Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1122
Peer-review started: November 1, 2022
First decision: December 19, 2022
Revised: December 31, 2022
Accepted: January 19, 2023
Article in press: January 19, 2023
Published online: February 16, 2023
Processing time: 104 Days and 14.7 Hours
Vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which were approved for emergency use have been administered on a large scale globally to contain the pandemic coronavirus disease 2019 (COVID-19) and to save lives. Vaccine safety is one of the issues under surveillance and a possible correlation between vaccines and thyroid function has been reported. However, reports of the impact of coronavirus vaccines on those with Graves’ disease (GD) are rare.
This paper presents two patients with underlying GD in remission, both developed thyrotoxicosis and one developed thyroid storm following the adenovirus-vectored vaccine (Oxford-AstraZeneca, United Kingdom). The objective of this article is to raise awareness regarding a possible association between COVID-19 vaccination and the onset of thyroid dysfunction in patients with underlying GD in remission.
Receiving either the mRNA or an adenovirus-vectored vaccine for SARS-CoV-2 could be safe under effective treatment. Vaccine induced thyroid dysfunction has been reported, but the pathophysiology still not well understood. Further investigation is required to evaluate the possible predisposing factors for developing thyrotoxicosis especially in patients with underlying GD. However, early awareness of thyroid dysfunction following vaccination could avoid a life-threatening event.
Core Tip: Thyroid storm is potentially life-threatening. If the diagnosis of thyroid storm is suspected, treatment should be initiated without delay. We report two cases with underlying Graves’ disease who experienced thyrotoxicosis and thyroid storm after receiving an adenovirus-vectored vaccine for coronavirus disease (COVID-19). Both had achieved disease remission before the vaccination. The pathophysiology of COVID-19 vaccine induced thyroid dysfunction still not well understood. However, early awareness of thyroid dysfunction following the vaccination could avoid the life-threatening events.
- Citation: Yan BC, Luo RR. Thyrotoxicosis in patients with a history of Graves’ disease after SARS-CoV-2 vaccination (adenovirus vector vaccine): Two case reports. World J Clin Cases 2023; 11(5): 1122-1128
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1122.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1122
It has been three years since the onset of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak. By December 20, 2022, the death toll from coronavirus disease 2019 (COVID-19) had surpassed 6.6 million, with more than 649 million confirmed cases[1]. Two mainstay diagnostic methods have been adopted-one is molecular diagnostic technique which takes 2-4 h with higher specificity and sensitivity, and the other is immunological diagnostic technique which generally considered inexpensive and more rapid[2]. To contain the SARS-CoV-2 pandemic, vaccination remains one of the most effective ways[3]. Although over 13 billion SARS-CoV-2 vaccine doses have been administered worldwide according to WHO statistics[1], there has been concern about the rapid development, authorization, and marketing of the vaccines which was required for the pandemic, one of which is thyroid dysfunction. Thyroid dysfunction can happen when certain viral infections occur. A previous review disclosed three potential mechanisms of the SARS-CoV-2 viral infection to cause thyroid dysfunction-nonthyroidal illness syndrome from infection, hypothalamic-pituitary-adrenal axis dysfunction due to hypothalamus/pituitary injury and thyroid cell destruction caused by direct thyroid cell infection[4]. However, there have been several reports of patients developing thyroid dysfunction after receiving the SARS-CoV-2 vaccine, and the autoimmune/inflammatory syndrome induced by adjuvants in genetically susceptible individuals has been widely discussed. However, there have been few reports on the impact of the SARS-CoV-2 vaccine on patients diagnosed with Grave’s Disease (GD). We report two cases of patients with underlying GD in remission who developed thyrotoxicosis following the administration of adenovirus-vectored vaccines (Oxford-AstraZeneca, United Kingdom). We hope to raise awareness regarding the possible association between SARS-CoV-2 vaccination and the onset of thyroid dysfunction in patients with remission of the underlying GD.
Case 1: A 50-year-old woman presented to the emergency room due to progressive dyspnoea and palpitations.
Case 2: A 31-year-old woman visited endocrinology outpatient department with the symptom of palpitations.
Case 1: The patient had received her first dose of AstraZeneca vaccine for SARS-CoV-2 on July 19, 2021. Ten days later, she developed palpitations, and the associated symptoms included progressive dyspnoea, dizziness, myalgia, distal tremors, and diarrhea. As the symptoms progressed, she visited the Far Eastern Memorial Hospital emergency department on July 31, 2021.
Case 2: The patient received her first dose of the AstraZeneca vaccine for SARS-CoV-2 on June 11, 2021 and began to have palpitations about two weeks later. The accompanying symptoms include a lump in the throat, hand tremors, dyspnoea, fatigue, increased bowel movements but no abdominal pain, nausea nor vomiting. There was no cough, sputum, sore throat, and no fever. Owing to the pandemic and fear of interhospital infection, the patient self-purchased propranolol to control her heart rate; however, there was no improvement and she began to be breathless when talking in August 2021. Finally, the patient made an appointment and presented to the endocrinology outpatient department on August 24, 2021.
Case 1: The patient had been diagnosed with GD and had received complete treatment during childhood. Her medical history revealed no other systemic disease.
Case 2: The patient had been diagnosed with GD in December 2018. She was on long-term methimazole until GD remission in November 2020. Apart from the diagnosis of GD, the patient was in good health and a non-smoker.
Case 1: No similar symptoms were noted in her family members.
Case 2: No similar symptoms were noted in her family members.
Case 1: Her initial vital signs revealed a blood pressure of 18.8/14.0 kPa, heart rate of 86 beats per minute, respiratory rate of 18 beats per minute, body temperature of 35.7 °C. Upon physical examination, proptosis in both eyes, neck supple without tenderness or palpable mass.
Case 2: Physical examination revealed hyperactivity, neck was supple without tenderness, distal tremors in both hands, and tachycardia. Her blood pressure was 17.7/12.0 kPa and heart rate was 109 beats per minute.
Case 1: A complete blood test and biochemical profiles including kidney function, hepatic markers, coagulation profile, cardiac markers, and urine and stool tests were normal but the aspartate aminotransferase was above normal (53 U/L) (normal range 13-39). Thyroid function tests indicated hyperthyroidism with thyrotropin (TSH) 0.015 IU/mL (normal range 0.400-4.000), thyroxine (free-T4) 3.4 ng/dL (normal range 0.8-2.00 ng/dL), and significantly elevated thyroid autoantibodies. The anti-thyroid peroxidase antibody (anti-TPO) was > 1000 (normal range 0.00-35.00) and anti-thyroid stimulating antibody (anti-TSHR) was 37.70% (normal 15.00%). The rapid antigen testing for COVID-19 was performed due to patient warning signs including dyspnoea, dizziness, myalgia and diarrhoea was negative.
Case 2: The laboratory results were normal, including routine blood tests, cardiac markers, liver and kidney function. Thyroid function tests revealed that her TSH level had decreased to 0.015 IU/mL, and free-T4 Level was 4.77 ng/dL. Her thyroid autoantibodies were raised significantly also. The anti-TPO was > 1000 and anti-TSHR was 41.29%. The rapid antigen testing for COVID-19 was performed as the patient had warning signs including dyspnoea and increase bowel movements and was negative.
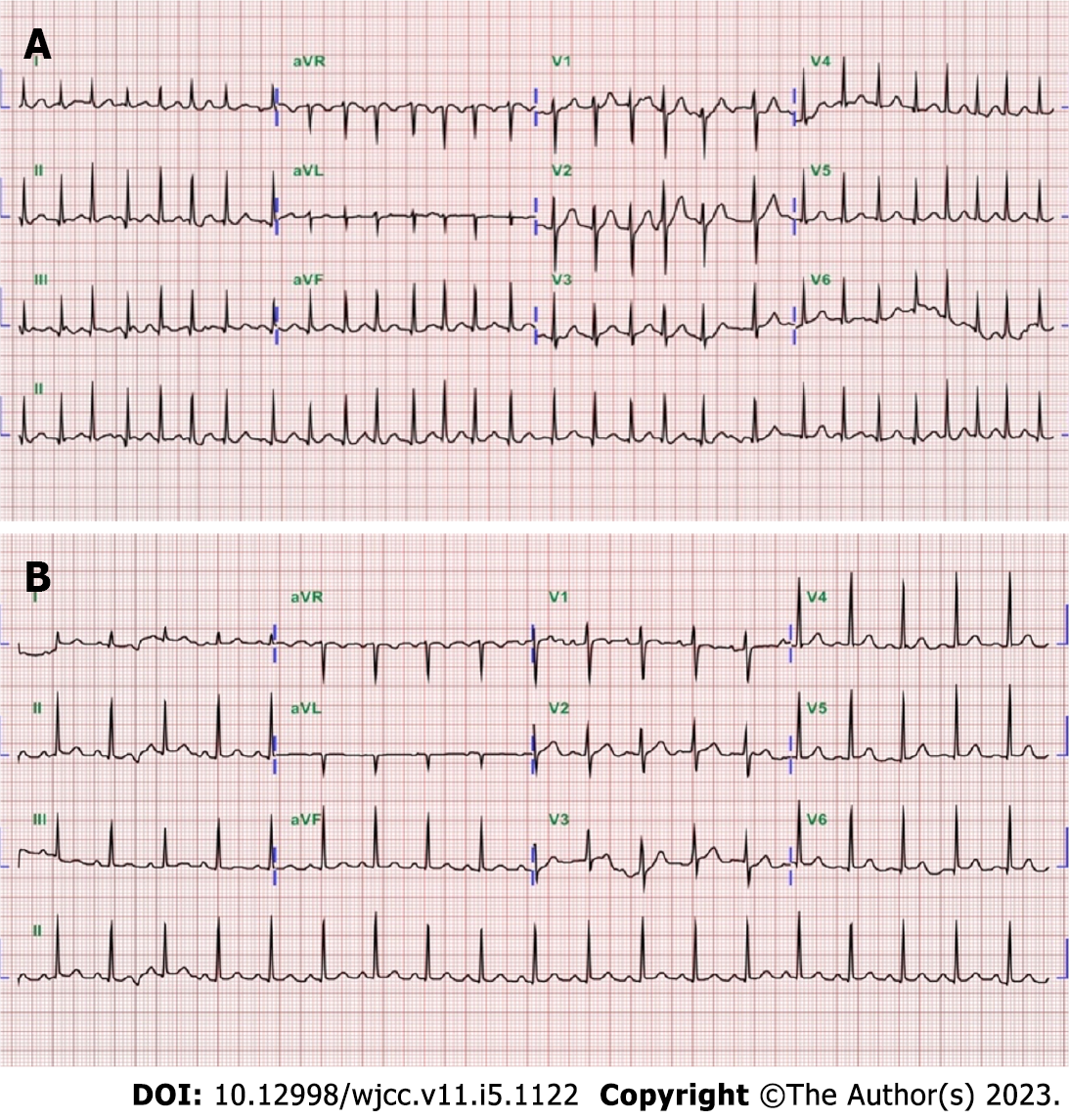
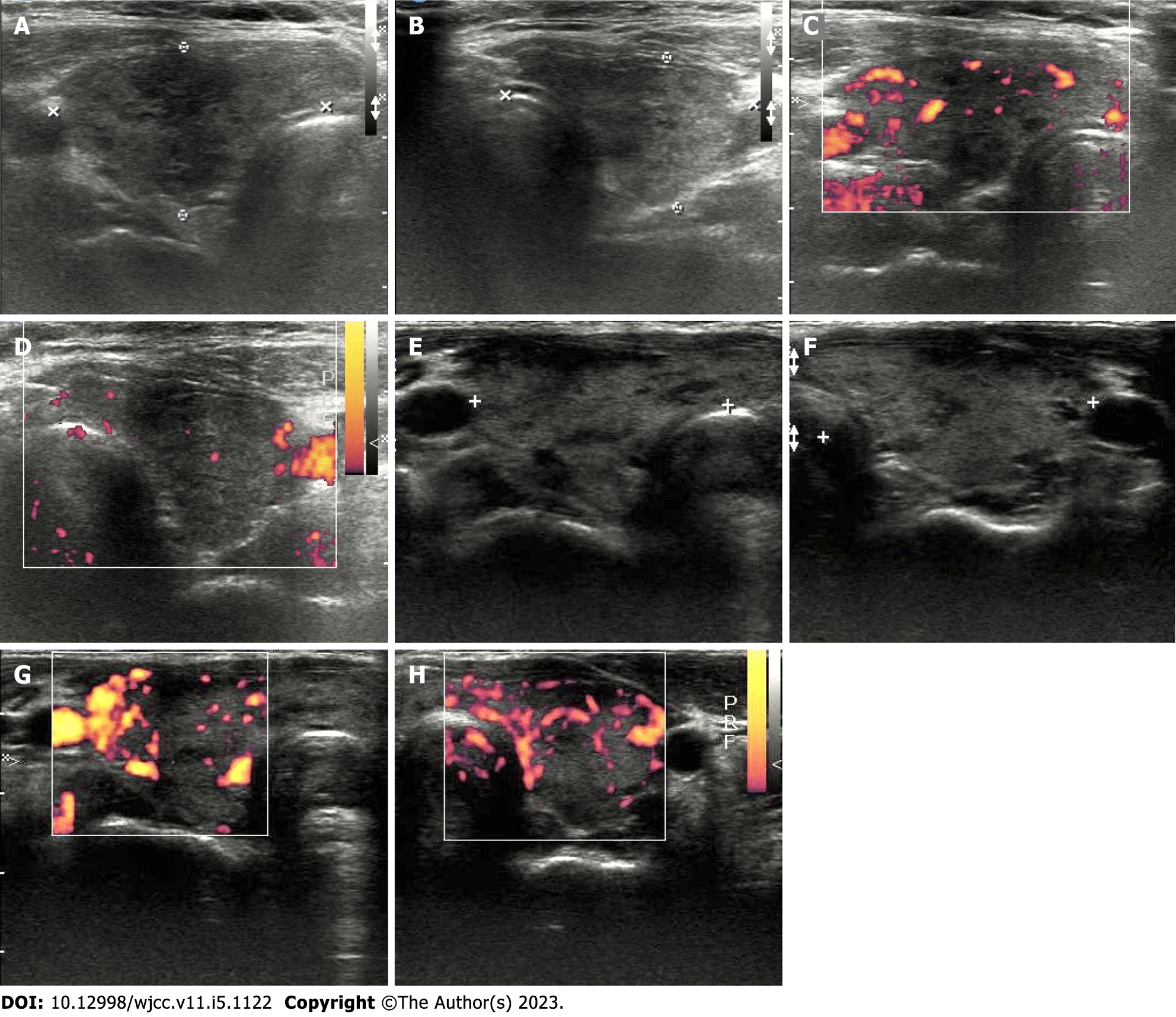
Case 1: Electrocardiography revealed atrial fibrillation with a rapid ventricular response of heart rate 170 beats per minute (Figure 1A). A chest film was taken which revealed non-specific findings of both lung zones and the heart size was within normal limits. Thyroid ultrasonography revealed a heterogeneous and hypoechoic thyroid with mildly increased vascularity over the right thyroid lobe, and no thyroid nodules were observed (Figure 2A-D).
Case 2: Electrocardiography revealed sinus tachycardia with a heart rate of 114 beats per minute (Figure 1B). The chest X-ray was normal. Thyroid ultrasonography revealed heterogeneous and hypoechoic echotexture with increased vascularity (Figure 2E-H).
The patient scored 55 points on the Burch–Wartofsky Point Scale (Table 1) which was highly suggestive of a thyroid storm following SARS-CoV-2 vaccination.
| Diagnostic criteria | Score | |
| Temperature (°F) | 99.6 | 0 |
| Central nervous system effects | Absent | 0 |
| Gastrointestinal-hepatic dysfunction | Diarrhea | 10 |
| Cardiovascular dysfunction (Tachycardia) | 170 beats/min | 25 |
| Congestive Heart Failure | Absent | 0 |
| Atrial fibrillation | Yes | 10 |
| Precipitant history | Yes | 10 |
| Total score | - | 55 |
Graves’ disease relapse following SARS-CoV-2 vaccination.
The patient received intravenous injections of diltiazem (15 mg), hydrocortisone (100 mg), and propranolol (30 mg) which resulted in rapid improvement in her symptoms. The patient was prescribed propranolol (30 mg/d) and carbimazole (30 mg/d). A repeat blood test performed four weeks later, on September 2, 2021, revealed subclinical hyperthyroidism with TSH 0.041 IU/mL, Free T4 1.2 ng/dL and normal liver function. Both propranolol and carbimazole were tapered to 20 mg/d during the regular follow-up visits. Another thyroid function test performed on October 20, 2021, showed that her free-T4 and TSH levels had returned to normal.
The patient was treated with carbimazole (20 mg/d) and propranolol (30 mg/d), with a good response. A thyroid function test repeated on October 11, 2021, demonstrated that her free-T4 Level had declined to 2.16 ng/dL and TSH to 0.06 IU/mL. Therefore, the regimen was tapered to carbimazole (10 mg/d) and propranolol (30 mg/). On December 02, 2021, her free-T4 and TSH levels had returned to normal. Therefore, the anti-thyroid drug was changed to tapzaole (5 mg/d) and propranolol use was discontinued.
Owing to thyrotoxicosis with a thyroid storm, the patient chose Moderna as her second SARS-CoV-2 vaccine on October 29, 2021. Thereafter, she had no recurrence. The patient was withdrawn from anti-thyroid drugs as of July, 2022, as her clinical condition was stable. Another thyroid function test performed on August 10, 2022, showed free-T4 1.050 ng/dL and TSH of 1.33 IU/mL. The patient received the Novavax Nuvaxovid® COVID-19 vaccine as booster on August 24, 2022. No discomfort was reported during the follow-up visits and free-T4 level 1.060 ng/dL; TSH 1.43 IU/mL on November, 2022.
The patient received a second dose of AstraZeneca vaccine on September 22, 2021, as scheduled and did not report any discomfort during the follow-up visit. Thereafter the patient had a regular follow-up at the endocrinology outpatient department and was taking tapzaole (2.5 mg/d). On September 20, 2022, the patient received a Moderna booster and her thyroid function tests on October 14, 2022, revealed a free-T4 level of 1.49 ng/dL; TSH 1.180 IU/mL.
GD, one of the most common types of hyperthyroidism worldwide, is an autoimmune disease, in which the body produces antibodies that attack the thyroid gland. Patients with GD can achieve remission if their antibody levels decrease or disappears. Patients with GD can choose between two general treatment methods: Removal of the thyroid either surgically or with radioactive iodine therapy and management with antithyroid drugs (ATDs). Treatment with ATDs is used to either achieve remission or prepare the patient for surgery or radioactive iodine treatment[5].
There have been several reports of thyroid dysfunction occurring after SARS-CoV-2 vaccination, with subacute thyroiditis being the most common and neck pain being a common presentation[6]. The pathophysiology of vaccine-induced thyroid dysfunction is not well understood. However, adjuvants used in different vaccines may trigger autoimmune responses such as thyroiditis[7-11] or GD[12,13], as mentioned previously. As adjuvants aim to increase the immune reaction in vaccine recipients, it is hypothesized that some pathogenic reactions could develop simultaneously[7,13]. In the majority of the previous reports patients had received mRNA vaccines (Pfizer/BioNTech or Moderna)[8,10,11,14,15], while others received inactive (CoronaVac)[9,16] or adenovirus-vectored vaccines (AstraZeneca)[7]. In contrast, the number of patients who developed hyperthyroidism with underlying GD after SARS-CoV-2 vaccination was low and may have been underreported[12]. To the best of our knowledge, the two cases reported herein might be the first to describe hyperthyroidism and thyroid storm with a known Graves diagnosis. Although GD recurrence rates can be high following discontinuation of medication, our patients did not develop palpitations, diarrhea, hand tremors, shortness of breath, or increased bowel movements before vaccination for SARS-CoV-2, and their symptoms occurred within weeks after receiving the adenovirus vector vaccine. Moreover, our cases demonstrate that receiving an adenovirus-vectored vaccine could be safe under effective ATDs treatment. However, further investigation is required to evaluate the possible predisposing factors for developing thyrotoxicosis with underlying GD after receiving SARS-CoV-2 vaccine.
Receiving either an mRNA or an adenovirus-vectored vaccine for SARS-CoV-2 could be safe under effective ATD treatment. Further investigation is required to evaluate the possible predisposing factors for developing thyrotoxicosis with underlying GD after receiving SARS-CoV-2 vaccines. However, early awareness of thyroid dysfunction following vaccination could prevent life-threatening events.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Agrawal P, United States; Fan Y, China; Rotondo JC, Italy S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | World Health Organization. Coronavirus disease (COVID-19) pandemic 2022. [cited Dec 21 2022]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.. |
| 2. | Rotondo JC, Martini F, Maritati M, Caselli E, Gallenga CE, Guarino M, De Giorgio R, Mazziotta C, Tramarin ML, Badiale G, Tognon M, Contini C. Advanced Molecular and Immunological Diagnostic Methods to Detect SARS-CoV-2 Infection. Microorganisms. 2022;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Anderson EJ, Rouphael NG, Widge AT, Jackson LA, Roberts PC, Makhene M, Chappell JD, Denison MR, Stevens LJ, Pruijssers AJ, McDermott AB, Flach B, Lin BC, Doria-Rose NA, O’Dell S, Schmidt SD, Corbett KS, Swanson PA 2nd, Padilla M, Neuzil KM, Bennett H, Leav B, Makowski M, Albert J, Cross K, Edara VV, Floyd K, Suthar MS, Martinez DR, Baric R, Buchanan W, Luke CJ, Phadke VK, Rostad CA, Ledgerwood JE, Graham BS, Beigel JH; mRNA-1273 Study Group. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383:2427-2438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1111] [Article Influence: 222.2] [Reference Citation Analysis (0)] |
| 4. | Chen W, Tian Y, Li Z, Zhu J, Wei T, Lei J. Potential Interaction Between SARS-CoV-2 and Thyroid: A Review. Endocrinology. 2021;162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Vos XG, Endert E, Zwinderman AH, Tijssen JG, Wiersinga WM. Predicting the Risk of Recurrence Before the Start of Antithyroid Drug Therapy in Patients With Graves’ Hyperthyroidism. J Clin Endocrinol Metab. 2016;101:1381-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Kyriacou A, Ioakim S, Syed AA. COVID-19 vaccination and a severe pain in the neck. Eur J Intern Med. 2021;94:95-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Oyibo SO. Subacute Thyroiditis After Receiving the Adenovirus-Vectored Vaccine for Coronavirus Disease (COVID-19). Cureus. 2021;13:e16045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Pandya M, Thota G, Wang X, Luo H. Thyroiditis After COVID-19 mRNA Vaccine: A Case Series. AACE Clin Case Rep. 2022;8:116-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Saygılı ES, Karakilic E. Subacute thyroiditis after inactive SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 10. | Schimmel J, Alba EL, Chen A, Russell M, Srinath R. Letter to the Editor: Thyroiditis and Thyrotoxicosis After the SARS-CoV-2 mRNA Vaccine. Thyroid. 2021;31:1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 11. | Sigstad E, Grøholt KK, Westerheim O. Subacute thyroiditis after vaccination against SARS-CoV-2. Tidsskr Nor Laegeforen. 2021;141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Sriphrapradang C, Shantavasinkul PC. Graves’ disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473-474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two Cases of Graves’ Disease Following SARS-CoV-2 Vaccination: An Autoimmune/Inflammatory Syndrome Induced by Adjuvants. Thyroid. 2021;31:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 152] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 14. | Franquemont S, Galvez J. Subacute Thyroiditis After mRNA Vaccine for Covid-19. J Endocr Soc. 2021;5 Suppl 1:A956-A957. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 15. | Jeeyavudeen MS, Patrick AW, Gibb FW, Dover AR. COVID-19 vaccine-associated subacute thyroiditis: an unusual suspect for de Quervain’s thyroiditis. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | İremli BG, Şendur SN, Ünlütürk U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J Clin Endocrinol Metab. 2021;106:2600-2605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |