Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1144
Peer-review started: October 27, 2022
First decision: November 25, 2022
Revised: December 3, 2022
Accepted: January 28, 2023
Article in press: January 28, 2023
Published online: February 16, 2023
Processing time: 109 Days and 17.4 Hours
Tocilizumab is a humanized monoclonal antibody against the interleukin-6 (IL-6) receptor that is commonly used to treat large vessel vasculitis and antineutrophil cytoplasmic antibody-related small vessel vasculitis. However, tocilizumab in combination with glucocorticoids for successfully treating granulomatosis with polyangiitis (GPA) has rarely been reported.
Here, we report a 40-year-old male patient who suffered GPA for 4 years. He was treated with multiple rounds of drugs, including cyclophosphamide, Tripter
Tocilizumab may be effective for treating GPA.
Core Tip: Granulomatosis with polyangiitis (GPA), formerly called Wegener's granulomatosis, is a necrotizing granulomatous vasculitis that involves small arteries, veins, and capillaries throughout the body. The upper respiratory tract, lower respiratory tract, and kidney are most commonly affected. Clinically, glucocorticoid combined with cyclophosphamide is the first treatment for GPA. Herein, we report a case of refractory GPA. Combined with the literature review, we found that interleukin-6 (IL-6) levels were generally elevated in GPA patients. IL-6 is involved in the pathogenesis of antineutrophil cytoplasmic antibody-related small vessel vasculitis. We successfully treated a patient with refractory GPA using the IL-6 inhibitor-tocilizumab. Tocilizumab is an option when conventional immunosuppressants and rituximab are not effective in treating GPA.
- Citation: Tang PF, Xu LC, Hong WT, Shi HY. Successful treatment of granulomatosis with polyangiitis using tocilizumab combined with glucocorticoids: A case report. World J Clin Cases 2023; 11(5): 1144-1151
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1144.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1144
Antineutrophil cytoplasmic antibody (ANCA)-related small vessel vasculitis (AAV) refers to a set of systemic small vessel vasculitis diseases associated with antineutrophil cytoplasmic antibodies, including microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Patients with GPA are mainly positive for classic ANCA (C-ANCA) and proteinase 3-ANCA (PR3-ANCA), and GPA tends to involve the upper and lower respiratory tracts, kidney, and systemic arterioles, venules, and capillaries. According to guideline recommendations[1], cyclophosphamide (CTX) or rituximab is the drug of choice for inducing remission in patients with AAV. Tocilizumab has been reported in the literature for treating primary and secondary small vessel vasculitis[2]. However, the use of tocilizumab for treating GPA has rarely been reported. Here, we report a case of successful treatment of GPA with tocilizumab in combination with glucocorticoids, suggesting that tocilizumab may be useful for treating GPA.
A 40-year-old man presented to the Rheumatology Department on October 8, 2021, with the main complaints of 4-year pulmonary nodules and 3-year repeated haemoptysis.
On October 8, 2021, the patient was admitted for recurrent haemoptysis without apparent cause.
Four years before admission (January 2017), the patient visited a local hospital for trauma (specific unknown). Pulmonary computed tomography (CT) revealed multiple bilateral pulmonary nodules and mass shadows, inflammatory flaked shadows, and significantly thickened pleura. Lung biopsy pathology showed granulomatous inflammatory flaked necrosis. Pulmonary tumours were excluded according to immunohistochemical findings. At follow-up, pulmonary CT demonstrated more pulmonary nodules and flaked shadows.
On June 2017, the patient visited a tertiary hospital in Beijing, China due to low fever, joint pain, and maculopapular rash. Laboratory examination showed an erythrocyte sedimentation rate (ESR) of 47 mm/h; C-reactive protein (CRP) level of 18.6 mg/L; rheumatoid factor (RF) level of 225 IU/mL; normal levels of antinuclear antibodies (ANAs), anti-ENA antibodies, anti-double-stranded DNA antibodies, immunoglobulins (IgA, IgM, and IgG), complement, and anti-cyclic citrullinated peptide antibodies; and C-ANCA and PR3-ANCA positivity. AAV was considered. Immunosuppression was obtained using methylprednisolone (80 mg/d) plus CTX 0.2 g qod. In addition, human immunoglobulins (20 g/d) were administered for 5 d with satisfactory results. Some pulmonary lesions disappeared, while the inflammatory indicators remained at a high level. After discharge from the hospital, the patient’s maintenance regimen consisted of oral prednisone 20 mg/d combined with CTX 0.2 g qod.
On July 2018, the patient returned to the tertiary hospital for cough and haemoptysis. He was positive for C-ANCA and exhibited significantly increased inflammatory levels, and more pulmonary nodules and mass shadows. According to the 2017 European League against Rheumatism/American College of Rheumatology classification criteria for granulomatosis with polyangiitis, GPA was diagnosed. Anti-inflammatory methylprednisolone 40 mg/d and oral CTX (150 mg for one day and 100 mg for two consecutive days) were administered. The symptoms improved, while he had intermittent cough and shortness of breath after activity.
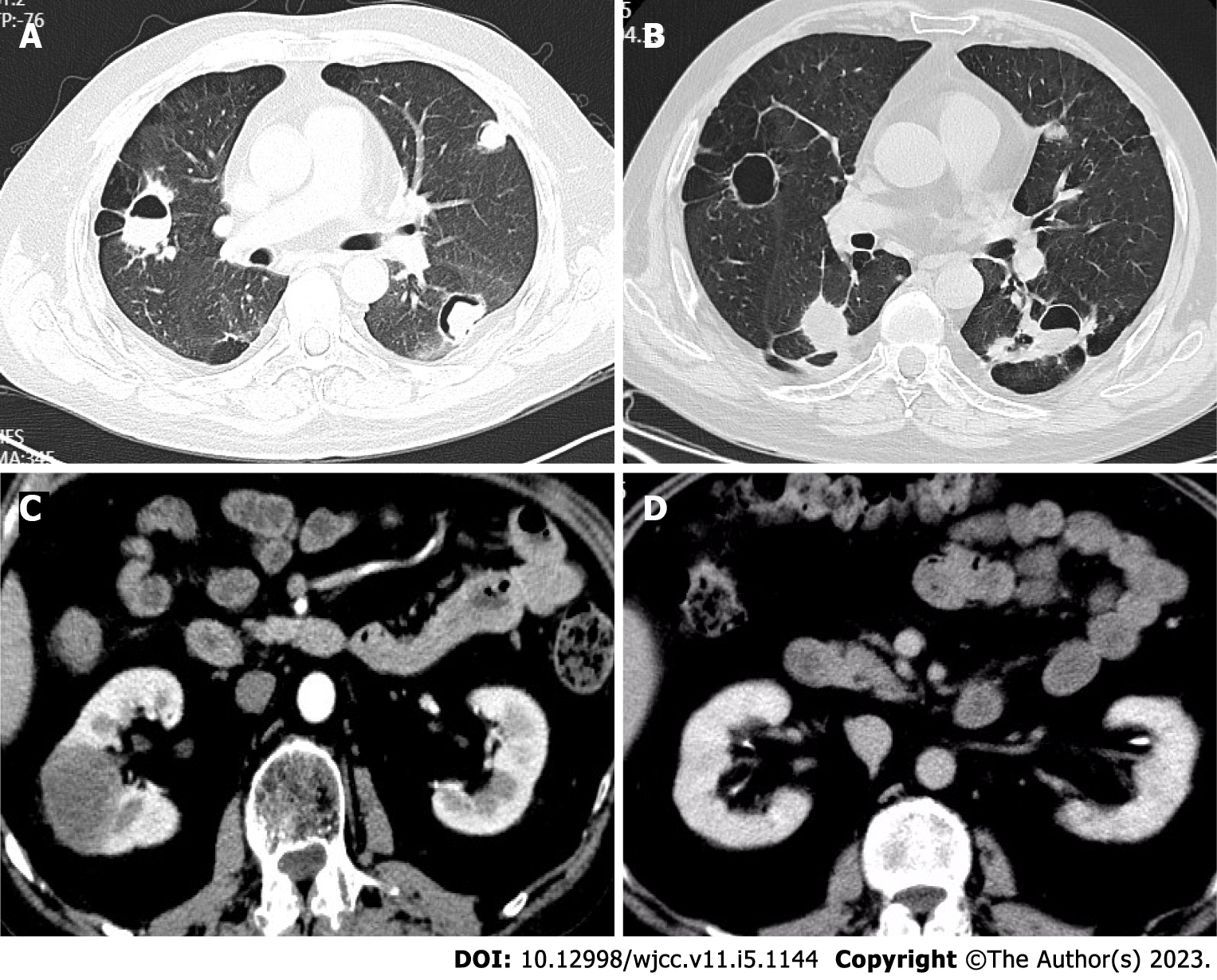
On August 13, 2018, the patient visited our hospital with the main complaint of cough with yellow sputum. Laboratory examination revealed a WBC count of 11.8 × 109/L, neutrophil count of 7.6 × 109/L, and lymphocyte count of 2.6 × 109/L. Analysis of lymphocyte subsets showed the following results: B lymphocyte antigen (CD3-CD19+) 102/μL, CD4 875/μL, CD8 1300/μL, and CD4/CD8 0.67. In addition, the patient exhibited C-ANCA positivity, PR3-ANCA 94.86 nmol/L (< 20 nmol/L), ESR 55 mm/h (normal, < 15.0), CRP 14.1 mg/L (normal, < 0.5), interleukin-6 (IL-6) 17.11 pg/mL (normal, < 7.0), and normal procalcitonin (PCT). Negative results were obtained for the sputum culture, serum G ((1-3)-β-d-glucan), galactomannan (GM), cytomegalovirus, Epstein-Barr virus (EBV) virus, and T-spot tests. Nine respiratory pathogen antibody IgM tests, namely, tests for Legionella pneumophila, Mycoplasma pneumoniae, Chlamydia pneumoniae, Rickettsia Q fever, influenza A and B viruses, parainfluenza virus, respiratory syncytial virus, and adenovirus, were normal. On pulmonary CT, multiple patchy shadows, nodular higher-density shadows, multiple cavities, heterogeneous wall thickness, and air crescent signs and flat lipids in some lesions were observed (Figure 1A). After Respiratory Department consultation, the possibility of lung infection (bacteria + fungi) was considered. Pneumocystis infection could not be ruled out. CTX was discontinued, and the glucocorticoid doses were reduced. In addition, moxifloxacin (antibacterial), voriconazole (antifungal), and prophylactic anti-Pneumocystis jiroveci treatments were administered for 2 wk with improved symptoms and decreased inflammatory levels. After discharge, CTX 100 mg was administered on alternate days, with a cumulative dose of 28 g. During the outpatient follow-up, the inflammatory marker levels were within the normal range, and the pulmonary lesions increasingly disappeared. However, the G test was positive twice, while the GM was negative. The vasculitis was considered to be controlled, but the pulmonary fungal infection persisted. Dose reduction was obtained, and methylprednisolone 6 mg/d was administered. CTX was replaced with Tripterygium wilfordii (TW) for immunosuppression. Oral antifungal agents were concurrently administered and discontinued within one year.
On March 19, 2019, September 5, 2019, November 23, 2019, and June 12, 2020, the patient visited the Rheumatology Department of our hospital for fever, fatigue, and systemic muscle soreness. Laboratory examination revealed increased CRP and ESR levels, IL-6 33.43 pg/mL, and normal PCT. EBV DNA was 5.09E + 4 copies/mL. Negative results were obtained for the pathological examination of tracheal lavage fluid, and TORCH test analysis of lymphocyte subsets revealed B lymphocytes (CD3-CD19+) 62/µL and CD4/CD8 0.6. On pulmonary CT, there were new and larger bilateral pulmonary nodules (Figure 1B). Anti-infectious moxifloxacin and antiviral peramivir were successively applied. The body temperature was normal, while the sensation of fatigue was retained. Elevation of the PR3-ANCA titre was examined, suggesting vasculitis activity. Since the patient had a low level of B lymphocytes and a history of deep mould infection, rituximab was not recommended. Therefore, the treatment plan was changed to a glucocorticoid dose increase combined with mycophenolate mofetil (MMF). The patient's fatigue symptoms improved. PR3-ANCA and inflammatory marker levels decreased.
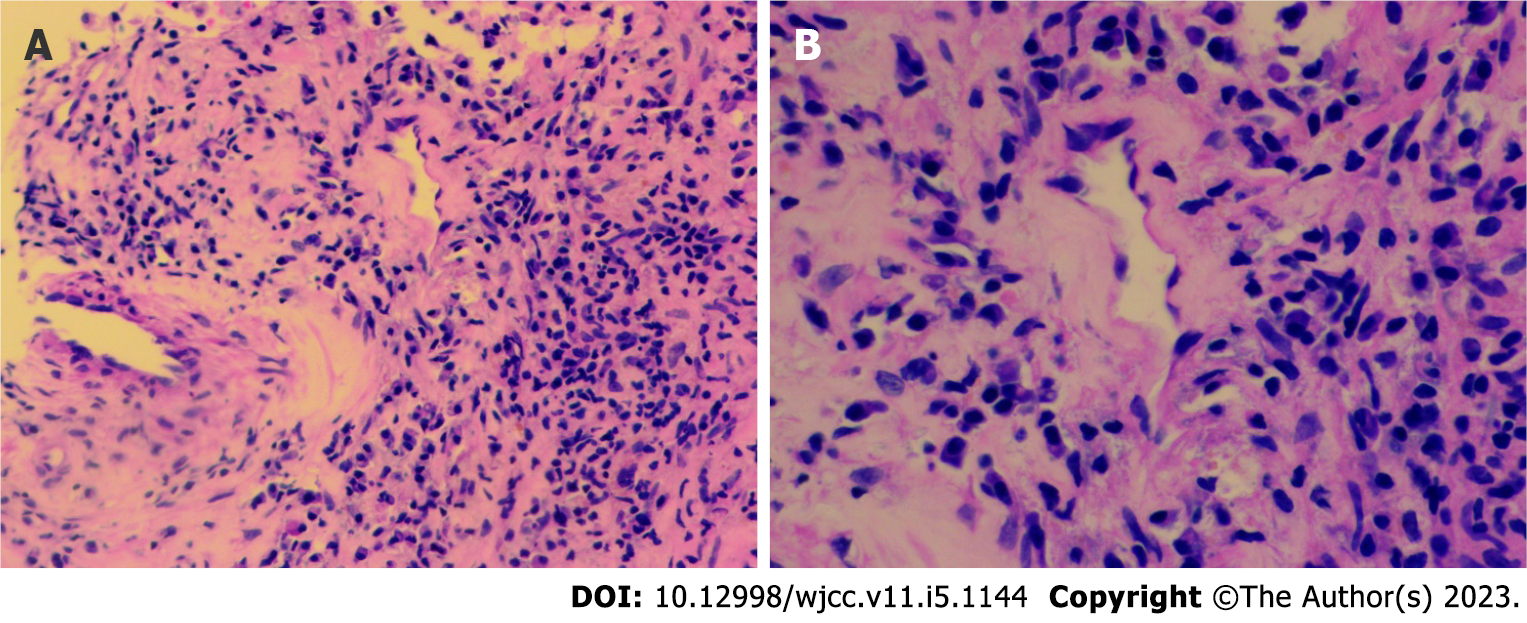
On September 15, 2020, the patient was treated at the Rheumatology Department of our hospital again for fatigue, muscle soreness at the extremities and joints, cough with yellow sputum, and shortness of breath. He exhibited CRP 51.09 mg/L, ESR 84 mm/h, C-ANCA positivity, PR3-ANCA (++), and G-test, GM-test, cryptococcal antigen, and TORCH test negativity. Pulmonary CT demonstrated more bilateral pulmonary nodules and cavities and some larger nodular lesions. Renal CT showed a space-occupying lesion in the right middle kidney. Puncture pathology of the space-occupying lesion revealed fibrous tissue proliferation with infiltration of tissue cells, plasma cells, and lymphocytes, focal necrosis, and no IGG4-positive plasma cell infiltration (Figure 2A and B). Vasculitis activity with the involvement of the kidney was considered. Referring to the literature, we noted that the level of circulating B lymphocyte factor increases in patients with AAV. Given the role of the B lymphocyte factor in the onset of AAV, the patient was treated with methylprednisolone 40 mg/d combined with MMF 500 mg bid and belimumab 840 mg (I.V.). The symptoms improved, but the fatigue and soreness at the extremities relapsed due to the dose reduction of glucocorticoids.
On March 21, 2021, the patient was admitted to our hospital for haemoptysis. Pulmonary CTA manifestations were normal, while the air crescent sign was observed on CT. The haemoptysis was then considered a result of pulmonary infection. MMF and belimumab were discontinued. Anti-infectious moxifloxacin was administered. There was no evidence of recurrence while reducing the use of glucocorticoids, and more pulmonary lesions disappeared. After discharge, the patient was treated with belimumab 840 mg (I.V.) once every month for a total of 8 times. Then, renal CT was performed and revealed a space-occupying lesion in the right middle kidney, which was larger than before (Figure 1C). In the meantime, the pulmonary nodules and cavities were similar. The patient experienced repeated elevation of inflammatory indicators, with a poorly controlled disease. The IL-6 Level during the treatment was high, ranging between 32 pg/mL and 49 pg/mL. Referring to the literature, tocilizumab 640 mg (I.V., once monthly) was administered. After dose reduction of the glucocorticoids, there was no evidence of recurrence of fatigue or muscle soreness at the extremities and joints. In addition, the right middle kidney-occupying lesion was smaller (Figure 1D), and the ESR recovered to normal (Figure 3A).
The patient had no previous underlying diseases, such as hypertension, nor a bad habit of smoking. He denied any family history of rheumatic diseases.
On physical examination, the vital signs were as follows: Body temperature, 36.8 °C; blood pressure, 130/70 mmHg; heart rate, 90 beats per min; respiratory rate, 20 breaths per min. In addition, heart, lung, and abdominal examinations showed no remarkable changes.
Second-generation gene sequencing revealed growth of Pseudomonas aeruginosa in bronchoalveolar lavage fluid.
Arteriography demonstrated more and thickened bilateral bronchial arterial branches.
GPA with pulmonary mixed infection (bacteria + fungi) was considered.
The bacteria were sensitive to piperacillin sodium and tazobactam sodium administered by injection. Tocilizumab was discontinued, and anti-infection therapy was administered due to drug susceptibility. There was no haemoptysis, and the infection was controlled. Tocilizumab was continued at 640 mg (I.V.) once every month, in combination with methylprednisolone 12 mg/d.
After treatment, there was no fatigue or muscle soreness, while there was occasional cough with yellow sputum. The patient was discharged after improvement on November 5, 2021. The ESR was normal (Figure 3A), and the levels of IL-6 (Figure 3B) and PR3-ANCA (Figure 3C) were decreased after the use of tocilizumab. The patient remains under outpatient follow-up. His general condition is normal, and his indicator levels are relatively stable.
At present, there is no literature review of the underlying mechanisms by which IL-6 suppression is effective; notably, IL-6 acts on T cells, B cells, monocyte macrophages, etc. The patient reported here mainly exhibited fever and pulmonary nodules/cavities. He had a significant increase in serum PR3-ANCA titre during the disease active phase, and the serum IL-6 Level was increased as well. Serum IL-6 elevation in AAV patients has been reported in the literature. For instance, Berti et al[3] reported abnormal IL-6 Levels in 81% of 78 patients with AAV and found associations with PR3-ANCA positivity, fever, pulmonary nodules/cavities, and urine red cell count. Similarly, the main clinical manifestations of the patient here were pulmonary nodules/cavities and fever, accompanying elevation of serum IL-6 and PR3-ANCA titres. IL-6 is one of the B-cell differentiation factors that can advance the in situ activation of macrophages, T lymphocyte differentiation, and synthesis of other proinflammatory cytokines. Patients with MPA and GPA have been reported to have serum IL-6 elevation during the active phase, and IL-6 expression has been documented in renal biopsy tissue samples[2]. These findings suggest that IL-6 plays a core role during the pathogenesis of AAV, but the specific involved pathways need to be further identified in a larger cohort.
According to the Joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of AAV (2016), a combination of glucocorticoids and CTX or rituximab is preferred for remission induction in cases with organ involvement or in critical medical conditions, followed by methotrexate, azathioprine, or MMF applied for maintenance therapy; other drugs can be considered upon recurrence. In the present case, sufficient CTX was initially administered. The patient then developed pulmonary mould infection, and CTX was replaced by Tripterygium glycosides with persistent disease activity. Referring to the literature[4], where MMF was proven to be effective for the induction of remission in AAV, the patient was then treated with MMF, but the efficacy remained poor. In addition, given the low B-lymphocyte level and a history of deep mould infection, rituximab was not considered. The literature has shown that IL-6 levels are increased in patients with AAV and are involved in the pathogenesis of this disease[5]. The patient was then treated with belimumab with recurrence of symptoms and repeated haemoptysis during the disease course. Pulmonary embolism and bronchial vascular malformation were ruled out. Pseudomonas aeruginosa was grown in BALF. Immunosuppressive agents were discontinued, and anti-infection treatment was intensified. There was no haemoptysis after the treatment. Combining the previous medical history, we considered that the repeated haemoptysis might be a result of AAV combined with infections. In addition, concurrent immunosuppression and infection could also be a major cause of multiple recurrences and poor therapeutic effects.
Tocilizumab is a humanized monoclonal antibody against the IL-6 receptor that has shown effectiveness in the treatment of large vessel vasculitis, including giant cell arteritis and Takayasu arteritis[6]. A foreign prospective, single-arm, single-centre clinical trial reported the therapeutic effect of tocilizumab in MPA patients[7]. Additionally, another literature review assessed the effectiveness and safety of tocilizumab in this population when combined with large-dose CS and reported its potential for treating primary and secondary vasculitis[8]. There was also a case of MPA that was successfully treated with tocilizumab alone[9]. Nevertheless, whether tocilizumab is still active in EGPA remains unclear. It has been established that EGPA tends to involve the respiratory tract (primarily the small- and medium-sized vessels) and is associated with asthma and eosinophilia. It was reported that the use of tocilizumab to treat rheumatoid arthritis could lead to the incidence of critical eosinophilia[10]. Thus, IL-6 inhibitors may not be a good option for treating EGPA. Sumida et al[11] reported good therapeutic outcomes in patients with MPO-ANCA-positive vasculitis and rheumatoid arthritis after receiving tocilizumab plus a rapid reduction in glucocorticoids. Tocilizumab as an induction therapy for leukocytoclastic vasculitis in rheumatoid arthritis has also been reported. The present paper describes a patient with repeated symptoms after multiple rounds of treatment with immunosuppressive agents. After treatment with tocilizumab, the patient was stable, with normal inflammatory indicator levels, decreased IL-6 levels, and smaller right kidney-occupying lesions.
This case report demonstrates the important role of IL-6 in the pathogenesis of AAV. In patients unresponsive to traditional immunosuppressive agents or with contraindications, tocilizumab can be a good treatment option. However, more case studies are required to verify the effectiveness and safety of tocilizumab for treating patients with AAV.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rostaing L, France; Shrestha MR, Nepal S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Yates M, Watts RA, Bajema IM, Cid MC, Crestani B, Hauser T, Hellmich B, Holle JU, Laudien M, Little MA, Luqmani RA, Mahr A, Merkel PA, Mills J, Mooney J, Segelmark M, Tesar V, Westman K, Vaglio A, Yalçındağ N, Jayne DR, Mukhtyar C. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583-1594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 920] [Cited by in RCA: 787] [Article Influence: 87.4] [Reference Citation Analysis (0)] |
| 2. | Berti A, Cavalli G, Campochiaro C, Guglielmi B, Baldissera E, Cappio S, Sabbadini MG, Doglioni C, Dagna L. Interleukin-6 in ANCA-associated vasculitis: Rationale for successful treatment with tocilizumab. Semin Arthritis Rheum. 2015;45:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Berti A, Warner R, Johnson K, Cornec D, Schroeder DR, Kabat BF, Langford CA, Kallenberg CGM, Seo P, Spiera RF, St Clair EW, Fervenza FC, Stone JH, Monach PA, Specks U, Merkel PA; RAVE-ITN Research Group. The association of serum interleukin-6 levels with clinical outcomes in antineutrophil cytoplasmic antibody-associated vasculitis. J Autoimmun. 2019;105:102302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Berti A, Alsawas M, Jawaid T, Prokop LJ, Lee JM, Jeong GH, Quintana LF, Moiseev S, Vaglio A, Tesar V, Geetha D, Shin JIL, Kronbichler A. Induction and maintenance of remission with mycophenolate mofetil in ANCA-associated vasculitis: a systematic review and meta-analysis. Nephrol Dial Transplant. 2022;37:2190-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 5. | Jayne D, Blockmans D, Luqmani R, Moiseev S, Ji B, Green Y, Hall L, Roth D, Henderson RB, Merkel PA; BREVAS Study Collaborators. Efficacy and Safety of Belimumab and Azathioprine for Maintenance of Remission in Antineutrophil Cytoplasmic Antibody-Associated Vasculitis: A Randomized Controlled Study. Arthritis Rheumatol. 2019;71:952-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Takenaka K, Ohba T, Suhara K, Sato Y, Nagasaka K. Successful treatment of refractory aortitis in antineutrophil cytoplasmic antibody-associated vasculitis using tocilizumab. Clin Rheumatol. 2014;33:287-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Sakai R, Kondo T, Kikuchi J, Shibata A, Chino K, Okuyama A, Takei H, Amano K. Corticosteroid-free treatment of tocilizumab monotherapy for microscopic polyangiitis: a single-arm, single-center, clinical trial. Mod Rheumatol. 2016;26:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Sakai R, Kondo T, Kurasawa T, Nishi E, Okuyama A, Chino K, Shibata A, Okada Y, Takei H, Nagasawa H, Amano K. Current clinical evidence of tocilizumab for the treatment of ANCA-associated vasculitis: a prospective case series for microscopic polyangiitis in a combination with corticosteroids and literature review. Clin Rheumatol. 2017;36:2383-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Sakai R, Shibata A, Chino K, Kondo T, Okuyama A, Takei H, Amano K. Corticosteroid- and cyclophosphamide-free treatment of anti-neutrophil cytoplasmic antibody-associated vasculitis using tocilizumab. Mod Rheumatol. 2015;25:810-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Morrisroe K, Wong M. Drug-induced hypereosinophilia related to tocilizumab therapy for rheumatoid arthritis. Rheumatology (Oxford). 2015;54:2113-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Sumida K, Ubara Y, Suwabe T, Hayami N, Hiramatsu R, Hasegawa E, Yamanouchi M, Hoshino J, Sawa N, Takemoto F, Takaichi K, Ohashi K. Complete remission of myeloperoxidase-anti-neutrophil cytoplasmic antibody-associated crescentic glomerulonephritis complicated with rheumatoid arthritis using a humanized anti-interleukin 6 receptor antibody. Rheumatology (Oxford). 2011;50:1928-1930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |