Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1198
Peer-review started: November 14, 2022
First decision: January 12, 2023
Revised: January 20, 2023
Accepted: January 28, 2023
Article in press: January 28, 2023
Published online: February 16, 2023
Processing time: 91 Days and 19 Hours
Regional anesthesia is a promising method in patients with post coronavirus disease 2019 (COVID-19) pulmonary sequelae for preserving pulmonary function and preventing postoperative pulmonary complications, compared with general anesthesia.
We provided surgical anesthesia and analgesia suitable for breast surgery by performing pectoral nerve block type II (PECS-II), parasternal, and intercostobrachial nerve blocks with intravenous dexmedetomidine administration in a 61-year-old female patient with severe pulmonary sequelae after COVID-19 infection.
Sufficient analgesia for 7 h was provided via PECS-II, parasternal, and intercostobrachial blocks perioperatively.
Core Tip: This is the first clinical case report of the application of multiple nerve blocks for breast cancer surgery in a patient with severe post coronavirus disease 2019 (COVID-19) pulmonary sequelae. The use of performing pectoral nerve block type II (PECS-II), parasternal, and intercostobrachial nerve blocks provided sufficient analgesic and anesthetic effects during the perioperative period. Therefore, this case report suggests an alternative anesthetic modality for post COVID-19 patients who are at a high risk of receiving general anesthesia for breast surgery.
- Citation: Jin Y, Lee S, Kim D, Hur J, Eom W. Combinations of nerve blocks in surgery for post COVID-19 pulmonary sequelae patient: A case report and review of literature. World J Clin Cases 2023; 11(5): 1198-1205
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1198.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1198
The coronavirus disease 2019 (COVID-19) is a fatal respiratory viral disease that causes severe respiratory symptoms in some patients, resulting in more than 6 million deaths worldwide to date[1,2]. In 1099 patients with COVID-19, 86% had chest computed tomography (CT) abnormalities, 41% required additional oxygen therapy, 3.4% were diagnosed with acute respiratory distress syndrome, and 2.3% required invasive mechanical ventilation[3].
Surgery within 7 wk of COVID-19 diagnosis is associated with an increased risk of postoperative pulmonary complications[4]. Patients with persistent COVID-19 symptoms have an increased perioperative risk compared to asymptomatic patients or those with completely resolved symptoms at the time of surgery[5]. For cases of pneumonia caused by COVID-19, regional anesthesia is promising for preserving pulmonary function and preventing pulmonary complications postoperatively, whereas general anesthesia, including airway management, has the potential to exacerbate the disease[6-8].
In this case, combined PECS-II, parasternal, and intercostobrachial nerve (ICBN) blocks were used as alternatives to general anesthesia for the anesthetic management of patients with COVID-19. In patients with COVID-19 pneumonia, regional blocks have advantages in terms of protecting the medical staff by minimizing the formation of aerosols and preventing the exacerbation of pneumonia in patients by preventing pulmonary complications caused by general anesthesia[9]. This case suggests that regional anesthesia is a safe alternative for managing breast surgery, especially during the COVID-19 pandemic.
A 61-year-old woman (height: 153 cm, weight: 47.8 kg) presented with a growing lump in her left breast, detected through ultrasound imaging and mammography.
The patient was diagnosed with invasive ductal carcinoma at the 6 and 10 o’clock positions of her left breast using core needle biopsy. The patient planned to undergo breast-conserving surgery and axillary dissection 2 wk after neoadjuvant chemotherapy. Fifteen days before the scheduled surgery, she had high fever (up to 39.3 °C), cough, and dyspnea. The patient was diagnosed with COVID-19 based on the polymerase chain reaction-positive results.
The patient denied any past history.
The patient denied any family history.
The patient's peripheral oxygen saturation level was 93% at 3 L/min of supplemental oxygen using a nasal prong.
Pulmonary function tests were not performed, considering the patient’s general condition and to minimize the contamination risk from the laboratory surroundings.
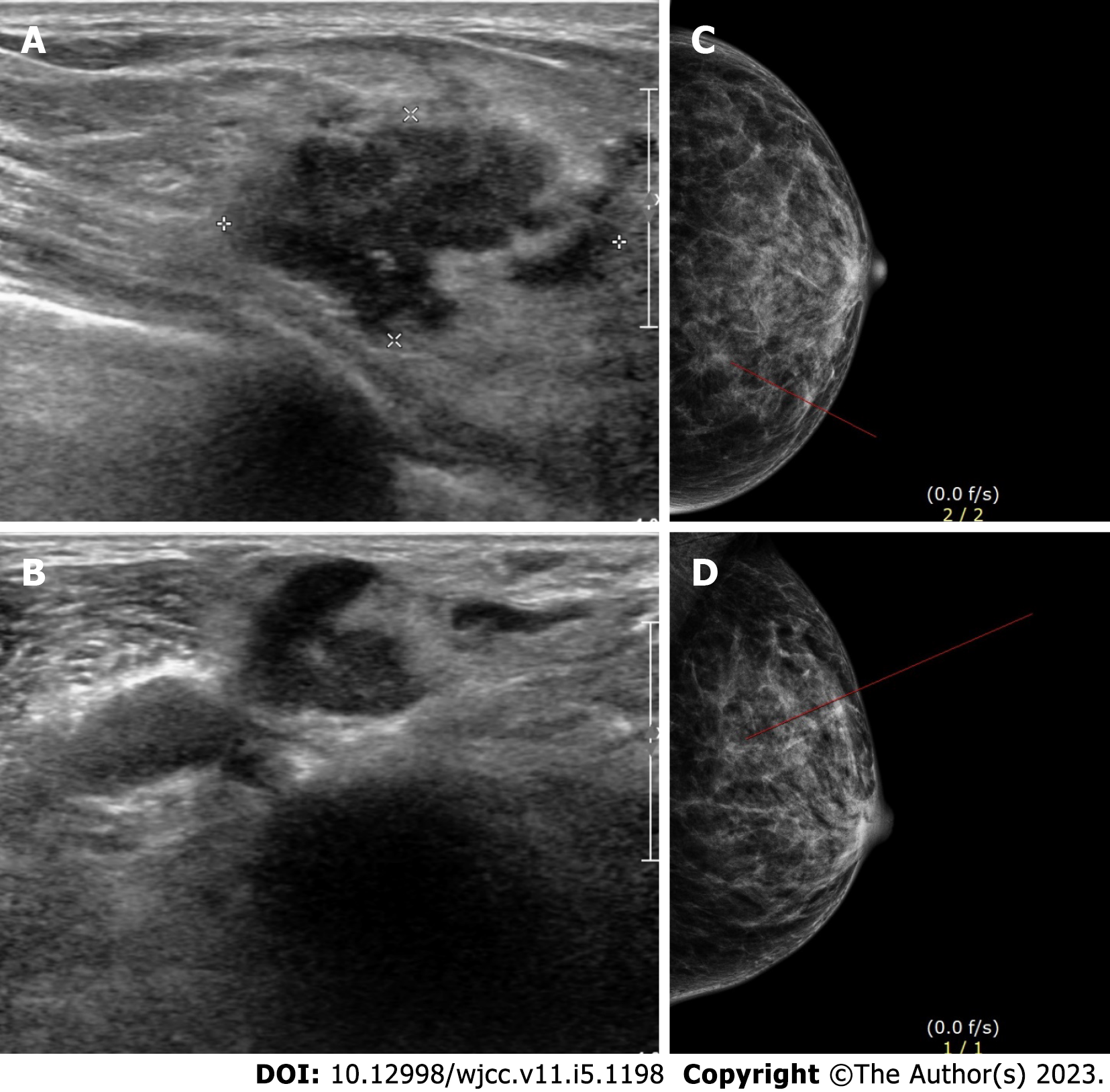
The left breast lump was detected through ultrasound imaging and mammography (Figure 1).
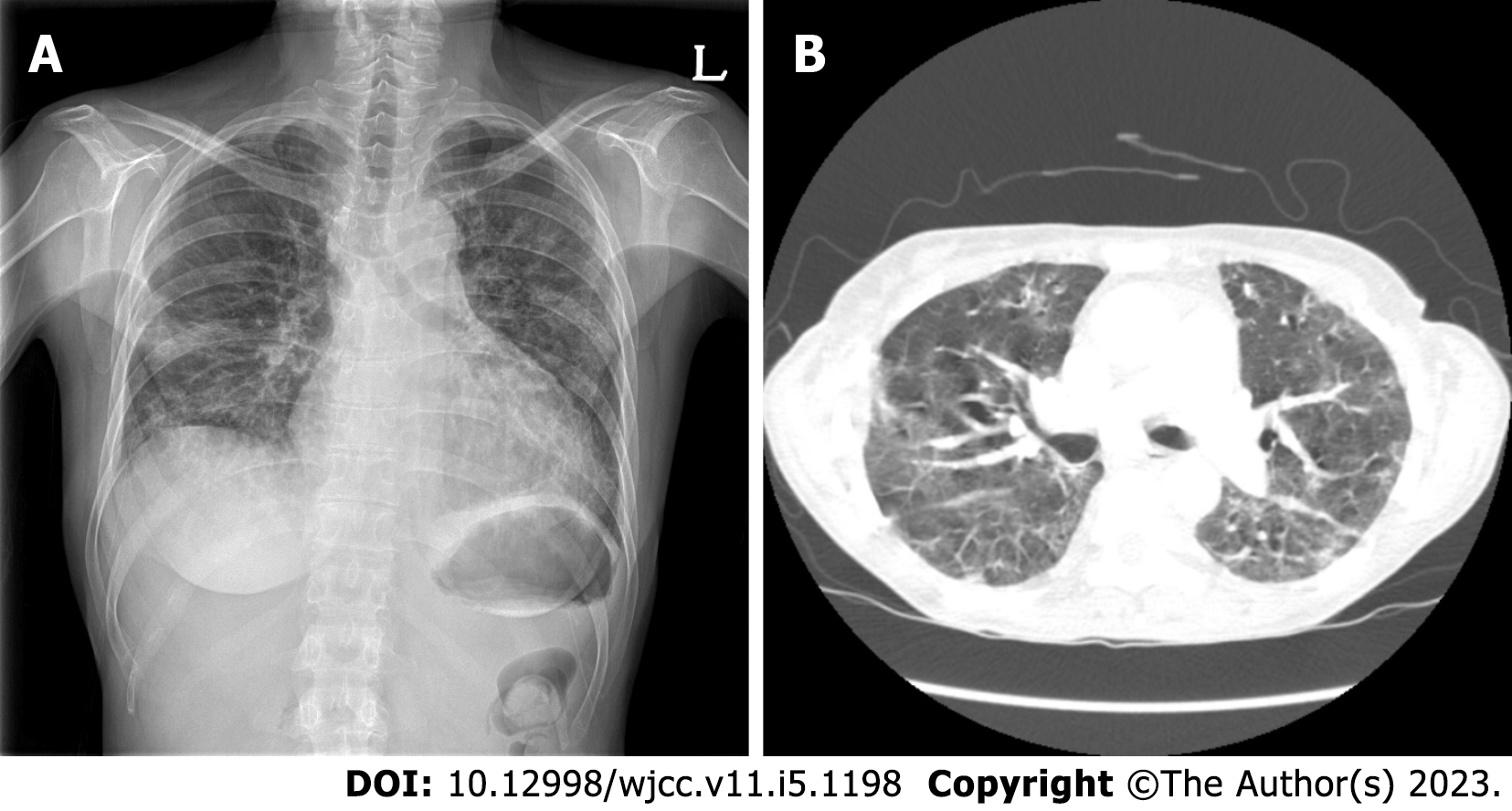
Because the patient had severe pulmonary fibrosis after the COVID-19 infection, a chest radiograph showed multiple patch opacities in both lungs, and a CT scan showed multifocal patchy ground-glass opacity consolidation in both lungs (Figure 2).
The patient had contracted COVID-19 pneumonia and received intensive care. Three weeks after treatment, the patient was hospitalized again for surgery.
Owing to the patient’s respiratory problems, we decided to proceed with the surgery under regional anesthesia rather than general anesthesia by combining PECS-II, parasternal, and ICBN blocks. Written informed consent for the combination of blocks and publications was obtained from the patient. Standard perioperative monitoring, including noninvasive blood pressure measurement, peripheral oxygen saturation measurement, and electrocardiography, was performed. Supplemental oxygen was administered via a facial mask.
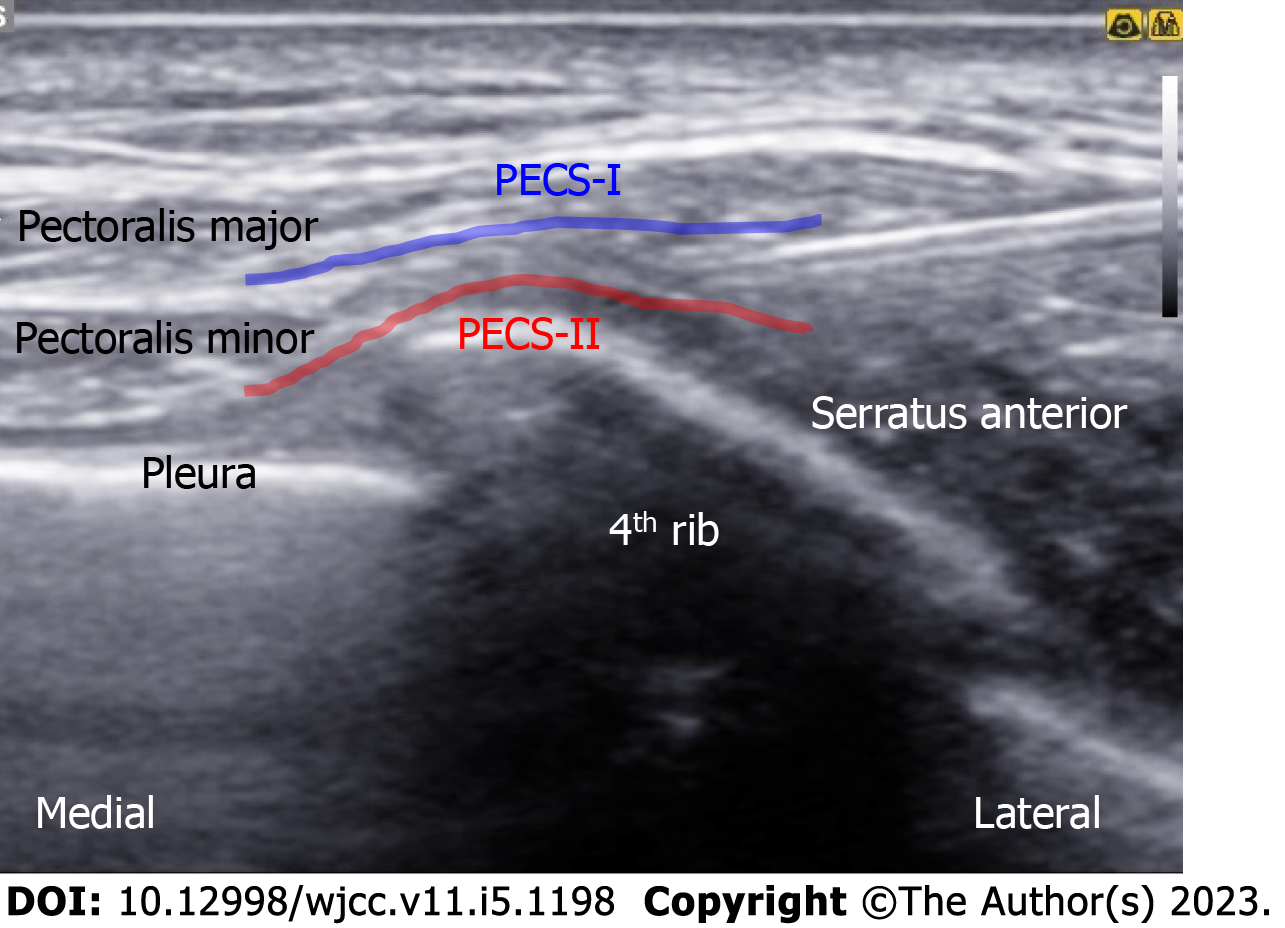
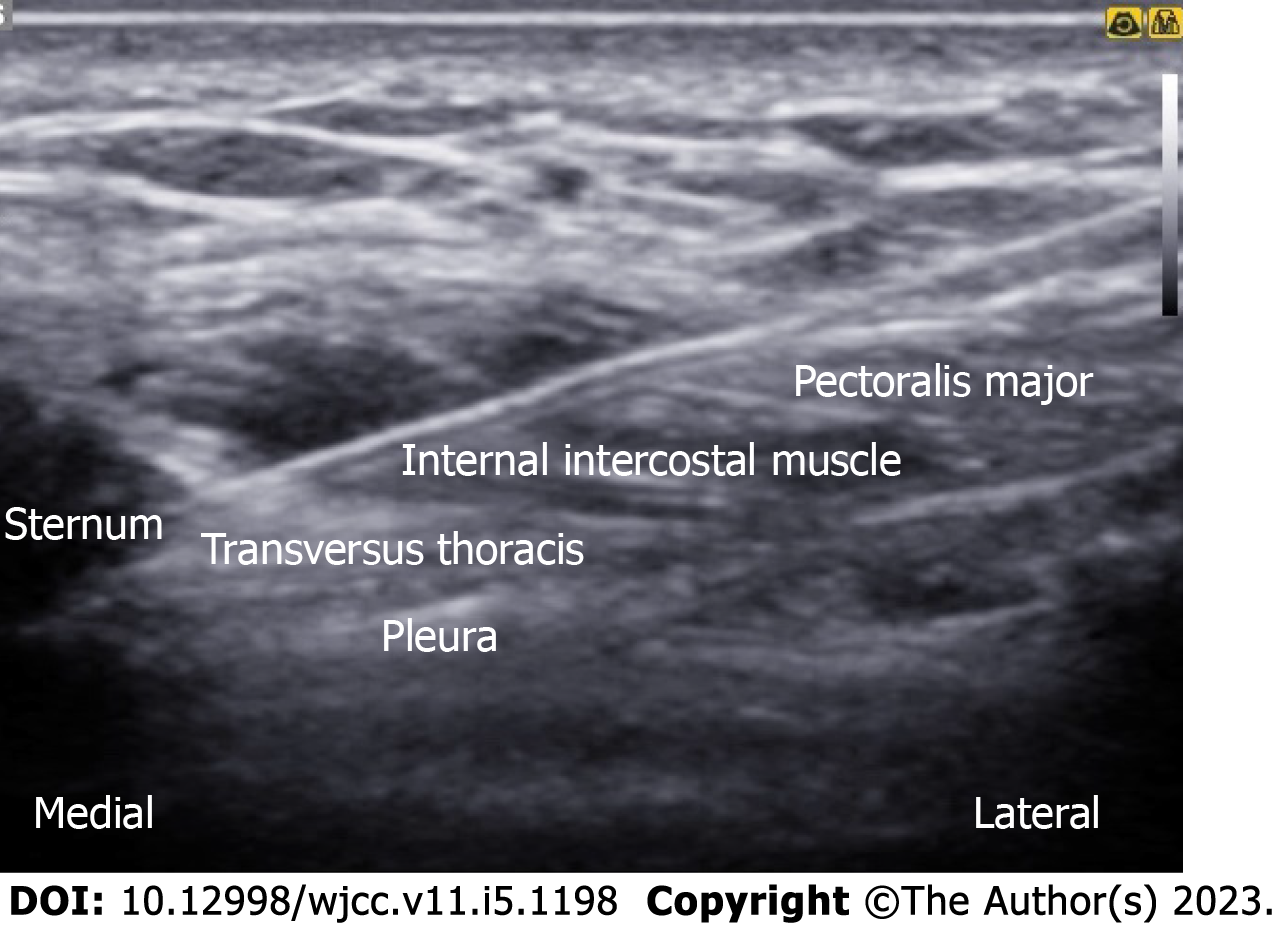
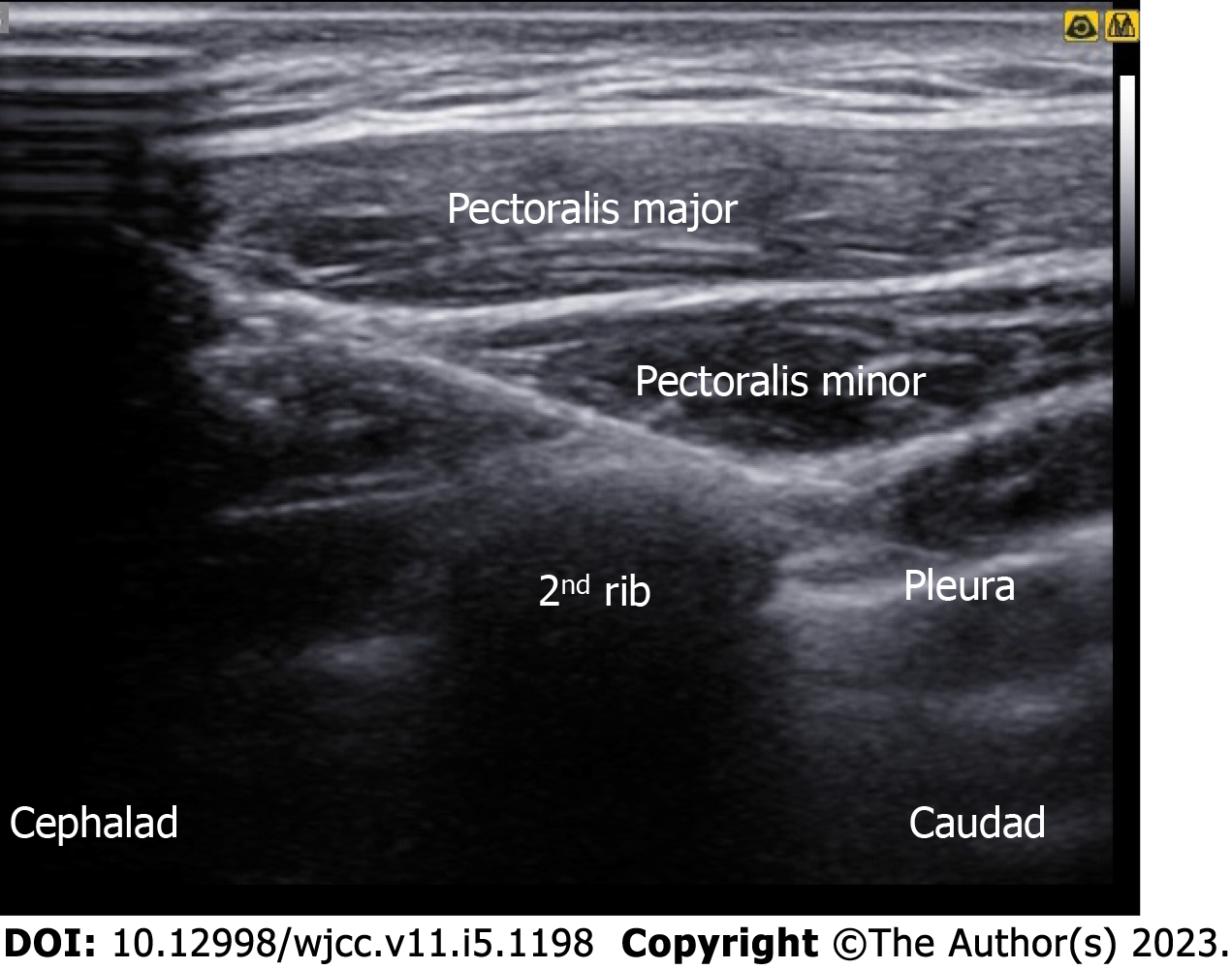
An in-plane needle (80 mm; B. Braun Medical Inc., Melsungen, Germany) was inserted under real-time ultrasound guidance using a 5-12 MHz high-frequency linear array transducer (Samsung Medison Co., Ltd., Seoul, Korea). After sterilization, the ultrasound probe was placed in a superomedial-to-inferolateral direction between the third and fourth ribs, below the lateral third of the clavicle. After local infiltration, 10 mL of 0.3% plain ropivacaine was injected into the interfascial plane between the pectoralis major and pectoralis minor muscles, without injuring the pectoral branch of the acromiothoracic artery. Subsequently, a needle was advanced a little more, and an additional 15 mL was injected between the pectoralis minor and serratus anterior muscles (Figure 3). For the parasternal nerve block, 5 mL of 0.3% ropivacaine was incrementally injected between the internal intercostal muscle and neurovascular bundles (Figure 4). For the ICBN block, 10 mL of 0.3% ropivacaine was injected medially into the medial border of the serratus anterior muscle at the inferior border of the second rib (Figure 5).
The total procedural time of the combined block was approximately 15 min, and no adverse events were reported during the procedure. After 20 min, sufficient analgesic effects were observed. Sensory impairment across the T2-T6 dermatomes was confirmed through the cold alcohol swab and pinprick tests in the medial and lateral areas of the left breast. Dexmedetomidine was administered as a loading dose of 1 μg/kg followed by a maintenance dose up to 0.5 μg/kg/h to target a Richmond agitation-sedation scale score of 2. The left breast was excised 1.5 cm × 1.8 cm at 4 cm from the nipple at the 6 o’clock position and 2.2 cm × 2.5 cm at the position 5 cm from the nipple at the 10 o’clock position. Because sentinel lymph node biopsy revealed a positive result for metastasis, an additional wide excision and axillary lymph node dissection were performed. The entire surgery was performed within 2 h and 10 min, without hemodynamic instability or complications. No supplemental opioids or additional local anesthetics were required during the surgery.
Postoperative pain scores, assessed using the numeric rating scale, were 1-2 in the post-anesthesia care unit. The duration of the combined block was approximately 7 h. No additional analgesics were administered for 1 d. Five days after the surgery, the patient was discharged.
Owing to the risk of aerosol generation and dispersion of viral particles, airway management in patients with COVID-19 undergoing surgery poses a great risk to healthcare providers[7,8]. For this reason, it is more appropriate for patients with COVID-19 to use regional anesthesia rather than general anesthesia, where applicable[7,8]. Additionally, regional anesthesia is a reasonable alternative for surgery in patients with COVID-19 because it has a lesser effect on pulmonary function involvement than general anesthesia and minimizes the incidence of postoperative pulmonary complications[7]. Regional techniques can also reduce the risk of opioid-induced hypoventilation[10]. Moreover, it provides excellent postoperative analgesic effects, attenuates the surgical stress response, and enables prompt postoperative recovery[11].
The sensory innervation of the breast is complex and is derived from several nerves. No single block can provide complete anesthesia, and complex chest wall blocks allow for more effective pain control[12]. The feasibility and risk of each block technique should be determined based on sufficient understanding of the surrounding structural and clinical anatomy.
The PECS-II block is an interfascial plane block that involves adding a second injection to the conventional PECS-I block under the pectoralis minor to broaden its coverage to the lateral and axillary regions[13]. Conventionally, the most commonly considered analgesic method in upper chest surgery is thoracic epidural block or thoracic paravertebral block[14]. The PECS block has garnered significant appeal due to its low risk of hypotensive effects and sympathetic block, as opposed to the thoracic epidural block or thoracic paravertebral block[15]. The PECS block also has a relatively low risk in patients with altered hemostasis or anticoagulant use compared to thoracic epidural block or thoracic paravertebral block[16]. In a meta-analysis comparing the analgesic effects of the PECS-II and paravertebral blocks, no significant difference in pain scores and rescue analgesic use was observed between the two blocks[17]. Some studies have reported that the PECS-II block has superior efficacy over the paravertebral block[18,19].
Furthermore, both the brachial plexus and the lateral branches of the superior intercostal nerve (intercostal nerves T2-T3) innervate the sensory distribution of the axilla[20]. The intercostobrachial nerve is the lateral cutaneous branch of the second and third intercostal nerves[21]. It crosses the serratus anterior muscle to enter the subcutaneous tissue of the axilla, at the midaxillary line[21]. It is located anatomically separate from the brachial plexus and, therefore, cannot be completely blocked by the brachial plexus block[22]. Ultrasound-guided technology provides better visualization and increases the success rate of selective nerve blockade.
Implementations of the PECS-II block were targeted to cover the intercostobrachial nerve, but did not completely block it. In some cases, even the intercostobrachial nerve can be blocked with the PECS-II block alone; however, the intercostobrachial nerve is often considered to be sparing[23]. The covered range varies depending on the volume, injection location, and skill level. Therefore, for more extensive surgical interventions in the axillary region, we performed an additional ICBN block.
The parasternal nerve block targets the anterior cutaneous branches of the thoracic intercostal nerve, which innervates the sternum and anteromedial thorax[24]. The nomenclature is based on whether the injection is administered in a more superficial plane between the ribs and internal intercostal muscles (pecto-intercostal plane block) or into a deeper plane between the transversus thoracis and internal intercostal muscles (transversus thoracis plane block)[25]. Because the PECS-II block alone did not affect sensory innervation of the medial side of the breast, a parasternal nerve block on the anterior branch of the intercostal nerve was necessary for medial breast anesthesia[26].
Dexmedetomidine, a centrally acting as a selective α2-adrenergic receptor agonist, has been actively used for its sedative and analgesic properties as an adjuvant therapy for regional anesthesia[27]. As a perineural adjuvant, dexmedetomidine has been demonstrated to be effective in prolonging the duration of local anesthesia in peripheral nerve blocks[28,29]. Furthermore, studies have shown that dexmedetomidine administration is associated with improved oxygenation in critically ill patients with COVID-19[30]. In addition, alterations in inflammatory and immune responses caused by dexmedetomidine have been suggested to reduce the severity of COVID-19 pneumonia[31].
In general, the brachial plexus block can be used to block the axillary region. However, we did not perform this because the interscalene approach of the brachial plexus block frequently results in phrenic nerve block and further diaphragmatic paralysis[7]. Regional techniques that have the potential to reduce the patient’s respiratory reserve should be avoided as much as possible in respiratory-compromised patients with coexisting pulmonary diseases[7]. A block with the least impact on the patient’s respiratory function should be selected, if possible[7,32].
Our study had several limitations. The complexity of combined blocks requires a specific learning curve to achieve time-efficient proficiency for the anesthesiologists. The untrained proficiency of the anesthesiologists in regional techniques can be a limiting factor in practice. Increasing the dosage of local anesthetics by implementing a combination of peripheral and subcutaneous blocks increases the risk of toxicity. Considering that the maximum tolerated dose of ropivacaine is approximately 3 mg/kg, the total volume of 40 mL of 0.3% ropivacaine is within the safe range for our patient weighing 47.8 kg[33]. Although the use of local anesthetics in this study is still within safe limits, the potential for local anesthetic systemic toxicity is still present. Therefore, the dose should be cautiously titrated depending on the patient’s underlying conditions. Hence, for the efficacy and safety of regional anesthesia in patients with COVID-19, further prospective studies on block-specific applications, dosing, and minimization of complications are needed.
To the best of our knowledge, this is the first case study to report the successful use of this novel approach in a patient with severe COVID-19 pulmonary sequelae. The combination of PECS-II, parasternal, and ICBN blocks had the expected analgesic and anesthetic effects on the medial to lateral region of the breast through the T2 to T6 dermatomes with an acceptable sensory blockade. The combination of these blocks appears to be a valid alternative modality for COVID-19-infected breast surgery patients who are at a high risk of receiving general anesthesia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He YF, China; Singh A, India; Zhang YN, China S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 12968] [Article Influence: 2593.6] [Reference Citation Analysis (1)] |
| 2. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14761] [Article Influence: 2952.2] [Reference Citation Analysis (0)] |
| 3. | Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS; China Medical Treatment Expert Group for COVID-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708-1720. [PubMed] [DOI] [Full Text] |
| 4. | Lal BK, Prasad NK, Englum BR, Turner DJ, Siddiqui T, Carlin MM, Lake R, Sorkin JD. Periprocedural complications in patients with SARS-CoV-2 infection compared to those without infection: A nationwide propensity-matched analysis. Am J Surg. 2021;222:431-437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 5. | COVIDSurg Collaborative; GlobalSurg Collaborative. Timing of surgery following SARS-CoV-2 infection: an international prospective cohort study. Anaesthesia. 2021;76:748-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 403] [Cited by in RCA: 366] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 6. | Memtsoudis SG, Cozowicz C, Bekeris J, Bekere D, Liu J, Soffin EM, Mariano ER, Johnson RL, Hargett MJ, Lee BH, Wendel P, Brouillette M, Go G, Kim SJ, Baaklini L, Wetmore D, Hong G, Goto R, Jivanelli B, Argyra E, Barrington MJ, Borgeat A, De Andres J, Elkassabany NM, Gautier PE, Gerner P, Gonzalez Della Valle A, Goytizolo E, Kessler P, Kopp SL, Lavand'Homme P, MacLean CH, Mantilla CB, MacIsaac D, McLawhorn A, Neal JM, Parks M, Parvizi J, Pichler L, Poeran J, Poultsides LA, Sites BD, Stundner O, Sun EC, Viscusi ER, Votta-Velis EG, Wu CL, Ya Deau JT, Sharrock NE. Anaesthetic care of patients undergoing primary hip and knee arthroplasty: consensus recommendations from the International Consensus on Anaesthesia-Related Outcomes after Surgery group (ICAROS) based on a systematic review and meta-analysis. Br J Anaesth. 2019;123:269-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 194] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 7. | Lie SA, Wong SW, Wong LT, Wong TGL, Chong SY. Practical considerations for performing regional anesthesia: lessons learned from the COVID-19 pandemic. Can J Anaesth. 2020;67:885-892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 8. | Hotta K. Regional anesthesia in the time of COVID-19: a minireview. J Anesth. 2021;35:341-344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Guven BB, Erturk T, Güner T, Ersoy A. Abdominal wall blocks for emergency ileostomy operation in a patient with COVID-19 pneumonia: a case report. Braz J Anesthesiol. 2021;71:572-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 10. | de la Torre PA, García PD, Alvarez SL, Miguel FJ, Pérez MF. A novel ultrasound-guided block: a promising alternative for breast analgesia. Aesthet Surg J. 2014;34:198-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 11. | Nadeem M, Sahu A. Ultrasound guided surgery under Dilutional Local Anaesthesia and no sedation in breast cancer patients. Surgeon. 2020;18:91-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Chin KJ, Versyck B, Pawa A. Ultrasound-guided fascial plane blocks of the chest wall: a state-of-the-art review. Anaesthesia. 2021;76 Suppl 1:110-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Blanco R, Fajardo M, Parras Maldonado T. Ultrasound description of Pecs II (modified Pecs I): a novel approach to breast surgery. Rev Esp Anestesiol Reanim. 2012;59:470-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 428] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 14. | Wild K, Chin KJ. Regional techniques for thoracic wall surgery. Curr Anesthesiol Rep. 2017;7:212-219. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kulhari S, Bharti N, Bala I, Arora S, Singh G. Efficacy of pectoral nerve block versus thoracic paravertebral block for postoperative analgesia after radical mastectomy: a randomized controlled trial. Br J Anaesth. 2016;117:382-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 16. | Narouze S, Benzon HT, Provenzano D, Buvanendran A, De Andres J, Deer T, Rauck R, Huntoon MA. Interventional Spine and Pain Procedures in Patients on Antiplatelet and Anticoagulant Medications (Second Edition): Guidelines From the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med. 2018;43:225-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Hussain N, Brull R, McCartney CJL, Wong P, Kumar N, Essandoh M, Sawyer T, Sullivan T, Abdallah FW. Pectoralis-II Myofascial Block and Analgesia in Breast Cancer Surgery: A Systematic Review and Meta-analysis. Anesthesiology. 2019;131:630-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Siddeshwara A, Singariya G, Kamal M, Kumari K, Seervi S, Kumar R. Comparison of efficacy of ultrasound-guided pectoral nerve block versus thoracic paravertebral block using levobupivacaine and dexamethasone for postoperative analgesia after modified radical mastectomy: A randomized controlled trial. Saudi J Anaesth. 2019;13:325-331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | Wahba SS, Kamal SM. Thoracic paravertebral block vs pectoral nerve block for analgesia after breast surgery. Egypt J Anaesth. 2014;30:129-135. [RCA] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 20. | Seidel R, Gray AT, Wree A, Schulze M. Surgery of the axilla with combined brachial plexus and intercostobrachial nerve block in the subpectoral intercostal plane. Br J Anaesth. 2017;118:472-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Loukas M, Hullett J, Louis RG Jr, Holdman S, Holdman D. The gross anatomy of the extrathoracic course of the intercostobrachial nerve. Clin Anat. 2006;19:106-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Thallaj AK, Al Harbi MK, Alzahrani TA, El-Tallawy SN, Alsaif AA, Alnajjar M. Ultrasound imaging accurately identifies the intercostobrachial nerve. Saudi Med J. 2015;36:1241-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Torre DE, Pirri C, Contristano M, Behr AU, De Caro R, Stecco C. Ultrasound-Guided PECS II + Serratus Plane Fascial Blocks Are Associated with Reduced Opioid Consumption and Lengths of Stay for Minimally Invasive Cardiac Surgery: An Observational Retrospective Study. Life (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 24. | Chin KJ. Thoracic wall blocks: From paravertebral to retrolaminar to serratus to erector spinae and back again - A review of evidence. Best Pract Res Clin Anaesthesiol. 2019;33:67-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Ueshima H, Kitamura A. Blocking of Multiple Anterior Branches of Intercostal Nerves (Th2-6) Using a Transversus Thoracic Muscle Plane Block. Reg Anesth Pain Med. 2015;40:388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med. 2015;40:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 243] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Ramadhyani U, Park JL, Carollo DS, Waterman RS, Nossaman BD. Dexmedetomidine: clinical application as an adjunct for intravenous regional anesthesia. Anesthesiol Clin. 2010;28:709-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Schnabel A, Reichl SU, Weibel S, Kranke P, Zahn PK, Pogatzki-Zahn EM, Meyer-Frießem CH. Efficacy and safety of dexmedetomidine in peripheral nerve blocks: A meta-analysis and trial sequential analysis. Eur J Anaesthesiol. 2018;35:745-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Xiong C, Han CP, Zhao D, Tang ZH, Zhang YF, Wang J. Comparing the effects of dexmedetomidine and dexamethasone as perineural adjuvants on peripheral nerve block: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2021;100:e27064. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Uusalo P, Valtonen M, Järvisalo MJ. Hemodynamic and respiratory effects of dexmedetomidine sedation in critically ill COVID-19 patients: A retrospective cohort study. Acta Anaesthesiol Scand. 2021;65:1447-1456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Jain A, Lamperti M, Doyle DJ. Dexmedetomidine: another arrow in the quiver to fight COVID-19 in intensive care units. Br J Anaesth. 2021;126:e35-e38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Uppal V, Sondekoppam RV, Landau R, El-Boghdadly K, Narouze S, Kalagara HKP. Neuraxial anaesthesia and peripheral nerve blocks during the COVID-19 pandemic: a literature review and practice recommendations. Anaesthesia. 2020;75:1350-1363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 33. | Leone S, Di Cianni S, Casati A, Fanelli G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008;79:92-105. [PubMed] |