Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1115
Peer-review started: October 4, 2022
First decision: December 19, 2022
Revised: January 8, 2023
Accepted: January 20, 2023
Article in press: January 20, 2023
Published online: February 16, 2023
Processing time: 112 Days and 22.7 Hours
Combined small cell lung cancer (C-SCLC) is a special subtype of small cell lung cancer that is relatively rare, aggressive, and prone to early metastasis and has a poor prognosis. Currently, there are limited studies on C-SCLC, and there is no uniform standard treatment, especially for extensive C-SCLC, which still faces great challenges. In recent years, the development and progress of immunotherapy have provided more possibilities for the treatment of C-SCLC. We used immunotherapy combined with first-line chemotherapy to treat extensive-stage C-SCLC to explore its antitumor activity and safety.
We report a case of C-SCLC that presented early with adrenal, rib, and mediastinal lymph node metastases. The patient received carboplatin and etoposide with concurrent initiation of envafolimab. After 6 cycles of chemot
Envafolimab combined with carboplatin and etoposide in the treatment of extensive-stage C-SCLC has preliminary antitumor activity and good safety and tolerability.
Core Tip: Combined small cell lung cancer (C-SCLC) is a special subtype of small cell lung cancer, which is relatively rare, aggressive, prone to early metastasis, and has a poor prognosis. With the development and progress of immunotherapy, several clinical studies have shown that programmed death ligand-1 combined with chemotherapy can effectively prolong the progression-free survival and median overall survival of patients with extensive stage small cell lung cancer, but its efficacy in C-SCLC is not exact. In this paper, we report a patient with extensive stage C-SCLC who was treated with envafolimab combined with carboplatin and etoposide with preliminary anti-tumor activity, safety and tolerability.
- Citation: Liu MH, Li YX, Liu Z. Envafolimab combined with chemotherapy in the treatment of combined small cell lung cancer: A case report. World J Clin Cases 2023; 11(5): 1115-1121
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1115.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1115
Combined small cell lung cancer (C-SCLC) is a special subtype of small cell lung cancer (SCLC) that was first proposed by the WHO in 2015 as a mixture of SCLC components and one or more non-SCLC components; it is relatively rare and accounts for approximately 2% to 23% of SCLC cases[1,2]. C-SCLC has similar clinical characteristics to SCLC. At present, there are few studies on C-SCLC, and there is no uniform standard treatment for C-SCLC, which has a poor prognosis and a median overall survival (OS) of approximately 9 mo[3].
In recent years, with the development of immunotherapy and the advent of immune checkpoint inhibitors, immunotherapy combined with chemotherapy has provided more possibilities for the treatment of extensive-stage C-SCLC. It effectively removes tumor cells and prevents their recurrence and metastasis by regulating the body's immune activation program and repairing immune checkpoint abnormalities. Several clinical studies have demonstrated the antitumor activity of programmed death ligand-1 (PD-L1) combined with chemotherapy in extensive-stage SCLC, which can significantly prolong the progression-free survival (PFS) and OS of patients; moreover atezolizumab in combination with carboplatin and etoposide has now been approved by the Food and Drug Administration (FDA) for the treatment of extensive-stage SCLC[4-6]. However, there are relatively few clinical studies on C-SCLC immunochemotherapy, and only a few case reports. Dong et al[7] reported a case of C-SCLC containing three different histological components; the solid tumor masses were significantly reduced after 2 cycles of treatment with camrelizumab combined with paclitaxel and cisplatin and met the Response Evaluation Criteria in Solid Tumors (RECIST) criteria for partial response, suggesting that immunochemotherapy may become a feasible regimen for the treatment of extensive-stage C-SCLC.
Envafolimab is a novel fusion of a humanized single-domain PD-L1 antibody and human IgG1 crystalline fragment formulated for subcutaneous injection. Phase I clinical trials have shown a favorable safety and pharmacokinetic profile, with promising preliminary antitumor activity in patients with advanced solid tumors[8]. In this paper, we report a case of extensive-stage C-SCLC and attempted to use envafolimab combined with carboplatin and etoposide to investigate its antitumor activity and safety. We also summarize the progress in the treatment of extensive-stage C-SCLC at this stage.
A 51-year-old man presented with fever, cough, and left-sided chest pain for half a month.
Half a month ago, the patient had irritable cough, yellow viscous sputum and fever, with a body temperature up to 39.3 °C, accompanied by chest pain in the left hypochondrium, without hemoptysis, wheezing, shortness of breath or palpitation syncope. He visited a local hospital and was treated with anti-infection, anti-tussive, expectorant, antipyretic and other treatments, with poor results, so he was transferred to our hospital for further treatment.
There was no significant medical history.
The patient smoked approximately 40 cigarettes a day for more than 20 years without a family history.
A body temperature of 38.6 ºC, a blood pressure of 130/85 mmHg, a heart rate of 82 beats/min, and a respiratory rate of 19 times/min were noted. Breath sounds in the left lower lung were decreased, moist rales could be heard, and the remaining physical examinations showed no significant abnormalities.
The laboratory data revealed the following: White blood cells 23.75 × 109/L, neutrophils 83.3%, and hemoglobin 117 g/L. Serum C-reactive protein was increased at 281.44 mg/L (normal range ≤ 8 mg/L), and the erythrocyte sedimentation rate was increased at 109 mm/h (normal range ≤ 15 mm/h). Lung cancer markers, including carcinoembryonic antigen, Cyfra21-1, neuron-specific enolase and squamous cell carcinoma, showed no significant abnormalities.
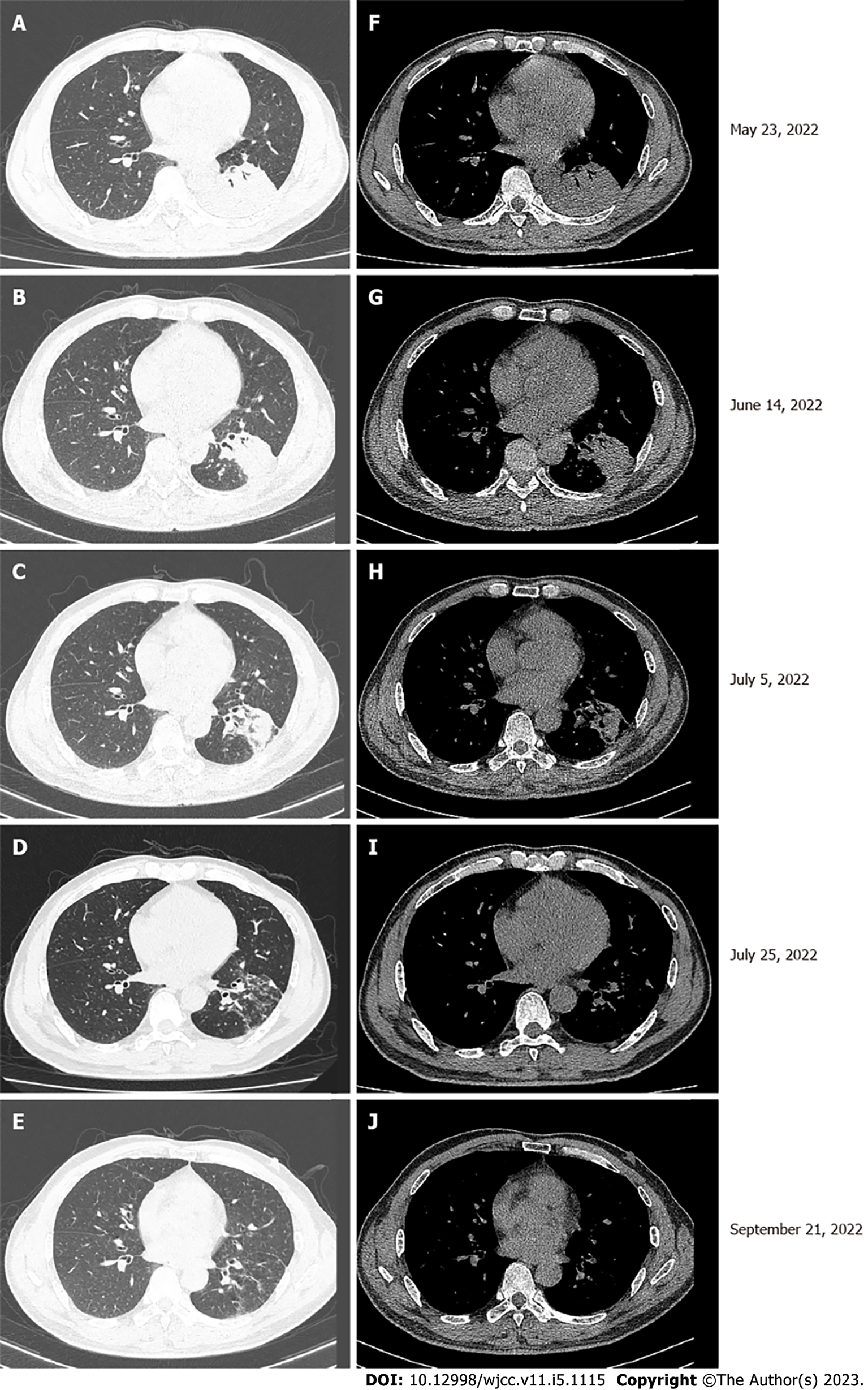
Chest computed tomography (CT) showed a mass in the left lower lobe and multiple enlarged lymph nodes in the left hilum and mediastinum (Figure 1A and F).
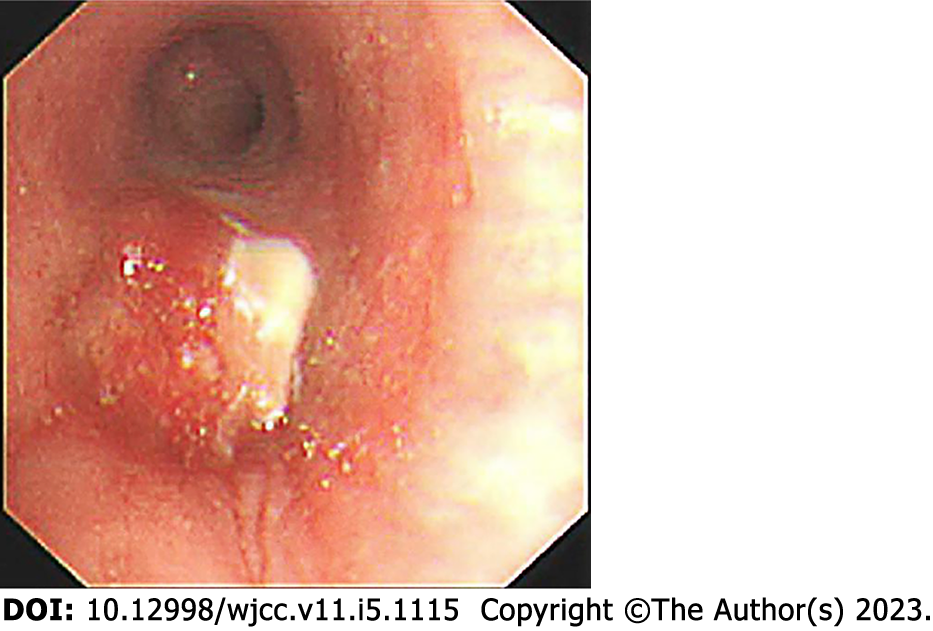
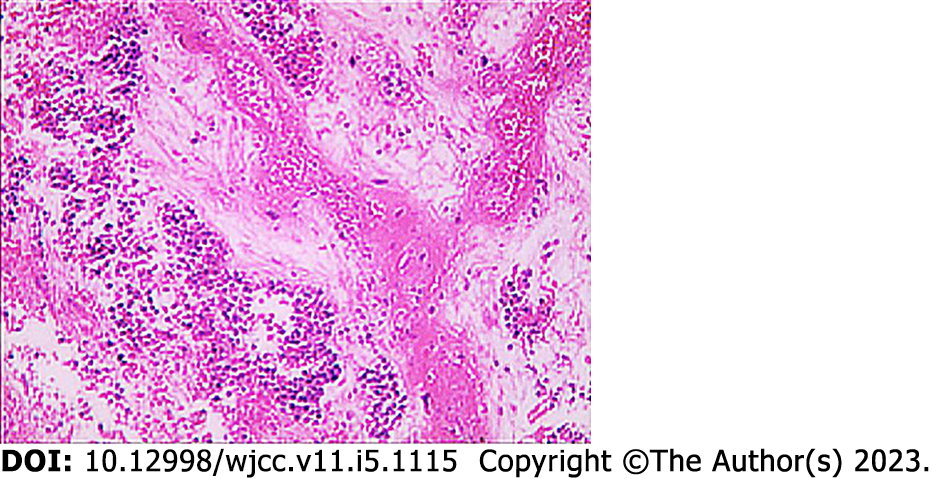
Fiberoptic bronchoscopy revealed a mass protruding from the orifice of the left lower lung inlet, covered with a necrotic white coating and bloody secretions, completely obstructing the left lower bronchus (Figure 2). Biopsy of the mass was performed, and a pathological diagnosis (pathological number: 202217190) of neuroendocrine carcinoma and poorly differentiated carcinoma was made (Figure 3). The immunohistochemistry findings were as follows: AE1/AE3: (large cell +), CD3: (-), CD31: (-), CD34: (-), CD56: (small cell +), CgA: (small cell +), CK5/6: (focus +), Desmin: (focus +), ERG: (+), HMB-45: (-), MyoD1: (-), Myogenin: (-), Napsin-A: (-), P40: (individual large cell +), S-100: (-), SMA: (focus +), SOX-10: (-), Syn: (small cell +), TTF-1: (-), Vimentin: (large cell +), CD20: (-), ALK: (-), Brg-1: (+), CD2: (-), CD30: (-), CD4: (-), CD68: (-), CD7: (-), CK8/18: (+), EBER: (), INI1: (+), Ki-67: (small cells + 40%, large cells + 70%), TIAI: (-), and ERG: (-). These results suggested C-SCLC. ECT showed slightly enhanced punctate radionuclide uptake in the right posterior 9th rib and left posterior 8th rib and possible bone metastasis. Abdominal CT showed a nodule in the adrenal region of the left kidney and possible adrenal metastasis.
C-SCLC with adrenal, rib and lymph node metastases.
Etoposide 100 mg/m2 combined with carboplatin 5 mg/(mL·min) was used for chemotherapy once every 3 wk for 6 cycles, while envafolimab was initiated for immunotherapy, and envafolimab 160 mg was injected subcutaneously once a week for a long period of time.
The lung lesion was significantly reduced after 6 cycles of immunotherapy combined with chemot
C-SCLC is a rare subtype of lung cancer with a diagnostic yield of only 2% in small pathological specimens or cytological specimens. Its clinical features are similar to SCLC, but C-SCLC has a slightly lower rate of early metastasis, is more likely to occur in peripheral lung tissue and is more common in the smoking male population than SCLC[9]. At present, there are few studies on C-SCLC, and there is no uniform standard treatment for C-SCLC. A large retrospective study analyzed the treatment regimens and clinical outcomes of C-SCLC from 2004 to 2016 and showed that C-SCLC had a better prognosis than SCLC in stage IA-IIIA; moreover, surgery was beneficial for C-SCLC in stage IA-IB, while chemotherapy-based treatment was the main choice for advanced patients[10]. However, C-SCLC has been found to have a lower response rate to chemotherapy than pure small cell lung cancer (P-SCLC) in clinical practice, and the response rate is maintained at only 40%-50%[11]. Men et al[12] also found a 5-year OS of 37.7% in patients with C-SCLC after chemotherapy, which was similar to that of patients who did not receive chemotherapy (35.4%), with no significant difference between the two groups, presumably because the mixed NSCLC component was not highly sensitive to chemotherapy. Therefore, the treatment of extensive-stage C-SCLC still faces great challenges at this stage.
With the development of immunotherapy, several clinical studies have comparatively observed the antitumor activity and safety of PD-L1 combined with chemotherapy in extensive-stage SCLC. In IMpower133, 201 patients received atezolizumab and carboplatin plus etoposide (CP/ET), and 202 patients received placebo and CP/ET (n = 202). The median follow-up OS was 22.9 mo at the time of the updated analysis; the median OS times were 12.3 months and 10.3 mo for atezolizumab plus CP/ET and placebo plus CP/ET, respectively, with median PFS times of 5.2 months and 4.3 mo, suggesting that patients could benefit from atezolizumab[4]. In the CAPSTONE-1 study, 230 (50%) patients received adebrelimab and chemotherapy (adebrelimab group), and 232 (50%) patients received placebo and chemotherapy (placebo group) from 26 December 2018 to 04 September 2020. At the time of the data cutoff (8 October 2021), the median follow-up was 13.5 mo. The median OS was significantly improved with adebrelimab (median OS 15.3 mo) compared to placebo (median OS 12.8 mo)[5]. In the KEYNOTE-604 study, of 453 participants, 228 were randomized to pembrolizumab and etoposide and platinum (EP), and 225 were randomized to placebo and EP. The PFS at 12 mo was estimated to be 13.6% in the pembrolizumab and EP group compared with 3.1% in the placebo and EP group, suggesting that pembrolizumab and EP significantly improved PFS; moreover, no unexpected toxicities were observed with pembrolizumab combined with EP, indicating that it had good safety[6]. In 2019, the FDA officially approved atezolizumab in combination with carboplatin and etoposide for the treatment of extensive-stage SCLC. The 2022 Chinese lung cancer guidelines also recommended combination with immunotherapy on the basis of chemotherapy for extensive-stage SCLC.
However, clinical studies of immunotherapy combined with chemotherapy in extensive-stage C-SCLC are very limited, with only a small number of individual case reports. Niitsu et al[13] reported a case of epidermal growth factor receptor mutation-positive C-SCLC that developed intrinsic resistance to the tyrosine kinase inhibitor oximotinib; this patient subsequently received 6 cycles of dose-adjusted durvalumab in combination with etoposide and carboplatin, which showed a sustained treatment response and disease control. Dong et al[7] reported a case of C-SCLC containing three different histological components; the solid tumor masses were significantly reduced after 2 cycles of treatment with camrelizumab combined with paclitaxel and cisplatin and met the RECIST criteria for partial response, suggesting that immunochemotherapy may be a feasible regimen for the treatment of extensive-stage C-SCLC. In this case, distant metastasis had already occurred at the time of diagnosis, and the opportunity for surgery was lost. Considering that this patient had C-SCLC, we combined envafolimab treatment on the basis of first-line chemotherapy with carboplatin and etoposide. To date, after 6 cycles of chemotherapy, the lung lesion was significantly reduced, the antitumor activity was significant, and only drug-related adverse events, such as mild nausea and vomiting, occurred, with good safety.
Additionally, we searched for other treatment options for extensive-stage C-SCLC. In terms of targeted therapy, although some C-SCLC patients can also have targeted gene mutations, because the SCLC component is more sensitive to chemotherapy, the effect of targeted therapy is not superior to that of chemotherapy. However, targeted therapy can also be tried in patients who have failed immunochemotherapy. Niitsu et al[13] reported a young female patient who was successfully treated with alectinib for ALK-positive C-SCLC after failure of immunochemotherapy for SCLC and cytotoxic chemotherapy for adenocarcinoma. Radiotherapy is rarely used in the treatment of C-SCLC and is often combined with other regimens. Wang et al[14] reported a case of a C-SCLC patient who had resistance to comprehensive treatment and then received nivolumab combined with radiotherapy, achieving clinical complete remission. In general, there are few studies on the treatment of C-SCLC, mainly case reports with limited persuasion and extrapolation, and more large-sample multicenter clinical studies are expected to provide more favorable evidence support in the future (Table 1).
| Ref. | Year | Intervention | Clinical outcome |
| Li[15] | 2022 | Durvalumab 15 mg/kg, etoposide, and carboplatin (at 70% of the standard doses) every 3 wk | The patient responded well to treatment and survived for 19 mo at the time of publication |
| Dong et al[7] | 2022 | Camrelizumab 200 mg ig d1, albumin paclitaxel 400 mg ig d1, cisplatin 60mg ig d1-2/q3w | After 4 cycles of chemotherapy, lung lesions were significantly reduced |
| Niitsu et al[14] | 2020 | Alectinib | The patient has been receiving alectinib treatment for five months, without disease progression or remarkable adverse events |
| Wang et al[15] | 2022 | Nivolumab combined with radiotherapy | Clinical complete remission |
In this paper, we report a case of extensive-stage C-SCLC treated with envafolimab combined with carboplatin and etoposide, which showed preliminary antitumor activity, safety and tolerability, suggesting that immunochemotherapy may be a feasible regimen for extensive-stage C-SCLC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng Y, China; Kamitani R, Japan S-Editor: Wang DM L-Editor: A P-Editor: Wang DM
| 1. | Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JHM, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I; WHO Panel. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol. 2015;10:1243-1260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2160] [Cited by in RCA: 3111] [Article Influence: 345.7] [Reference Citation Analysis (0)] |
| 2. | Babakoohi S, Fu P, Yang M, Linden PA, Dowlati A. Combined SCLC clinical and pathologic characteristics. Clin Lung Cancer. 2013;14:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Xu L, Zhang G, Song S, Zheng Z. Prognostic factors, treatment, and outcomes in combined small cell lung cancer: a SEER survey from 2004 to 2015. Transl Cancer Res. 2020;9:5292-5303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol. 2021;39:619-630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 518] [Article Influence: 129.5] [Reference Citation Analysis (0)] |
| 5. | Wang J, Zhou C, Yao W, Wang Q, Min X, Chen G, Xu X, Li X, Xu F, Fang Y, Yang R, Yu G, Gong Y, Zhao J, Fan Y, Liu Q, Cao L, Yao Y, Liu Y, Wu J, He Z, Lu K, Jiang L, Hu C, Zhao W, Zhang B, Shi W, Zhang X, Cheng Y; CAPSTONE-1 Study Group. Adebrelimab or placebo plus carboplatin and etoposide as first-line treatment for extensive-stage small-cell lung cancer (CAPSTONE-1): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:739-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 218] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 6. | Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, Cheema PK, Rodriguez-Abreu D, Wollner M, Yang JC, Mazieres J, Orlandi FJ, Luft A, Gümüş M, Kato T, Kalemkerian GP, Luo Y, Ebiana V, Pietanza MC, Kim HR; KEYNOTE-604 Investigators. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J Clin Oncol. 2020;38:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 526] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 7. | Dong Y, Li Q, Li D, Fang Y, Wang C. Whole-Process Treatment of Combined Small Cell Lung Cancer Initially Diagnosed as "Lung Squamous Cell Carcinoma": A Case Report and Review of the Literature. Front Immunol. 2022;13:831698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Papadopoulos KP, Harb W, Peer CJ, Hua Q, Xu S, Lu H, Lu N, He Y, Xu T, Dong R, Gong J, Liu D. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist. 2021;26:e1514-e1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 9. | Qin J, Lu H. Combined small-cell lung carcinoma. Onco Targets Ther. 2018;11:3505-3511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | He J, Xu S, Pan H, Li S, He J. Treatments for combined small cell lung cancer patients. Transl Lung Cancer Res. 2020;9:1785-1794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Sundstrøm S, Bremnes RM, Kaasa S, Aasebø U, Hatlevoll R, Dahle R, Boye N, Wang M, Vigander T, Vilsvik J, Skovlund E, Hannisdal E, Aamdal S; Norwegian Lung Cancer Study Group. Cisplatin and etoposide regimen is superior to cyclophosphamide, epirubicin, and vincristine regimen in small-cell lung cancer: results from a randomized phase III trial with 5 years' follow-up. J Clin Oncol. 2002;20:4665-4672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Men Y, Hui Z, Liang J, Feng Q, Chen D, Zhang H, Xiao Z, Zhou Z, Yin W, Wang L. Further understanding of an uncommon disease of combined small cell lung cancer: clinical features and prognostic factors of 114 cases. Chin J Cancer Res. 2016;28:486-494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Niitsu T, Shiroyama T, Miyake K, Noda Y, Kido K, Hara R, Enomoto T, Adachi Y, Amiya S, Suga Y, Fukushima K, Koyama S, Iwahori K, Hirata H, Nagatomo I, Takeda Y, Kumanogoh A. Combined small cell lung carcinoma harboring ALK rearrangement: A case report and literature review. Thorac Cancer. 2020;11:3625-3630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Wang W, Li L, Wu S, Shen J, Huang C, Chen Y, Li S. Abscopal effect of radiation therapy and nivolumab in a patient with combined small-cell lung cancer: a case report. Immunotherapy. 2022;14:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Li YC. Durable response to durvalumab-based immunochemotherapy in small-cell lung carcinoma transformation from EGFR-mutant non-small cell lung cancer: A case report. Thorac Cancer. 2022;13:775-779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |