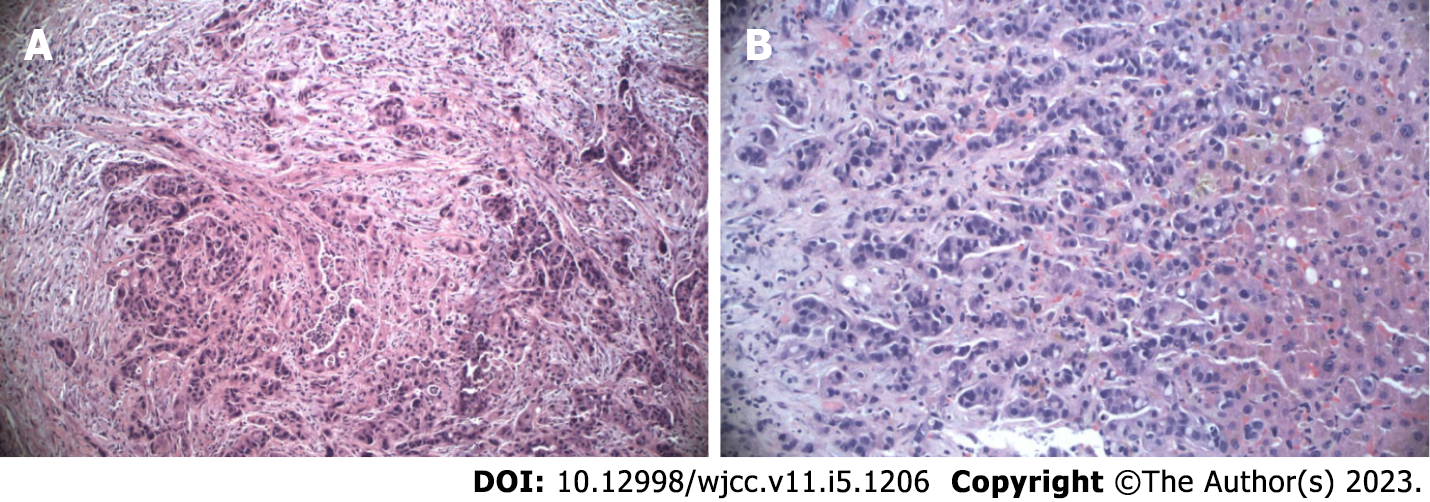
The patient had jaundice due to choledochal obstruction from a pancreatic mass and underwent drainage via an endoprosthesis. Biopsy revealed stage IV pancreatic ductal adenocarcinoma of the head with liver metastases (Figure 1). After central line placement in the right subclavian vein, chemotherapy with nab-paclitaxel (125 mg/m2) plus gemcitabine (1000 mg/m2) was administered on days 1, 8 and 15 of a 28-d cycle. Due to cancer progression, a second-line treatment was started with 5-fluorouracil (2400 mg/m2 continuous infusion for 44 h), folic acid (200 mg/m2), oxaliplatin (85 mg/m2) and irinotecan (150 mg/m2). The patient was sensitive to this regimen because pretreatment pharmacogenomic evaluation of 5-fluorouracil degradation by DPYD and UGT1A1 yielded positive results.
The patient had undergone right mastectomy and lymphadenectomy for an infiltrating ductal carcinoma with axillary lymph node metastases 9 years prior. She had received adjuvant treatment with cyclophosphamide (600 mg/m2), methotrexate (40 mg/m2) and 5-fluorouracil (600 mg/m2) for 12 cycles and hormone therapy with tamoxifen 20 mg/d for 5 years.
The patient mentioned a positive family history for breast cancer (maternal aunt), whereas her personal and family history of cardiovascular disease was unremarkable. The patient was married without offspring. She complained of abdominal pain and social isolation due to the cancer diagnosis.
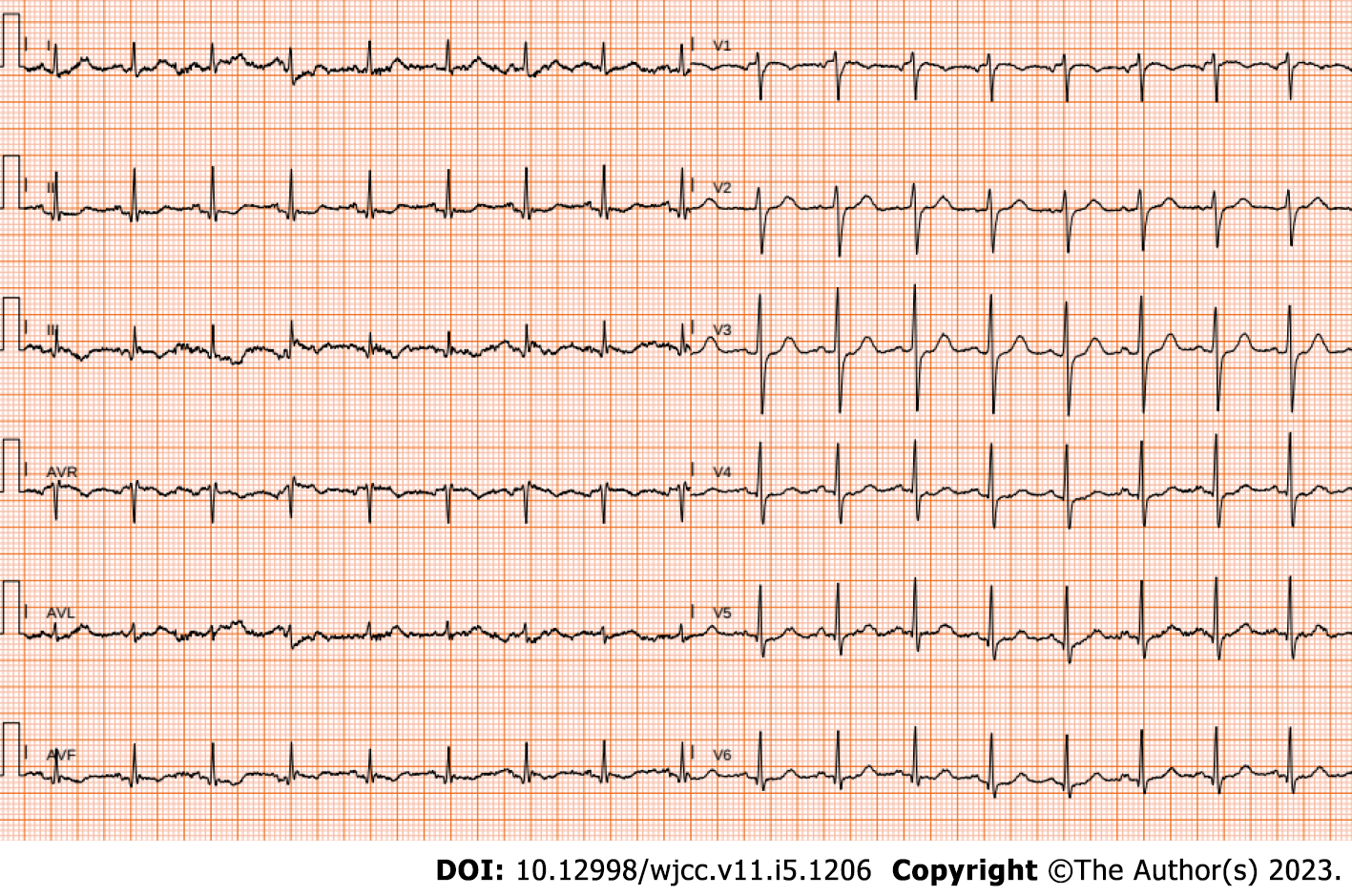
Upon physical examination, blood pressure was 110/80 mmHg at rest, while body temperature was 36.1 °C. Heart rate was 103 beats/min with a regular heart rhythm. Intensity of the first heart sound (S1) did not vary, and a regurgitant murmur was heard at the apex. The patient’s physical examination revealed no other notable clinical findings. Electrocardiogram showed sinus tachycardia and biphasic T waves in the inferior leads (Figure 2). Once the patient’s general clinical condition significantly declined, blood pressure was 95/70 mmHg and heart rate was 143 beats/min.
Hemoglobin was 10.0 g/dL (normal range: 13.5-17.5 g/dL). Platelet count (PC) was 50 × 103/μL (normal range: 150-450 × 103/μL) and progressively declined to 40 × 103/μL and to 20 × 103/μL.
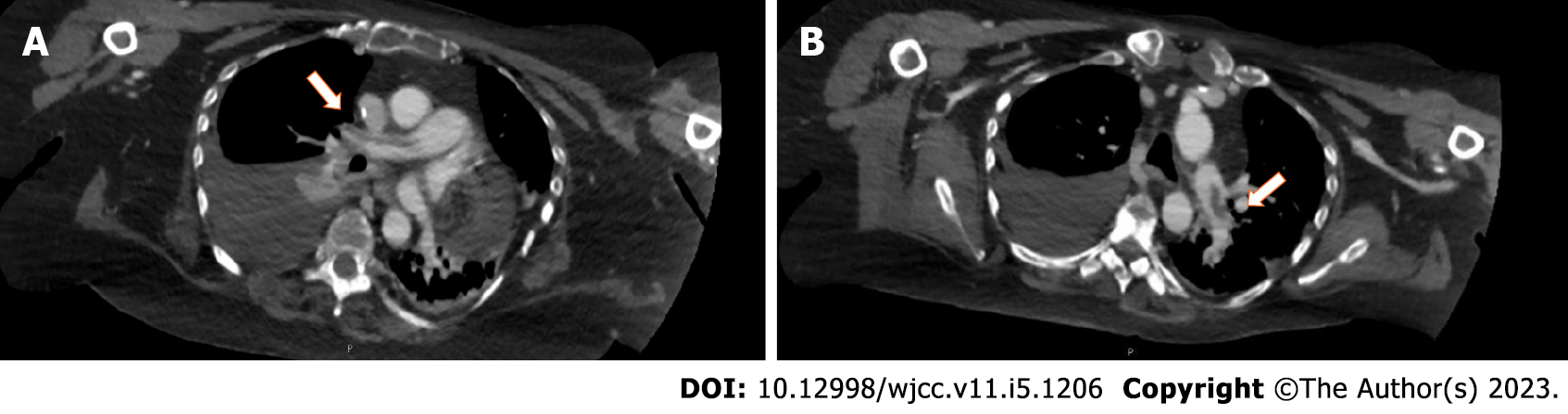
Computed tomography (CT) identified enlargement of the hepatic metastases, partial occlusion of both branches of the pulmonary artery (Figure 3) and deep vein thrombosis (DVT) of the right external iliac vein and both common femoral veins. Furthermore, left pulmonary infarction and mild right pleural effusion were detected. After 3 mo of anticoagulant treatment, CT documented persistence of lower limb DVT and pulmonary embolism (PE) and a worsening of the liver metastases.
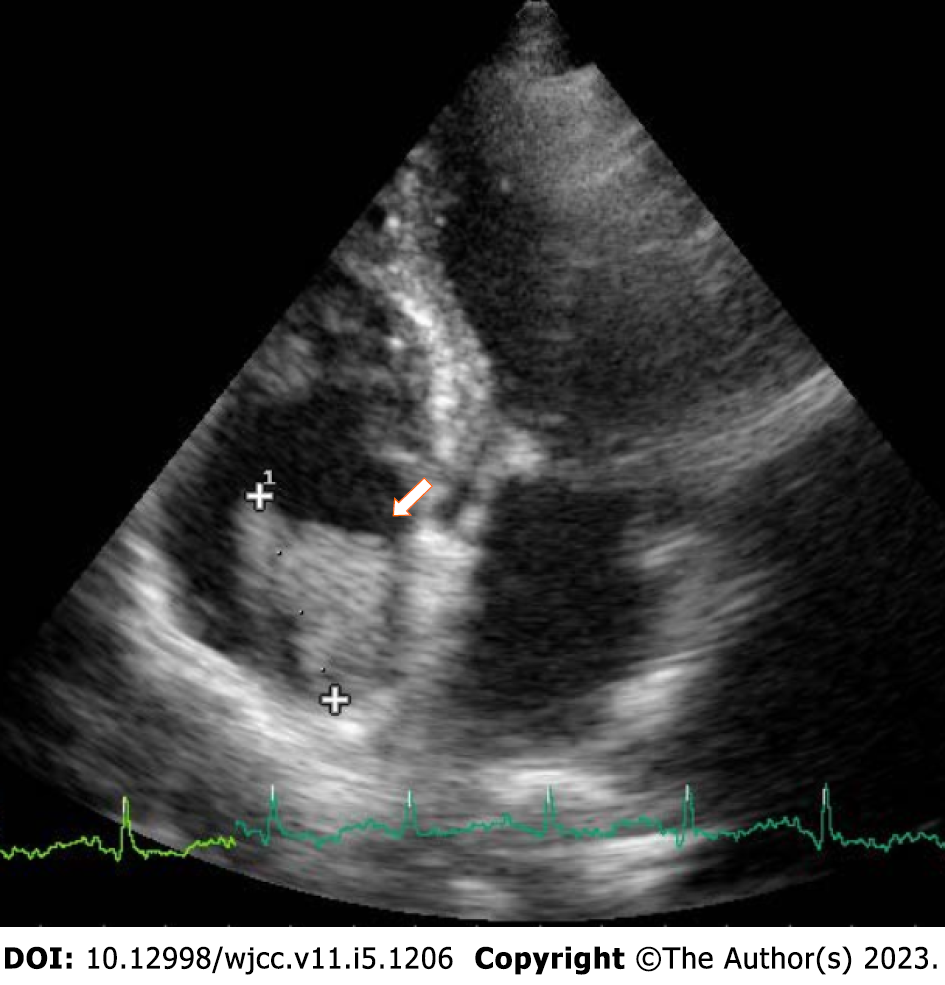
Transthoracic echocardiogram (TTE) showed a normal systolic function (ejection fraction: 55%) and dilated right chambers (diameters of the right atrium and the right ventricle were 46 mm and 50 mm, respectively). An isoechoic/hyperechoic large solid right atrial mass (2 cm × 3 cm) with irregular and uneven margins was observed, with no apparent pedicle to the atrial roof and close to the orifice of the inferior cava vein (ICV) (Figure 4). The mass was highly mobile and non-vascularized. It partially prolapsed into the tricuspid orifice toward the ventricle during diastole, without evidence of functional obstruction or stenosis. Mild mitral and tricuspid valve regurgitations and grade II diastolic dysfunction were also revealed.
Due to the patient’s worsening clinical condition, further investigations were suspended (despite the value of a complete diagnostic workup). Given the patient’s history and the overall echocardiographic features, a new venous thromboembolism (VTE) event was suspected. In addition, the recent diagnosis of CAT strongly indicated the presence of a clot.
After the diagnosis of DVT with PE, the patient refused hospitalization and was treated at home with anticoagulant therapy. Low molecular weight heparin (LMWH) treatment was started by her oncologist, consisting of enoxaparin sodium 100 IU anti-Xa 1 mg/kg twice daily. However, it did not resolve the CAT. After a telephone interview with the patient’s husband, we verified the patient’s poor adherence due to difficulties with daily self-injections. Accordingly, due to lack of therapeutic efficacy and patient’s preference for oral administration, we replaced LMWH with a direct oral anticoagulant (edoxaban 60 mg once daily)[3]. The patient’s PC decreased to 60 × 103/μL, and we replaced edoxaban with enoxaparin sodium 100 IU anti-Xa 1 mg/kg twice daily. The patient’s PC continued to decrease (40 × 103/μL). Therefore, we decreased the LMWH dose by half. Anticoagulant therapy was completely discontinued when the patient’s PC decreased to 20 × 103/μL[4].
The patient’s disease continued to progress and worsen. While the patient was under our care, we surveyed her emotional well-being via questions on her perspectives related to her clinical case history (Table 1). Afterwards, the patient’s medical information was provided to us by her husband and her general practitioner. Chemotherapy treatment with carboplatin (250 mg/m2) was started. However, after 3 mo the oncologist recommended discontinuation of any further anticancer treatment and suggested palliative care (Table 2).
| Questions on patient’s perspectives | Patient’s answers |
| Patient’s own feelings | I feel very upset, anxious and sad. I already passed through cancer in the past and now I have to face it again. Why is this happening to me? |
| Patient’s sorrow for her family | My husband accompanies me to the hospital for visits and exams, but our life is devastated for the second time |
| Patient’s discomfort for treatments | I really don’t like these injections every day and I can’t pay someone to do it for me; therefore, my husband has to do it. During infusions I feel very weak |
| Patient’s complaints | I feel exhausted and for every effort I need my husband’s help, because of “missing air” sensation. I can’t breathe properly |
| Patient’s life insights | I hope the oncological treatments will help me. I should ask for psychological support. Now a second evil is in my lungs and all these complications are worsening my belief in life anymore |
| Date | Event |
| 2011 | Breast cancer (right mastectomy and lymphadenectomy + adjuvant chemotherapy with cyclophosphamide, methotrexate, 5-fluorouracil + hormone therapy with tamoxifen) |
| 2020 | Pancreatic cancer with hepatic metastases (chemotherapy with nab-paclitaxel and gemcitabine) |
| February 2021 | Second-line treatment with 5-fluorouracil, folic acid, oxaliplatin, irinotecan |
| May 2021 | Deep vein thrombosis (right external iliac vein and both common femoral veins) and pulmonary embolism |
| Start LMWH | |
| June 2021 | Persistence of deep vein thrombosis and pulmonary embolism |
| LMWH replaced with edoxaban for inefficacy and patient’s preference | |
| August 2021 | Chemotherapy with carboplatin for worsening of liver metastases |
| September 2021 | Dyspnea at rest and fatigue |
| Echocardiogram: Incidental large right atrial mass, highly mobile and not vascularized, close to the ICV orifice | |
| Edoxaban replaced with LMWH due to thrombocytopenia and anemia | |
| October 2021 | Halved LMWH dose due to further platelet count decrease |
| November 2021 | Marked decline of clinical general condition |
| Discontinuation of anticancer drugs for severe disease progression | |
| Discontinuation of LMWH for severe thrombocytopenia | |
| Start palliative care |
Herein, we have described the very unusual finding of a large right atrial mass detected by TTE in a patient with a history of breast cancer and secondary metastatic pancreatic cancer who had developed CAT. Up to 12% of primary cardiac tumors are clinically silent and are detected during incidental cardiovascular workup or at autopsy[5]. For our patient, the suspicion for vegetations was quickly excluded due to clinical history, mass site, absence of vegetation-related presenting symptoms and absence of prosthetic valves. About 75% of infective endocarditis occurs in patients with underlying structural heart disease[6], which was absent in this patient.
The risk of VTE is up to 50-fold higher in patients with cancer than in those without[7]. Patients with advanced cancer have a higher risk of VTE than patients with localized cancers. Pancreatic cancer, gastric cancer and myeloma are associated with a higher risk of VTE as well[8]. VTE is associated with high morbidity and mortality rates[9]. CAT is the second leading cause of death in malignancy after the progression of cancer itself, with an incidence of 20%[10]. Cancer patients are also prone to bleeding[11] and renal failure, which typically leads to surgery and interventional procedures. Notably, cancer patients have a higher risk of both thrombosis relapse and bleeding while on anticoagulant therapy compared to patients without cancer[12]. In addition, the presence of indwelling lines increases the chance of developing a clot due to blood stasis around the port and the high viscosity of the drugs administered through the catheter[13].
According to the Khorana Risk Score, the following parameters increase VTE risk: Site of cancer (stomach, pancreas); PC ≥ 350 × 103/μL; hemoglobin level < 10 g/dL; leukocyte count > 11 × 103/μL; and body mass index > 35 kg/m2[14]. The recently established Protecht Risk Score determined that gemcitabine-based and platinum-based therapies increase the risk of VTE[15].
In 2015, the World Health Organization updated the classification of tumors of the heart and the pericardium[16]. Cardiac tumors are extremely rare diseases, with an incidence of approximately 0.02% in an autopsy series[17]. By contrast, cardiac thrombi are more common. Benign cardiac tumors produce severe hemodynamic instability, leading to mechanical issues or the onset of embolic or arrhythmic events. Presenting symptoms include fever, weakness, fatigue and weight loss[18].
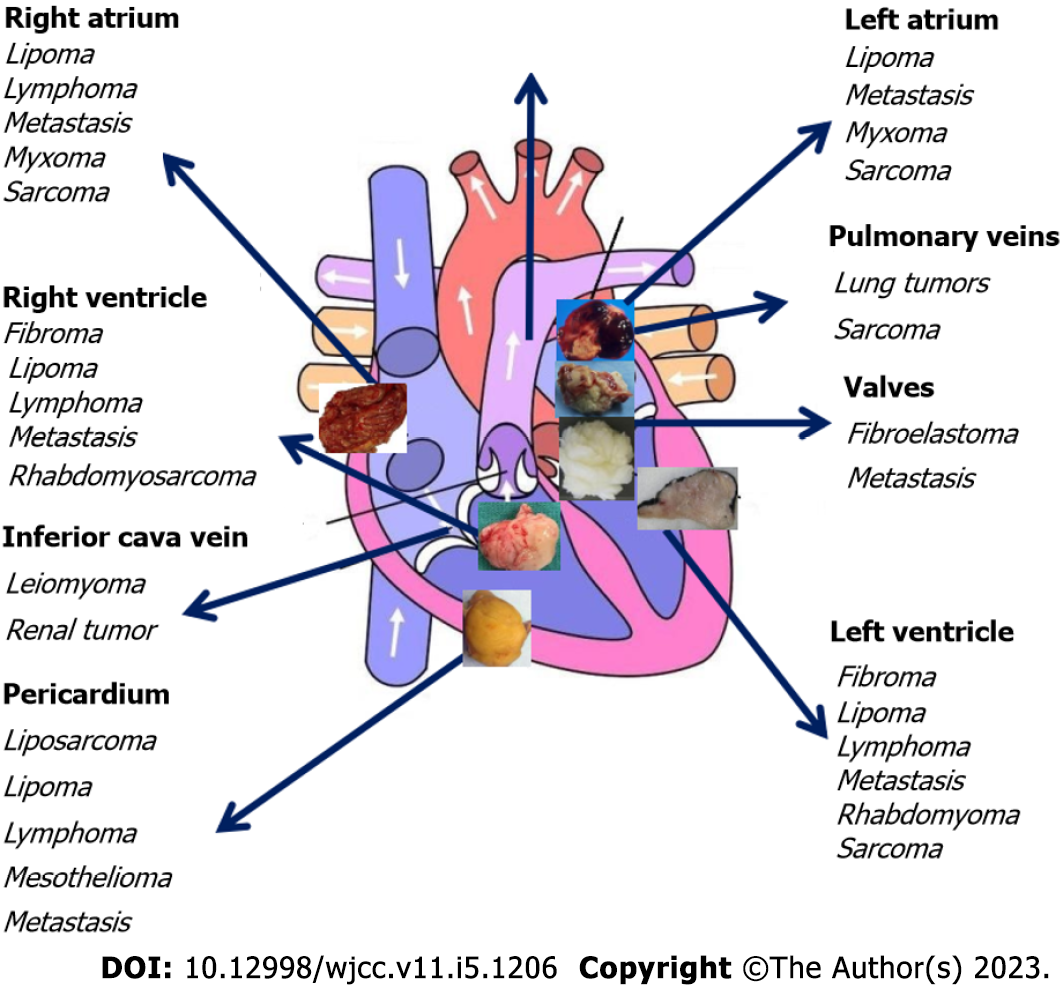
About 75% of all primary cardiac tumors are benign. The most frequent tumors in the adult population are myxomas (50%), papillary fibroelastomas (20%), lipomas (15%-20%) and hemangiomas (5%). The other 25% of primary cardiac tumors are malignant, with 95% of those being sarcomas and 5% being lymphomas. By far, metastases involving the heart and pericardium (secondary cardiac tumors) are 20-40 times more common than primary cardiac tumors and originate primarily from breast, esophageal or lung cancers, melanoma or renal cell carcinoma (RCC)[19].
Malignant cancers metastasize to the heart through direct extension from mediastinal tumors, hematogenous spread, lymphatic spread or intracavitary extension arising from the ICV (RCC, adrenal carcinoma and hepatocellular carcinoma)[20] and rarely through the pulmonary vein. Particularly, the right atrium is a typical location for malignant cardiac tumors such as angiosarcoma, lymphoma and melanoma and for intracardiac metastases from RCC due to spread via the ICV. Chordoma commonly metastasizes to the right atrium[21]. Myxomas and lipomas are also typically localized to the atria, whereas rhabdomyosarcomas and lymphomas are commonly located in the ventricles and fibroelastomas are localized to the valves[22] (Figure 5).
Thrombi located in the right atrium are seldom visualized by echocardiography (ECO) and account for only 4% of cases. Atrial thrombi are more often located in the left atrium or appendage and are generally accompanied by structural heart disease[23]. They are usually highly mobile, serpiginous and without the typical myxoma features[24,25] such as the attachment via a stalk and the presence of intramass calcification[26].
The diagnostic pathway should be based on the tumor type epidemiology, imaging features and histopathological diagnosis. In this patient, the third component was missing due to her worsening prognosis. The non-invasive diagnostic armamentarium to discriminate thrombi from cancers includes ECO, CT, magnetic resonance imaging (MRI) and positron emission tomography (PET)[27].
ECO is the first-line modality to quantify size, shape, location, attachment and mobility of the intracardiac and extracardiac mass. Its diagnostic sensitivity is 93% for TTE and 97% for transesophageal ECO[28]. Additionally, three-dimensional ECO (3DECO) offers notable advantages. The entire mass can be visualized compared to a thin slice obtained by the two-dimensional ECO (2DECO). 3DECO can also evaluate the volume instead of just linear dimensions, making the measurement more accurate (Table 3). 3DECO can also identify clues, such as homogeneity, vascularity, calcification and necrosis, and it can outline the attachment point by the cropping technique. 3DECO sensitivity for identifying thrombi is > 90%, while specificity is > 85% compared to 2DECO[29]. Moreover, echocardiographic contrast perfusion is regarded as a mainstay to characterize the vascularity of cardiac masses. It typically detects differences among neovascularized malignant or highly vascular cardiac tumors, poorly vascularized stromal tumors, avascular thrombi and normal myocardium[30].
| Thrombus | Tumor |
| Smaller size | Larger size |
| Single lesion | Single lesion (benign tumor) |
| Multiple lesions (malignant tumor) | |
| Mostly left atrium/left ventricle | Left atrium (benign tumor) |
| Right atrium/multiple chambers/pulmonary artery trunk (malignant tumor) | |
| Homogeneous tissue pattern, areas of lysis, “donut hole” appearance | Heterogeneous tissue pattern, irregular and patchy texture, areas of echolucency |
| Highly mobile and serpiginous | Not mobile |
| Broad based attachment | Attached by a stalk |
| Absence of intramass calcifications | Intramass calcifications, ossification, hemorrhage |
| Not infiltrating adjacent tissues | Intracardiac/extracardiac infiltration |
| Avascular | Vascularized |
| Poorly defined borders | Lobulated margins, definite edges |
| Absence of pericardial effusion | Associated pericardial effusion |
| Absence of metastases | Metastases may be present |
CT provides a precise delineation of the lesion margins, and it optimally evaluates calcified masses. Chest and lung tissue can also be evaluated. Besides, CT may exclude obstructive coronary artery disease or masses in the coronary arteries as well[31]. When the diagnosis by CT is uncertain and discrimination between benign and malignant lesions is challenging, 18F-fludeoxyglucose (FDG)-PET/CT is an extremely powerful tool to elucidate substantial information regarding the nature of the mass. 18F-FDG-PET is a molecular imaging method to visualize cell metabolism and to assess metabolic activity[32]. Although its availability is limited, 18F-FDG-PET is an insightful tool to visualize the metabolic rate of glycolysis in tumors. Malignant cardiac tumors typically exhibit a high 18F-FDG uptake, while benign cardiac tumors exhibit a slight 18F-FDG uptake[33].
The combination of ECO and MRI can be used for the diagnosis and monitoring of cardiac masses[34]. Compared to CT, MRI offers higher temporal resolution and additional tissue characterization. Moreover, it does not require ionizing radiation exposure[35]. Importantly, MRI may evaluate the age of a thrombus. Acute thrombi usually present intermediate signal intensity on both T1- and T2-weighted images, subacute thrombi have a lower T1-weighted signal intensity and increased T2-weighted signal intensity, and chronic organized thrombi have a lower signal intensity on both T1- and T2-weighted images. Unlike tumors, thrombi appear with an absence of contrast material uptake in early and late gadolinium enhancement due to their avascular nature[36]. Contrast MRI with first-pass perfusion and late gadolinium enhancement are particularly useful to define benign vs malignant tumors. All malignant tumors have first-pass perfusion due to their highly vascular nature and they also present late gadolinium enhancement.
In contrast to current guidelines that regard ECO, CT, MRI and PET as equal diagnostic methods[27], our diagnostic algorithm (Figure 6) proposes ECO as the only first-line tool due to its widespread availability and lack of radiation exposure. If ECO is inconclusive, equivocal or unreliable, CT, MRI and PET are second-line options to allow differential diagnosis between cancers and thrombi in a timely manner.
Our patient was at high risk for a prothrombotic burden due to metastatic pancreatic cancer, an indwelling line and gemcitabine and platinum therapy. Furthermore, the atrial mass did not appear to infiltrate other structures and was not vascularized. A cardiac tumor was excluded in our patient due to the lack of infiltrative growth, lobulated margins, pericardial effusion and large size. We recognize that the patient’s workup was suboptimal, and the mass was in the right atrium, suggestive of a malignant tumor. However, the location of cardiac masses may be misleading. Due to the patient’s age, mass localization, general clinical context (cancer site and stage, cancer treatment, recent extended CAT) and ultrasound features, the mass was likely a new VTE event.
We have reported herein the rare incidental finding of a notable intracardiac mass diagnosed as a VTE in a patient with secondary metastatic pancreatic cancer and CAT. Differential diagnosis of the mass was challenging due to the location of the mass. We suspected a new CAT event due to the patient’s cancer history, echocardiographic characteristics of the mass and the previously discovered CAT. Our patient was unable to adhere to injectable anticoagulant treatment with LMWH. Due to worsening prognosis, palliative care was recommended.
Cardioncological surveillance is of utmost importance in patients with active cancer to detect incidental VTE, which may otherwise be missed and untreated. We presented a non-invasive evidence-based flow chart to guide the differential diagnosis of atrial masses to improve patient care and outcomes.
We are grateful to Simon Noble, Athina Papa and Alfredo Zito for their helpful comments and careful reading of this manuscript. The authors affiliated with the IRCCS Istituto Tumori “Giovanni Paolo II” in Bari Italy are responsible for the views expressed in this article, which do not necessarily represent those of the Institute.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: He X, China; Xu X, China; Yang L, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Lestuzzi C. Primary tumors of the heart. Curr Opin Cardiol. 2016;31:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Tomlinson JS, El-Gaaly M, Khan S, Papouchado M. Right atrial mass: a challenging diagnosis. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, Meyer G, Segers A, Shi M, Wang TF, Yeo E, Zhang G, Zwicker JI, Weitz JI, Büller HR; Hokusai VTE Cancer Investigators. Edoxaban for the Treatment of Cancer-Associated Venous Thromboembolism. N Engl J Med. 2018;378:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 1090] [Article Influence: 155.7] [Reference Citation Analysis (0)] |
| 4. | Samuelson Bannow BR, Lee AYY, Khorana AA, Zwicker JI, Noble S, Ay C, Carrier M. Management of anticoagulation for cancer-associated thrombosis in patients with thrombocytopenia: A systematic review. Res Pract Thromb Haemost. 2018;2:664-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 472] [Cited by in RCA: 528] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 6. | L'Angiocola PD, Donati R. Cardiac Masses in Echocardiography: A Pragmatic Review. J Cardiovasc Echogr. 2020;30:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Mulder FI, Bosch FTM, Young AM, Marshall A, McBane RD, Zemla TJ, Carrier M, Kamphuisen PW, Bossuyt PMM, Büller HR, Weitz JI, Middeldorp S, van Es N. Direct oral anticoagulants for cancer-associated venous thromboembolism: a systematic review and meta-analysis. Blood. 2020;136:1433-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 8. | Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 637] [Cited by in RCA: 840] [Article Influence: 70.0] [Reference Citation Analysis (0)] |
| 9. | Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, Gates LE, Kakkar AK, Levine MN, Liebman HA, Tempero MA, Lyman GH, Falanga A. Venous Thromboembolism Prophylaxis and Treatment in Patients With Cancer: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2020;38:496-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 946] [Article Influence: 157.7] [Reference Citation Analysis (0)] |
| 10. | Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41:81-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 11. | Schulman S, Shrum J, Majeed A. Management of bleeding complications in patients with cancer on DOACs. Thromb Res. 2016;140 Suppl 1:S142-S147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Prandoni P, Lensing AW, Piccioli A, Bernardi E, Simioni P, Girolami B, Marchiori A, Sabbion P, Prins MH, Noventa F, Girolami A. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484-3488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1246] [Cited by in RCA: 1323] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 13. | Nair RM, Maroo A. The concoction of cancer, catheter, and intracardiac clot: a case report describing a potential treatment strategy. Eur Heart J Case Rep. 2020;4:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902-4907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1526] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 15. | van Es N, Di Nisio M, Cesarman G, Kleinjan A, Otten HM, Mahé I, Wilts IT, Twint DC, Porreca E, Arrieta O, Stépanian A, Smit K, De Tursi M, Bleker SM, Bossuyt PM, Nieuwland R, Kamphuisen PW, Büller HR. Comparison of risk prediction scores for venous thromboembolism in cancer patients: a prospective cohort study. Haematologica. 2017;102:1494-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 16. | Burke A, Tavora F. The 2015 WHO Classification of Tumors of the Heart and Pericardium. J Thorac Oncol. 2016;11:441-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 166] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 17. | Casavecchia G, Lestuzzi C, Gravina M, Corrado G, Tusa M, Brunetti ND, Manuppelli V, Monte IP. Cardiac Tumors. J Cardiovasc Echogr. 2020;30:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Maleszewski JJ, Anavekar NS, Moynihan TJ, Klarich KW. Pathology, imaging, and treatment of cardiac tumours. Nat Rev Cardiol. 2017;14:536-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 19. | Maleszewski JJ, Bois MC, Bois JP, Young PM, Stulak JM, Klarich KW. Neoplasia and the Heart: Pathological Review of Effects With Clinical and Radiological Correlation. J Am Coll Cardiol. 2018;72:202-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Khurana A, Tak T. Venous thromboembolic disease presenting as inferior vena cava thrombus extending into the right atrium. Clin Med Res. 2004;2:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Shah Z, Alraies MC, Harp A, Khalil S, Kaki A, Elder M. A right atrial mass. Cleve Clin J Med. 2019;86:445-447. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 22. | Saric M, Armour AC, Arnaout MS, Chaudhry FA, Grimm RA, Kronzon I, Landeck BF, Maganti K, Michelena HI, Tolstrup K. Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. J Am Soc Echocardiogr. 2016;29:1-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 257] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 23. | Benjamin MM, Afzal A, Chamogeorgakis T, Feghali GA. Right atrial thrombus and its causes, complications, and therapy. Proc (Bayl Univ Med Cent). 2017;30:54-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Habibi R, Altamirano AJ, Dadkhah S. Clot in Lung, Clot in Heart: A Case Report of Tumor-Like Thrombus in Right Atrium. Clin Med Insights Case Rep. 2017;10:1179547617698460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Ahmadi-Renani S, Alidoosti M, Salehi-Omran A, Shahbazi N, Hosseinsabet A. A Right Atrial Appendage Thrombus Mimicking a Tumor. J Cardiovasc Echogr. 2020;30:231-233. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 26. | Fan C, Zhang H, Zhuang H, Jiang Z, Tan H, Iroegbu CD, Song L, Liu L. Case Report: Giant Biatrial Myxoma Mimicking Malignant Cardiac Tumor in a Patient With a Hepatic Angiomatous Mass. Front Cardiovasc Med. 2021;8:676807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 27. | Lyon AR, López-Fernández T, Couch LS, Asteggiano R, Aznar MC, Bergler-Klein J, Boriani G, Cardinale D, Cordoba R, Cosyns B, Cutter DJ, de Azambuja E, de Boer RA, Dent SF, Farmakis D, Gevaert SA, Gorog DA, Herrmann J, Lenihan D, Moslehi J, Moura B, Salinger SS, Stephens R, Suter TM, Szmit S, Tamargo J, Thavendiranathan P, Tocchetti CG, van der Meer P, van der Pal HJH; ESC Scientific Document Group, Lancellotti P, Thuny F, Abdelhamid M, Aboyans V, Aleman B, Alexandre J, Barac A, Borger MA, Casado-Arroyo R, Cautela J, Čelutkienė J, Cikes M, Cohen-Solal A, Dhiman K, Ederhy S, Edvardsen T, Fauchier L, Fradley M, Grapsa J, Halvorsen S, Heuser M, Humbert M, Jaarsma T, Kahan T, Konradi A, Koskinas KC, Kotecha D, Ky B, Landmesser U, Lewis BS, Linhart A, Lip GYH, Løchen ML, Malaczynska-Rajpold K, Metra M, Mindham R, Moonen M, Neilan TG, Nielsen JC, Petronio AS, Prescott E, Rakisheva A, Salem JE, Savarese G, Sitges M, Ten Berg J, Touyz RM, Tycinska A, Wilhelm M, Zamorano JL. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS). Eur Heart J. 2022;43:4229-4361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 1332] [Article Influence: 444.0] [Reference Citation Analysis (0)] |
| 28. | Cohen A, Donal E, Delgado V, Pepi M, Tsang T, Gerber B, Soulat-Dufour L, Habib G, Lancellotti P, Evangelista A, Cujec B, Fine N, Andrade MJ, Sprynger M, Dweck M, Edvardsen T, Popescu BA; Reviewers: This document was reviewed by members of the 2018–2020 EACVI Scientific Documents Committee; chair of the 2018–2020 EACVI Scientific Documents Committee. EACVI recommendations on cardiovascular imaging for the detection of embolic sources: endorsed by the Canadian Society of Echocardiography. Eur Heart J Cardiovasc Imaging. 2021;22:e24-e57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 29. | Zaragoza-Macias E, Chen MA, Gill EA. Real time three-dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2012;29:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 30. | Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, DeCara JM, Weinert L, Krausz T, Lang RM. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 31. | Kassop D, Donovan MS, Cheezum MK, Nguyen BT, Gambill NB, Blankstein R, Villines TC. Cardiac Masses on Cardiac CT: A Review. Curr Cardiovasc Imaging Rep. 2014;7:9281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | D'Angelo EC, Paolisso P, Vitale G, Foà A, Bergamaschi L, Magnani I, Saturi G, Rinaldi A, Toniolo S, Renzulli M, Attinà D, Lovato L, Lima GM, Bonfiglioli R, Fanti S, Leone O, Saponara M, Pantaleo MA, Rucci P, Di Marco L, Pacini D, Pizzi C, Galiè N. Diagnostic Accuracy of Cardiac Computed Tomography and 18-F Fluorodeoxyglucose Positron Emission Tomography in Cardiac Masses. JACC Cardiovasc Imaging. 2020;13:2400-2411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 33. | Rahbar K, Seifarth H, Schäfers M, Stegger L, Hoffmeier A, Spieker T, Tiemann K, Maintz D, Scheld HH, Schober O, Weckesser M. Differentiation of malignant and benign cardiac tumors using 18F-FDG PET/CT. J Nucl Med. 2012;53:856-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 34. | Armstrong GT, Plana JC, Zhang N, Srivastava D, Green DM, Ness KK, Daniel Donovan F, Metzger ML, Arevalo A, Durand JB, Joshi V, Hudson MM, Robison LL, Flamm SD. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876-2884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 228] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 35. | Motwani M, Kidambi A, Herzog BA, Uddin A, Greenwood JP, Plein S. MR imaging of cardiac tumors and masses: a review of methods and clinical applications. Radiology. 2013;268:26-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 257] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 36. | Kassi M, Polsani V, Schutt RC, Wong S, Nabi F, Reardon MJ, Shah DJ. Differentiating benign from malignant cardiac tumors with cardiac magnetic resonance imaging. J Thorac Cardiovasc Surg. 2019;157:1912-1922.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |