Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1165
Peer-review started: November 4, 2022
First decision: December 13, 2022
Revised: December 25, 2022
Accepted: January 20, 2023
Article in press: January 20, 2023
Published online: February 16, 2023
Processing time: 101 Days and 20.5 Hours
Invasive urothelial carcinoma (UC) with squamous and glandular differentiation is a highly malignant and complicated pathological subtype, and the standard care is radical cystectomy (RC). However, urinary diversion after RC significantly reduces patient quality of life, thus bladder-sparing therapy has become a research hotspot in this field. Recently, five immune checkpoint inhibitors have been approved for systemic therapy of locally advanced or metastatic bladder cancer by the Food and Drug Administration, but the efficacy of immunotherapy combined with chemotherapy for invasive UC is still unknown, especially for pathological subtypes with squamous and glandular differentiation.
We report the case of a 60-year-old male who complained of repetitive painless gross hematuria and was diagnosed with muscle-invasive bladder cancer with squamous and glandular differentiation, defined as cT3N1M0 according to the American Joint Committee on Cancer, who had a strong desire to preserve the bladder. Immunohistochemical staining revealed that programmed cell death-ligand 1 (PD-L1) expression in the tumor was positive. Thus, a transurethral resection to maximize removal of the bladder tumor was performed under cystoscopy, and the patient subsequently received a combination of chemot
This case shows that the combination of chemotherapy and immunotherapy might be an effective and safe treatment strategy for PD-L1 expression positive UC with divergent histologic differentiation.
Core Tip: Urothelial carcinoma (UC) with complicated differentiation is highly malignant, and radical cystectomy (RC) is the preferred treatment. Because of the complications related to RC, bladder-sparing therapy has become a research hotspot. We here report a patient who was diagnosed with muscle-invasive bladder cancer with squamous and glandular differentiation and positive programmed cell death-ligand 1 (PD-L1) expression. The patient received a combination of chemotherapy and immunotherapy and achieved bladder preservation and has maintained tumor-free for over two years. This case highlights that the combination treatment might be an effective and safe strategy for UC with divergent histologic differentiation and positive PD-L1 expression.
- Citation: Yang R, Chen JX, Luo SH, Chen TT, Chen LW, Huang B. Bladder preservation in complicated invasive urothelial carcinoma following treatment with cisplatin/gemcitabine plus tislelizumab: A case report. World J Clin Cases 2023; 11(5): 1165-1174
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1165.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1165
Urothelial carcinoma (UC), originating in the bladder or upper urinary tract, is the most common histological type of cancer[1]. UC has a wide range of clinical and morphological characteristics, including the unique ability to differentiate into all histological variants, including squamous, glandular, micropapillary, sarcomatoid, small cell, clear cell and so on[2]. The biological behavior and treatment strategy of a bladder tumor with variant histology differs from that of conventional UC, which is an uncertain and difficult challenge for clinicians[3]. Although radical cystectomy (RC) is the preferred standard treatment for muscle-invasive bladder cancer (MIBC) according to the National Comprehensive Cancer Network (NCCN), some patients still have a strong desire to preserve the bladder due to the marked decline in quality of life after RC. Unfortunately, effective bladder-sparing treatments are limited.
Platinum-based cytotoxic chemotherapy is the standard treatment for metastatic or locally advanced UC and the preferred treatment option in the perioperative (neoadjuvant and/or adjuvant) setting for MIBC. However, the overall significance of the mixed histological features of MIBC remains unclear. In addition, with the increased understanding of cancer immunobiology, systematic immunotherapy targeting immune checkpoint suppression has been explored and clinically applied in the field of UC[4].
Herein, we report a case of programmed cell death-ligand 1 (PD-L1) expression positive MIBC with squamous and glandular differentiation, who refused total cystectomy. The patient received combination treatment with chemotherapy and immunotherapy after successful transurethral resection of the bladder tumor (TURBT). A marked response to combination treatment was achieved and the patient successfully preserved bladder function.
In August 2020, a 60-year-old male patient was admitted to the Department of Urology of The First Affiliated Hospital of Sun Yat-Sen University (Guangzhou, China) with repetitive painless gross hematuria and a 6-mo history of blood clots.
The patient had no history of chronic respiratory, cardiovascular, or other medical conditions.
There was no history of genetic disease or UC in his family.
On admission, the patient’s temperature was 36.6 ℃, heart rate was 70 bpm, respiratory rate was 15 breaths/min, and blood pressure was 136/83 mmHg. The abdomen was soft without masses or organomegaly, and no tenderness or percussed pain was observed at lower abdomen.
Routine blood tests revealed red blood cell, white blood cell, and platelet counts of 4.75 × 1012/L, 7.47 × 109/L, and 229 × 109/L, respectively, and hemoglobin level of 147 g/L. Prothrombin and partial thromboplastin times were normal, but the level of fibrinogen was 4.82 g/L (2.00-4.00 g/L). The results of blood biochemistry tests were within normal ranges. Serum creatinine level was 78 μmol/L and blood urea nitrogen level was 3.9 mmol/L, indicating normal renal function. Routine urine test revealed higher amounts of protein, red blood cells and white blood cells in urine. The electrocardiography and chest X-ray were normal.
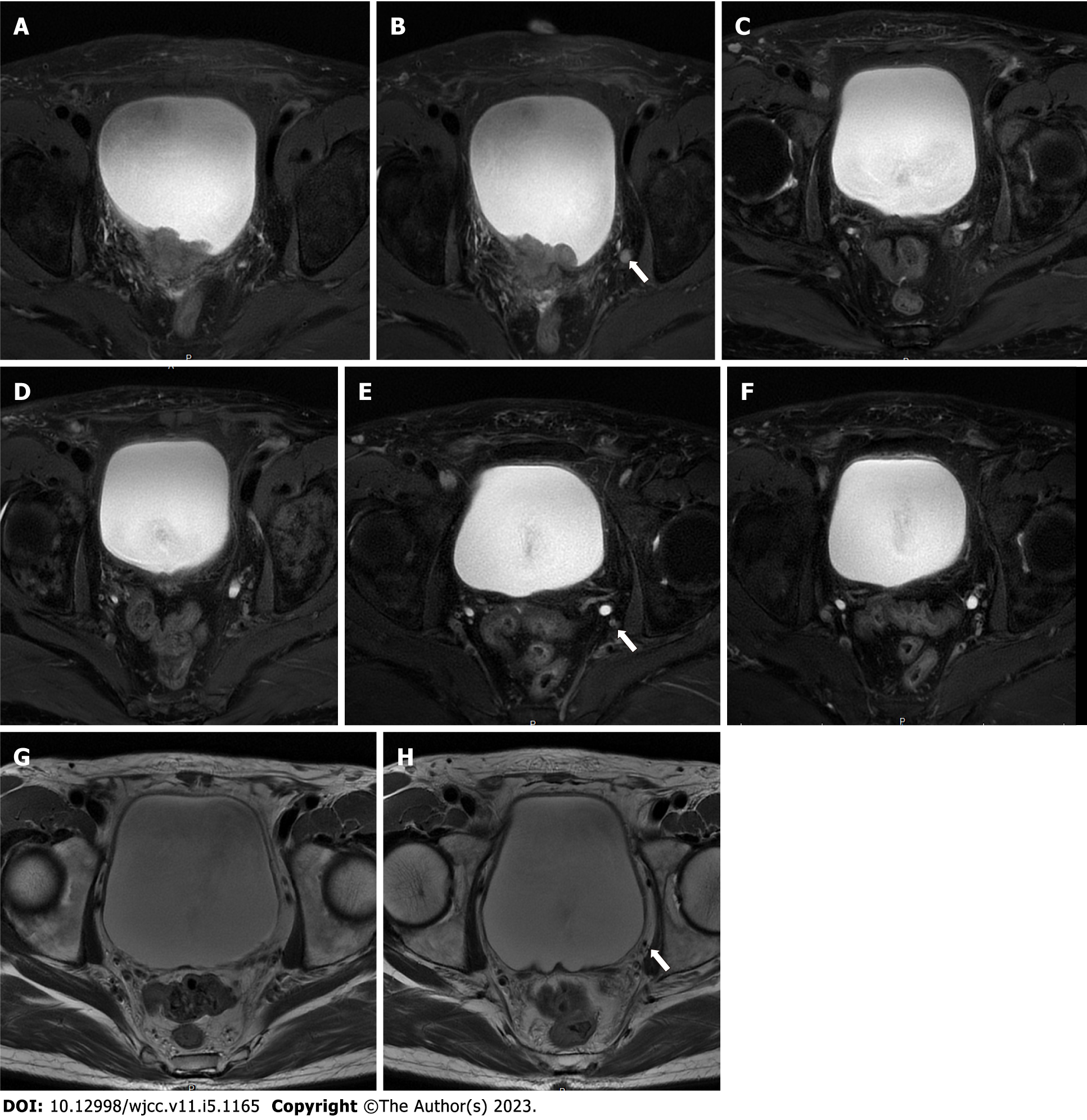
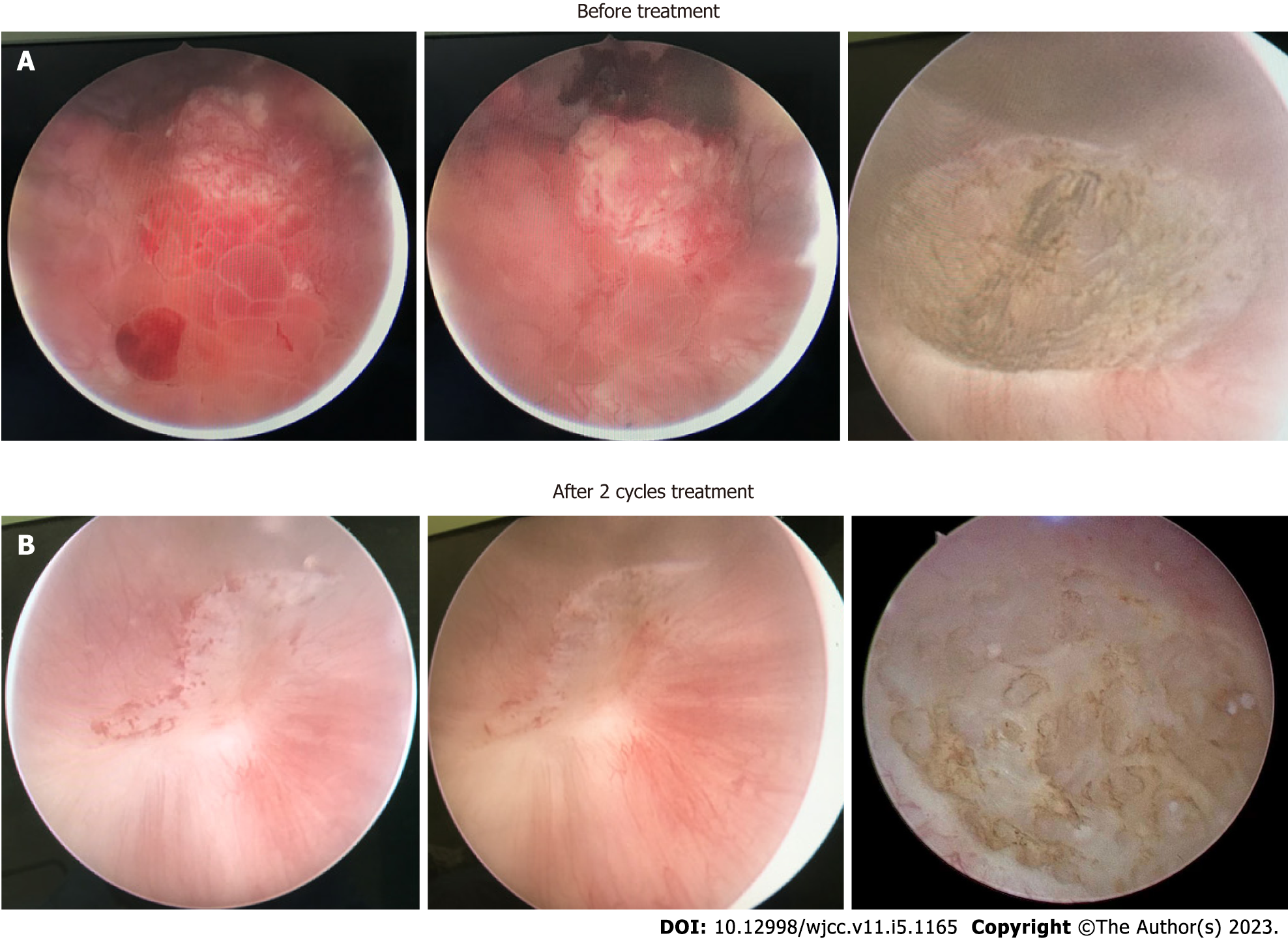
Pelvic magnetic resonance imaging (MRI) revealed a 24 mm × 42 mm × 29 mm mass located at the posterior wall of the bladder, which invaded the entire muscular layer and the serosa (Figure 1A). Suspicious enlarged lymph nodes adjacent to the left internal iliac vessels in the pelvic cavity were observed (Figure 1B). Cystoscopy and diagnostic TURBT were performed for further examination. Cystoscopy revealed a mass (approximately 45 mm in diameter) at the posterior wall of the bladder, with necrotic tissue and blood clots on the surface (Figure 2A). Pathological examination revealed high-grade invasive UC with squamous and adenoid differentiation (Figure 3A and B), and immunohistochemistry results showed positive expression of some intestinal adenocarcinoma markers. Based on the imaging, pathological and other examinations, the patient was staged as cT3N1M0 according to the American Joint Committee on Cancer.
The surgical tumor specimen and paired blood sample obtained from the patient were sent for genetic testing of 733 tumor-related genes (3D Medicines Inc., a College of American Pathologists-accredited and Clinical Laboratory Improvement Amendment-certified laboratory, Shanghai, China). Formalin-fixed paraffin-embedded tissue sections were subjected to PD-L1 expression assessment using the VENTANA PD-L1 (SP263) Assay (Roche Diagnostics). PD-L1 expression was also determined using the Tumor Proportion Score (TPS), which is the proportion of viable tumor cells showing partial or complete membrane PD-L1 staining at any intensity. Tumor mutational burden (TMB) was defined as the number of non-synonymous and synonymous somatic single nucleotide variations and indels in the examined coding regions, excluding driver mutations.
Next generation sequencing results revealed that the tumor harbored six somatic mutations: ERBB2 p.S310F, RB1 p.F404Lfs*6, ARID1A p.S733*, TERT c.-124C>T, TP53 p.R282W and TP53 p.P152T (Table 1). Immunohistochemical (IHC) staining revealed that PD-L1 expression in the tumor was positive (TPS = 30%, Figure 3C). The microsatellite status was stable and the TMB was classified as intermediate with 10.06 mutations per megabase, which was less than 51% of MIBCs in the detection database (Figure 4).
| Gene | Mutation | Abundance |
| ERBB2 | p.S310F | 3.62% |
| RB1 | p.F404Lfs*6 | 10.42% |
| ARID1A | p.S733* | 11.14% |
| TERT | c.-124C>T | 14.83% |
| TP53 | p.R282W | 3.70% |
| p.P152T | 24.48% |
The patient was preliminarily diagnosed with MIBC, whose pathological type was complicated UC with squamous and glandular differentiation.
RC is the preferred choice of treatment for UC. However, the patient strongly wished to preserve the bladder. We decided to administer preoperative neoadjuvant chemotherapy first, and evaluate whether RC should be performed according to the treatment effect. The standard treatment of preoperative neoadjuvant chemotherapy for MIBC is platinum-based cytotoxic chemotherapy, but the benefits in this complicated pathological type are unknown. In addition, IHC testing of tumor tissue showed that PD-L1 expression was positive, indicating that the patient might benefit from immunotherapy. Considering the high treatment efficacy of anti-programmed cell death-1 (PD-1) immunotherapy for MIBC and the patient’s circumstances, he subsequently received chemotherapy combined with immunotherapy for bladder-sparing therapy. TURBT was performed to remove as much tumor tissue as possible, with the operative wound in the serosal layer of the bladder. The patient then received immunotherapy (tislelizumab) combined with chemotherapy (cisplatin/gemcitabine) every three weeks for four cycles. Cisplatin was administered at 70 mg/m2 intravenously from the second day of the three-week cycle, gemcitabine was administered at 1000 mg/m2 intravenously on day one and eight every three weeks and tislelizumab was administered 200 mg intravenously on day one of the three-week cycle.
Side effects during the treatment period included low-grade anemia, leukopenia and subclinical hypothyroidism. To evaluate the efficacy of immunotherapy combined with chemotherapy, the patient underwent pelvic MRI, cystoscopy and TURBT after two cycles of therapy. Pelvic MRI revealed no obvious nodules or masses on the posterior wall of the bladder and no enlarged lymph nodes around the iliac vessels (Figure 1C and D). Cystoscopy showed that the primary tumor site had been replaced by scar tissue (Figure 2B). The tissue sample collected during cystoscopy for pathological and IHC analysis showed no malignant characteristics. After four cycles of combination therapy, MRI demonstrated that there were still no nodules or masses in the bladder and the lymph nodes were the same as previously described (Figure 1E and F). Full-body positron emission tomography/computed tomography showed that the lesion described previously was obviously reduced without evidence of distant metastasis. Pathological examination results were also negative. All results indicated a marked response following the combination of chemotherapy and immunotherapy; thus, the next treatment strategy developed was immunotherapy, where tislelizumab was administered 200 mg intravenously on day one of the three-week cycle alone.
The patient was advised to undergo MRI or cystoscopy every three months for the first year and every six months for the next two years to assess his condition.
The entire treatment period was two years, the patient was reexamined by MRI, and there was no recurrence of the tumor or enlarged lymph nodes (Figure 1G and H), which indicated that the patient was tumor-free with preserved bladder for almost two years. The overall treatment timeline is shown in Figure 5.
Bladder cancer is one of the most common malignant neoplasms worldwide. More than 90% of these cancers are transitional cell carcinomas, which can also present mixed with other malignant components[5]. The mixed type of bladder histology includes squamous differentiation (20%-60% of bladder cancers), adenocarcinoma or glandular differentiation (10%), sarcomatoid (7%), micropapillary (3.7%), and lymphoepithelioma-like carcinoma[2]. Thus, the identification of a histological variant type in UC is essential, which may be associated with different clinical outcomes and treatment strategies[6,7]. Herein, we report a case of PD-L1 positive invasive UC with squamous and glandular differentiation. Considering the patient’s strong desire for bladder preservation, bladder-sparing therapy with the combination of cisplatin/gemcitabine and tislelizumab was administered after TURBT. A marked response with no tumor or malignant features was observed following imaging and pathological examinations. Several studies have reported that the clinical phenotype of squamous and glandular differentiation was different to pure pathological types of UC. Adenocarcinoma is thought to have a poorer prognosis but has not been adequately studied to make definitive statements[8]. It has been shown that pure adenocarcinoma is relatively resistant to chemotherapy and radiation, and there is no evidence that these treatments are beneficial in mixed tumors[9]. Besides, the significance of invasive UC with squamous and glandular differentiation at TURBT is still not confirmed, meaning no satisfactory and effective treatment has been found for this pathological type of UC[10]. However, research on a series of patients with variant histology found that UC with squamous or glandular or both types of differentiation often present with a higher pathologic stage, and significantly longer overall survival with cisplatin-based neoadjuvant chemotherapy (P = 0.024)[11].
Although RC is the standard treatment for MIBC, some patients have a strong desire to preserve their native bladders due to poor quality of life after RC. Other treatment options for these patients are chemoradiotherapy or radiotherapy. In the present patient, the bladder tumor was relatively small with a maximum diameter of 42 mm. Radiotherapy will inevitably irradiate normal tissue, leading to adverse events such as gastrointestinal reactions, radioactive cystitis, skin damage, and some systemic side effects. In addition, RC is an alternative in the case of failed drug treatment, and the side effects of radiotherapy have great effects on the surgery. The effects of radiotherapy on operating room tissue can also cause fibrosis, which leads to fixation of pelvic organs, making blunt dissection more difficult, and causes disruption of surgical landmarks and loss of tissue planes[12,13]. Postoperative complications such as intestinal obstruction, poor wound healing, ureteral ileal anastomotic leakage and stenosis, lymphatic leakage, lower extremity venous embolism and cardiopulmonary, liver and kidney dysfunction can also increase. Some studies revealed that the rate of one or more complications in patients with RC and a history of pelvic radiotherapy is as high as 48%-76%, which is significantly higher than that in patients without a history of pelvic radiotherapy (25.5%-35.8%)[13,14]. In other studies, the perioperative mortality (0.8%) of RC in patients without a history of pelvic radiotherapy was lower than that (5.7%) in patients with a history of pelvic radiotherapy[14,15]. Overall, radiotherapy was not considered the preferred treatment option for this patient.
PD-1/PD-L1 blockade has been approved as a promising and effective treatment option for patients with advanced stage UC, and PD-L1 IHC expression has been shown to be involved in predicting immune checkpoint inhibitor therapy efficacy[1]. Five different anti-PD-1 (durvalumab, nivolumab and pembrolizumab) or anti-PD-L1 antibodies (atezolizumab and avelumab) have currently been approved for treatment of locally advanced or metastatic UC as second-line post-platinum-based chemotherapy, including atezolizumab[16] or pembrolizumab[17] and may be considered for cisplatin ineligible patients as first-line therapy if PD-L1 expression is positive. Moreover, in a single-arm phase 2 trial (NCT04004221/CTR20170071), tislelizumab (anti-PD-1 monoclonal antibody) as second-line therapy in PD-L1 positive (SP263) UC patients who progressed during/following platinum-containing therapy and had no prior PD-1/L1 inhibitor treatment achieved a confirmed objective response rate of 24% in 104 evaluable patients, including 10 complete and 15 partial responses. Median progression-free survival and overall survival were 2.1 and 9.8 mo, respectively[18].
Different genetic mutations can influence immunotherapy efficacy. Six somatic mutations in our patient, including ERBB2 p.S310F, RB1 p.F404Lfs*6, ARID1A p.S733*, TERT c.-124C>T, TP53 p.R282W, and TP53 p.P152T, were considered tumorigenic. ERBB2 encodes an epidermal growth factor receptor, and its amplification can regulate cell growth by activating downstream signaling pathways[19]. A phase 2 trial showed that 18 patients with lung cancer carrying ERBB2 mutations were treated with trastuzumab emtansine (T-DM1), with an overall response rate of 44% and a median progression free survival of 5 mo[20] Cells carrying the S310F mutation are sensitive to the ERBB2 inhibitor and ERBB2 monoclonal antibody[21]. However, there have been few studies on the other somatic mutations as biomarkers for the treatment of UC.
TMB can be used as a marker for evaluating the effects of immunotherapy. Tumor cells with higher TMB are more easily recognized by the immune system and may have a stronger immune response to checkpoint inhibitors. In a clinical study, patients with non-small cell lung cancer were divided into high and low TMB groups according to the median TMB, and the clinical benefit rates of the high and low TMB groups receiving pembrolizumab were 73% and 13% in the discovery cohorts, 83% and 22% in the validation cohorts[22]. Additionally, the TMB of melanoma patients who received ipilimumab and achieved long-term clinical benefits was significantly higher than that of melanoma patients who did not achieve long-term clinical benefits[23].
In the present case, considering that PD-L1 IHC staining of tumor tissue was positive, the somatic mutation of ERBB2 p.S310F, and 51% TMB, a combination of chemotherapy and immunotherapy was finally chosen.
There are some limitations in this study. As the patient had a strong desire for bladder preservation, he did not undergo RC as the initial treatment which is recommended by the NCCN. Pathological specimens used for efficacy assessment were obtained by TURBT and thus there was possible undetected tumor tissue. Therefore, the pathology and follow-up results should be combined to comprehensively evaluate the treatment efficacy. In addition, the Food and Drug Administration has currently approved various PD-L1 assays for clinical use, usually corresponding to specific immune checkpoint inhibitors[24].
Analyses of the same sample using different assays may lead to discordant results, which might influence clinical treatment decisions. Furthermore, assuming that the cut-off value of the PD-L1 score applied in clinical trials is intended to achieve statistically predefined results, it may not reflect a uniform tumor biological state[7].
For accurate analysis, we reviewed a series of previous studies, based on PD-L1 assays (SP263) and cut-off values (TPS ≥ 25%) that were used in clinical trials of tislelizumab, and applied them to our case[18,25]. Furthermore, to minimize manual error, automated quantification and interpretation of the PD-L1 IHC results were adopted in this case.
We report a case of invasive UC with squamous and glandular differentiation and positive PD-L1 expression, for which there is currently no established therapy for such differentiation types. The tumor showed a marked response to the treatment with the combination of cisplatin/gemcitabine and tislelizumab, and the patient’s bladder was successfully preserved. This case provides a new strategy for the treatment of patients with complex mixed histology MIBC. The combination of immunotherapy and chemotherapy for MIBC patients with positive PD-L1 expression may postpone the time to complete bladder resection and improve patient quality of life.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: El Tayebi HM, Egypt; Mohammadi S, Iran S-Editor: Li L L-Editor: A P-Editor: Li L
| 1. | Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA. 2020;324:1980-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 1048] [Article Influence: 209.6] [Reference Citation Analysis (0)] |
| 2. | Shanks JH, Iczkowski KA. Divergent differentiation in urothelial carcinoma and other bladder cancer subtypes with selected mimics. Histopathology. 2009;54:885-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Willis DL, Porten SP, Kamat AM. Should histologic variants alter definitive treatment of bladder cancer? Curr Opin Urol. 2013;23:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Kim HS, Seo HK. Immune checkpoint inhibitors for urothelial carcinoma. Investig Clin Urol. 2018;59:285-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 781] [Cited by in RCA: 822] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 6. | Amin MB. Histological variants of urothelial carcinoma: diagnostic, therapeutic and prognostic implications. Mod Pathol. 2009;22 Suppl 2:S96-S118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 280] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 7. | Kim B, Lee C, Kim YA, Moon KC. PD-L1 Expression in Muscle-Invasive Urinary Bladder Urothelial Carcinoma According to Basal/Squamous-Like Phenotype. Front Oncol. 2020;10:527385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Black PC, Brown GA, Dinney CP. The impact of variant histology on the outcome of bladder cancer treated with curative intent. Urol Oncol. 2009;27:3-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 173] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 9. | Pons F, Orsola A, Morote J, Bellmunt J. Variant forms of bladder cancer: basic considerations on treatment approaches. Curr Oncol Rep. 2011;13:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Daneshmand S, Nazemi A. Neoadjuvant Chemotherapy in Variant Histology Bladder Cancer: Current Evidence. Eur Urol Focus. 2020;6:639-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 11. | Mitra AP, Bartsch CC, Bartsch G Jr, Miranda G, Skinner EC, Daneshmand S. Does presence of squamous and glandular differentiation in urothelial carcinoma of the bladder at cystectomy portend poor prognosis? Urol Oncol. 2014;32:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Kim HL, Steinberg GD. Complications of cystectomy in patients with a history of pelvic radiation. Urology. 2001;58:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Ramani VA, Maddineni SB, Grey BR, Clarke NW. Differential complication rates following radical cystectomy in the irradiated and nonirradiated pelvis. Eur Urol. 2010;57:1058-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Chang SS, Cookson MS, Baumgartner RG, Wells N, Smith JA Jr. Analysis of early complications after radical cystectomy: results of a collaborative care pathway. J Urol. 2002;167:2012-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 234] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Novotny V, Hakenberg OW, Wiessner D, Heberling U, Litz RJ, Oehlschlaeger S, Wirth MP. Perioperative complications of radical cystectomy in a contemporary series. Eur Urol. 2007;51:397-401; discussion 401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 209] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 16. | Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, Dawson NA, van der Heijden MS, Dreicer R, Srinivas S, Retz MM, Joseph RW, Drakaki A, Vaishampayan UN, Sridhar SS, Quinn DI, Durán I, Shaffer DR, Eigl BJ, Grivas PD, Yu EY, Li S, Kadel EE 3rd, Boyd Z, Bourgon R, Hegde PS, Mariathasan S, Thåström A, Abidoye OO, Fine GD, Bajorin DF; IMvigor210 Study Group. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1689] [Article Influence: 211.1] [Reference Citation Analysis (0)] |
| 17. | Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 726] [Cited by in RCA: 1003] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 18. | Ni K, Wang Z, Yu S, Zheng J, Li G. Camrelizumab monotherapy leading to partial remission for relapsed upper tract urothelial carcinoma after radical nephroureterectomy: a case report. Transl Androl Urol. 2021;10:1821-1826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2397] [Cited by in RCA: 2482] [Article Influence: 124.1] [Reference Citation Analysis (0)] |
| 20. | Li BT, Shen R, Buonocore D, Olah ZT, Ni A, Ginsberg MS, Ulaner GA, Offin M, Feldman D, Hembrough T, Cecchi F, Schwartz S, Pavlakis N, Clarke S, Won HH, Brzostowski EB, Riely GJ, Solit DB, Hyman DM, Drilon A, Rudin CM, Berger MF, Baselga J, Scaltriti M, Arcila ME, Kris MG. Ado-Trastuzumab Emtansine for Patients With HER2-Mutant Lung Cancers: Results From a Phase II Basket Trial. J Clin Oncol. 2018;36:2532-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 399] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 21. | Greulich H, Kaplan B, Mertins P, Chen TH, Tanaka KE, Yun CH, Zhang X, Lee SH, Cho J, Ambrogio L, Liao R, Imielinski M, Banerji S, Berger AH, Lawrence MS, Zhang J, Pho NH, Walker SR, Winckler W, Getz G, Frank D, Hahn WC, Eck MJ, Mani DR, Jaffe JD, Carr SA, Wong KK, Meyerson M. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109:14476-14481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6065] [Cited by in RCA: 6308] [Article Influence: 630.8] [Reference Citation Analysis (0)] |
| 23. | Chan TA, Wolchok JD, Snyder A. Genetic Basis for Clinical Response to CTLA-4 Blockade in Melanoma. N Engl J Med. 2015;373:1984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 150] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 24. | Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS, Hirsch FR. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat Rev Clin Oncol. 2021;18:345-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 897] [Article Influence: 224.3] [Reference Citation Analysis (0)] |
| 25. | Gulinac M, Dikov D, Velikova T, Belovezhdov V. Increased PD-L1 expression in high-grade bladder cancer with squamous cell differentiation in Bulgarian and French patients' samples. Ann Diagn Pathol. 2020;49:151640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |