Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1099
Peer-review started: September 24, 2022
First decision: December 13, 2022
Revised: December 20, 2022
Accepted: January 19, 2023
Article in press: January 19, 2023
Published online: February 16, 2023
Processing time: 143 Days and 3.5 Hours
Maturity-onset diabetes of the young (MODY) is the most common monogenic type of diabetes. Recently, 14 gene mutations have been found to be associated with MODY. In addition, the KLF11 gene mutation is the pathogenic gene of MODY7. To date, the clinical and functional characteristics of the novel KLF11 mutation c. G31A have not yet been reported.
We report of a 30-year-old male patient with a one-year history of nonketosis-prone diabetes and a 3-generation family history of diabetes. The patient was found to carry a KLF11 gene mutation. Therefore, the clinical data of family members were collected and investigated. A total of four members of the family were found to have heterozygous mutations in the KLF11 gene c. G31A, which resulted in a change in the corresponding amino acid p.D11N. Three patients had diabetes mellitus, and one patient had impaired glucose tolerance.
The heterozygous mutation of the KLF11 gene c.G31A (p. D11N) is a new mutation site of MODY7. Subsequently, the main treatment included dietary interventions and oral drugs.
Core Tip: We describe a patient with maturity-onset diabetes of the young 7 caused by KLF11 mutation (NM_003597), where the mutation of nucleotide 31 was replaced from guanine to adenine in the coding region, and p.D11N was the mutation of amino acid 11 from aspartic acid to asparagine. Excellent blood glucose control can be achieved by using metformin.
- Citation: Zhang N, Zhao H, Li C, Zhang FZ. Novel gene mutation in maturity-onset diabetes of the young: A case report. World J Clin Cases 2023; 11(5): 1099-1105
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1099.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1099
Maturity-onset diabetes of the young (MODY) is an autosomal dominant inherited type of diabetes that usually occurs at a young age and has a family history of inheritance within three generations. MODY was first reported in 1975[1]. To date, a total of 14 genes have been found to be related to MODY (HNF4α, GCK, HNF1α, IPF1, HNF1β, NEUROD1, KLF11, CEL, PAX4, INS, BLK, ABCC8, KCNJ11, and APPL1)[2]. The pathogenic genes correspond to the MODY1-14 subtypes. MODY7 is one of the subtypes associated with mutations in the KLF11 gene.
KLF11 is a member of the Kruppel-like factor (KLF) family, which is a family of zinc finger proteins that are widely distributed in mammals. KLF11 can bind to different factors to exert different transcriptional regulation functions. In 2005, two rare KLF11 variants (Ala347Ser and Thr220Met) in an early-onset type 2 diabetes family were discovered, which significantly impaired the transcriptional activity of KLF11[3]. This effect is related to the involvement of KLF11 in the regulation of islet β-cell function. Moreover, by inhibiting insulin gene promoter activity, the insulin gene cannot be normally expressed, which reduces insulin levels. Additionally, high glucose stimulation can inhibit the function of the peroxidase promoter and reduce the ability of islet β cells to scavenge oxidative free radicals, which eventually leads to MODY7[4]. Another report identified a novel KLF11 Pro193Thr variant in a three generation family with MODY7[4]. These findings shed light on the molecular mechanisms underlying the pathogenesis of MODY7. Therefore, MODY7 is caused by mutations in the transcription factor KLF11 gene.
A new KLF11 variant was associated with early childhood-onset type 1B diabetes in 2019[5]. As such, KLF11 is a valid candidate gene to determine the genetic predisposition to early onset and type 2 diabetes, as defects in this gene may lead to early onset diabetes[6]. However, KLF11, due to its role as a MODY gene, is a potential therapeutic target for maturity-onset diabetes.
A novel mutation of the KLF11 (c. G31A) gene has been reported and analyzed in conjunction with the literature. In this study, a suspected MODY patient was found to carry a KLF11 gene mutation via gene detection methods. Therefore, the clinical characteristics and treatment of MODY7 were further investigated.
A 30-year-old male patient with a previous history of diabetes was admitted to the Liaocheng Third People's Hospital on October 7, 2021, due to polydipsia, polyuria, and weight loss being experienced for more than 1 year.
The patient experienced polydipsia, polyuria, and weight loss without obvious inducement in August 2020. The weight loss was approximately 5 kg over 3 mo. At that time, the patient reported no blurred vision, numbness of the limbs, fatigue, or discomfort. The patient’s fasting blood glucose level was 11.9 mmol/L, the postprandial glucose level was 15 mmol/L, the HbA1c levels were 9.5%, and the C-peptide level was normal, as reported in the local hospital.
The patient had no history of previous illness or diabetic ketoacidosis.
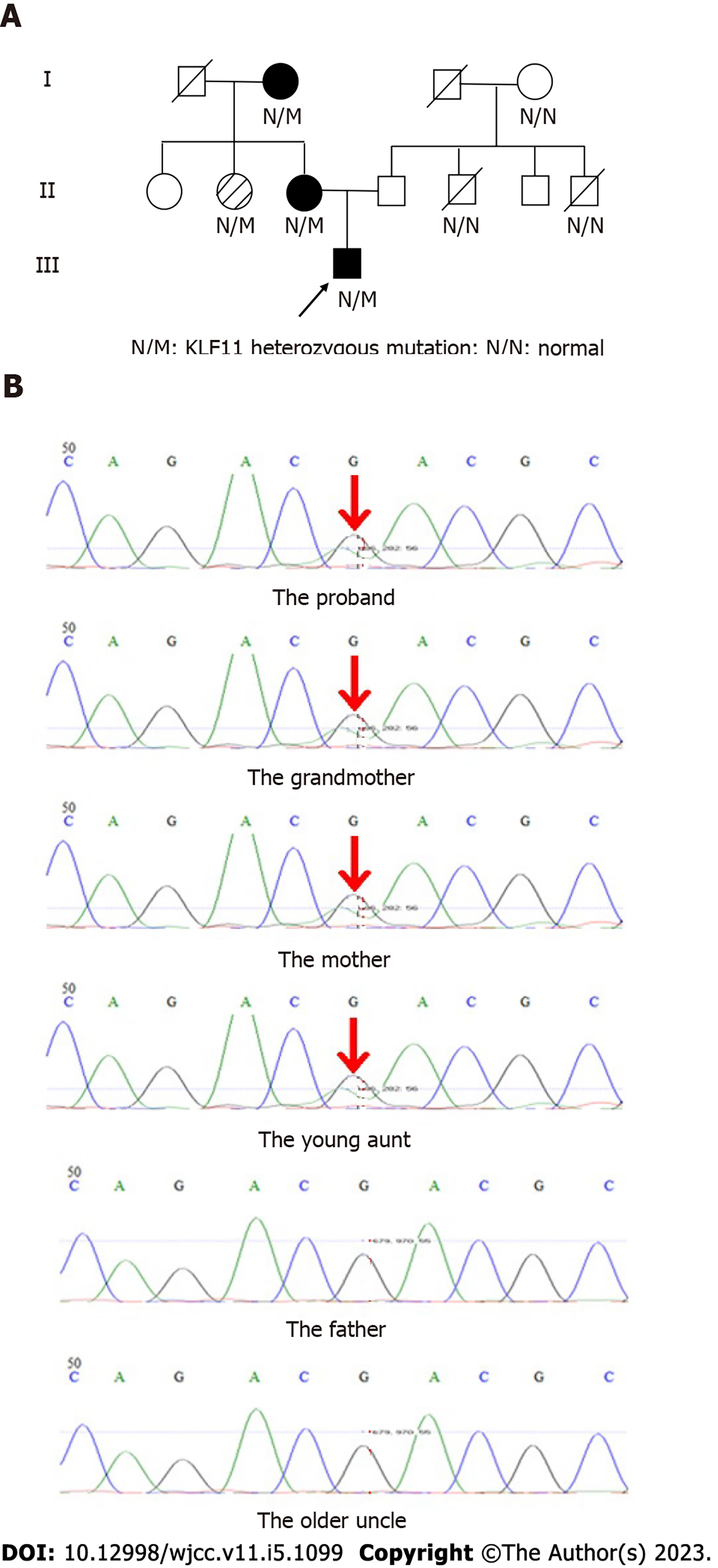
The patient was the first parturient of the first child and had a full-term natural birth. His birth weight was 3.5 kg, and his length was unknown. The growth and development of the patient were the same as those of other children of the same age. His mother had no history of special drug use during pregnancy. In addition, the patient’s mother and grandmother were diagnosed with diabetes (Figure 1A).
The patient’s height was 170 cm on physical examination; he weighed 65 kg and had a body mass index (BMI) of 22.5 kg/m2. His waist circumference and hip circumference were 74 cm and 79 cm, respectively. Moreover, the patient did not exhibit any obvious abnormalities of the thyroid, heart, lungs, or abdomen, and there was no edema in either of the lower limbs.
A steamed bread meal test was simultaneously performed with insulin and C-peptide release experiments to assess islet function (Table 1). Islet autoantibody screening demonstrated an absence of glutamic acid decarboxylase, anti-insulin cell antibodies (ICA-IgG), and insulin autoantibodies (IAAs).
| Time (min) | Glucose (mmol/L) | Insulin (μU/mL) | C-peptide (ng/mL) |
| 0 | 7.6 | 5.55 | 1.27 |
| 120 | 9.2 | 64.87 | 7.04 |
Fundus examination, electromyography, and ultrasonography of both lower limbs showed no abnormalities.
The characteristics of the patient could not exclude the presence of a unique type of diabetes. Therefore, further clinical information and genetic testing of seven people in his family were collected and conducted (Table 2). One of the individuals who had been living abroad all year round without a history of diabetes refused to be checked. This study was approved by the ethics committee of our hospital, and the consent of the patient and his family was obtained.
| Case | Age (yr) | Diagnosis age (yr) | Symptom | BMI (kg/m2)
| FBG (mmol/L) | 2hPG (mmol/L) | HbA1c (%) | Gene | TC (mmol/L) | TG (mmol/L) | LDL (mmol/L) | Hepatorenal function |
| Patient | 30 | 29 | Yes | 22.5 | 7.6 | 9.2 | 6.8 | + | 3.58 | 0.66 | 2.0 | |
| Grandmother | 79 | 59 | None | 20.8 | 7.8 | 11.5 | 7.5 | + | 5.9 | 1.9 | 3.0 | - |
| Mother | 58 | 55 | None | 24.2 | 6.3 | 9.5 | 5.8 | + | 5.2 | 2.9 | 3.8 | - |
| Young aunt | 50 | None | None | 21.6 | 5.3 | 8.1 | 6.3 | + | 5.5 | 1.8 | 2.7 | - |
| Father | 59 | None | None | 22.6 | 5.4 | 7.2 | 5.9 | - | 4.9 | 1.9 | 2.5 | - |
| Older uncle | 63 | None | None | 28.9 | 5.1 | 7.4 | 6.0 | - | 5.1 | 2.1 | 2.7 | - |
Seven persons of the family were sampled with a mouth swab. A panel of 14 MODY genes was sequenced by using high-throughput DNA sequencing. The detection region included the exon region of 14 genes related to MODY, and the minimum coverage was 30×. Genetic testing of the patient, mother, grandmother, and young aunt showed that KLF11 (NM_003597, c. G31A, p. D11N mutation) was positive (Figure 1B), as well as the fact that c. G31A was the mutation of nucleotide 31 from guanine to adenine in the coding region, and p. D11N was the mutation of amino acid 11 from aspartic acid to asparagine. The genetic testing results supported a diagnosis of MODY7. The sequences of the other 13 MODY genes are normal.
The patient was initially treated with metformin and diabetes pills, and his blood glucose was controlled within 1 wk. Studies have reported that patients with MODY7 can be treated with metformin and rosiglitazone[7]. Subsequently, treatment was changed to metformin and dietary interventions. The level of blood glucose was controlled and stable. The patient’s mother and grandmother were also treated with metformin.
Half a year later, the patient's glycemic control was excellent (fasting blood glucose: 5.1 mmol/L; Hb1Ac level: 6%).
According to the 2019 World Health Organization classification of diabetes[8], monogenic diabetes is a special type of diabetes caused by a single gene mutation, which is divided into two categories: β-cell function defects and insulin action defects, which accounts for 1% to 5% of the total number of diabetes cases. Among them, MODY is the most common type of monogenic β-cell function defect. It accounts for 2% to 15% of young diabetic patients[9]. At present, the following diagnostic criteria of MODY proposed by Ellard et al[10] are mostly used: (1) Autosomal dominant inheritance of diabetes mellitus for more than three consecutive generations; (2) At least one patient in the family having the disease before the age of 25-year-old; (3) Insulin therapy was not required for 5 years after diagnosis; and (4) Combined with islet β cell dysfunction. The criteria for the diagnosis of high specificity, the low sensitivity, the clinical diagnosis of difficult MODY, and fewer people (in strict accordance with the criteria for the diagnosis of MODY patients) were missed diagnoses; additionally, foreign studies will require MODY diagnoses to be extended beyond the standard and the diagnosis of patients with MODY, also pointed out that 30 years before the diagnosis of diabetes, patients require molecular genetic monitoring[11]. The onset age of MODY varies widely, and it can occur in different stages from childhood to early adulthood. Different subtypes have different clinical characteristics and are easily diagnosed as being type 1 or type 2 diabetes, thus resulting in a missed diagnosis. British studies have shown that some MODY patients have been diagnosed for an average of 13 years from the diagnosis of diabetes to the final diagnosis of MODY[12]. As a result, inappropriate treatment with insulin and/or insulin sensitizers for MODY patients can occur over a long time period. Therefore, genetic testing is particularly important for the diagnosis and classification of MODY. According to the screening pathway in the expert consensus on the screening, diagnosis, and treatment of MODY [13], when the patient's BMI is ≤ 24 kg/m2, the age is between 25- and 45-year-old, there is a family history of more than 3 generations, no metabolic syndrome, and a negative islet autoantibodies, genetic testing should be performed to diagnose MODY.
A study found that the KLF11 gene in islet β cells is a PDX-1 transcriptional regulator dependent on P300, which promotes and maintains insulin synthesis and plays an important role in the normal development of the pancreas and the maintenance of islet β cell function, which is the core mechanism of MODY7 occurrence[14]. In 2012, Lomberk et al[15] found that mutations in the KLF11 gene cause MODY7 and neonatal diabetes; in this study, the A347S gene variant was found in MODY7 patients, which disrupts KLF11-mediated increases in basal insulin levels and promoter activity and attenuates glucose-stimulated insulin secretion. This mechanism contributes to our understanding of the complex gene regulation in MODY. Moreover, Wu et al[16] document a novel heterozygous KLF11 variant (p. Pro349Ser) as a potential monogenic mutation associated with MODY7 in a family. This variant impairs insulin secretion from pancreatic beta cells, possibly by repressing insulin promoter regulation activity. Therefore, KLF11, as a transcription factor that is widely expressed in a variety of tissues in vivo, regulates blood glucose homeostasis by a very complex mechanism. It not only directly regulates insulin gene expression, but it also interacts with different target genes to jointly regulate the level of glucose metabolism in the body, thus ultimately leading to the occurrence of MODY7.
Recently, a novel KLF11 (c.1061G>T) mutation associated with MODY7 has been reported for the first time, which impairs the regulatory activity of the insulin promoter and impairs insulin expression and secretion in islet β cells. Moreover, in the study of the genetic factors that can also lead to MODY propositus, the mother and grandfather were carrying disease-causing genes, the grandfather had a history of diabetes, and the mother had no history of diabetes. Therefore, even in the same family, the clinical features and differences caused by the same mutation are associated with incomplete penetrance KLF11 mutations. In addition, it was confirmed that KLF11 (c.1061G>T) is associated with diabetes in this family[17]. In another study, the clinical characteristics of MODY subjects were reported. These subjects included those patients without a family history of diabetes, which was not a diagnostic feature of MODY. Even without a typical family history, the diagnosis of the patient was confirmed as MODY[11]. In this paper, the patient's mother, second aunt, and maternal grandmother had the same gene mutation, thus confirming the characteristics of dominant inheritance of the gene mutation. The patient's mother and maternal grandmother had a history of diabetes, and the young aunt had a history of impaired glucose tolerance, thus indicating that the clinical characteristics caused by the same mutation were different.
The pathogenic genes and clinical characteristics of different MODY subtypes are different, and the treatment is not the same. Due to the small number of patients with MODY7 and the lack of treatment research data, there is currently no clear treatment plan. Most scholars administer oral sulfonylureas for MODY7[18,19], and the effect is generally ideal. Some studies have also indicated that insulin is used in the initial stage of MODY7 treatment[3,8], and sulfonylureas, including oral hypoglycemic drugs, can also be used for metformin and rosiglitazone[3]. In addition, studies have found that oral hypoglycemic drugs and dietary interventions are beneficial for MODY7 patients, and the control of the intake of staple food carbohydrates helps to control blood glucose[17]. The patient also confirmed that the treatment was effective. Sulfonylureas and metformin were used in the initial stage, and hypoglycemic drugs were discontinued in the later stage.
The number of patients with MODY is small, especially regarding MODY7, which rarely occurs. The clinical characteristics of MODY are different, and the diagnosis is mainly based on clinical characteristics; therefore, some patients with MODY are missed and not diagnosed. It is of great significance for the precise treatment, prognosis, and genetic counseling of monogenic diabetes. The KLF11 (c. G31A) mutation in this patient expand the genotype and clinical spectrum of MODY7, but more data are needed to provide a basis for the study of MODY7 in the future.
The work was supported by Professor Zhou KX and his team from the Big Data Center of Institute of Biophysics at the University of Chinese Academy of Sciences, and we thank them for their help in the genetic testing.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lang F, United States; Stefanaki C, Greece S-Editor: Gao CC L-Editor: A P-Editor: Gao CC
| 1. | Tattersall RB, Fajans SS. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes. 1975;24:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 196] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Tallapragada DS, Bhaskar S, Chandak GR. New insights from monogenic diabetes for "common" type 2 diabetes. Front Genet. 2015;6:251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Neve B, Fernandez-Zapico ME, Ashkenazi-Katalan V, Dina C, Hamid YH, Joly E, Vaillant E, Benmezroua Y, Durand E, Bakaher N, Delannoy V, Vaxillaire M, Cook T, Dallinga-Thie GM, Jansen H, Charles MA, Clément K, Galan P, Hercberg S, Helbecque N, Charpentier G, Prentki M, Hansen T, Pedersen O, Urrutia R, Melloul D, Froguel P. Role of transcription factor KLF11 and its diabetes-associated gene variants in pancreatic beta cell function. Proc Natl Acad Sci U S A. 2005;102:4807-4812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 4. | Guan G, Qin T, Zhao LL, Jin P. Genetic and Functional Analyses of the Novel KLF11 Pro193Thr Variant in a Three-Generation Family with MODY7. Horm Metab Res. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Ushijima K, Narumi S, Ogata T, Yokota I, Sugihara S, Kaname T, Horikawa Y, Matsubara Y, Fukami M, Kawamura T; Japanese Study Group of Insulin Therapy for Childhood and Adolescent Diabetes. KLF11 variant in a family clinically diagnosed with early childhood-onset type 1B diabetes. Pediatr Diabetes. 2019;20:712-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Matyka KA, Beards F, Appleton M, Ellard S, Hattersley A, Dunger DB. Genetic testing for maturity onset diabetes of the young in childhood hyperglycaemia. Arch Dis Child. 1998;78:552-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Zhang QY, Li JY, Zhang YZ. The Prevalence and Precision Medicine of Maturity Onset Diabetes of the Young. Her Med. 2022;41:86-91. |
| 8. | World Health Organization. Classification of diabetes mellitus 2019. [cited 2 Sep 2022]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/325182/9789241515702-eng.pdf. |
| 9. | Zhang H, Colclough K, Gloyn AL, Pollin TI. Monogenic diabetes: a gateway to precision medicine in diabetes. J Clin Invest. 2021;131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (1)] |
| 10. | Ellard S, Bellanné-Chantelot C, Hattersley AT; European Molecular Genetics Quality Network (EMQN) MODY group. Best practice guidelines for the molecular genetic diagnosis of maturity-onset diabetes of the young. Diabetologia. 2008;51:546-553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 323] [Cited by in RCA: 282] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 11. | Thanabalasingham G, Pal A, Selwood MP, Dudley C, Fisher K, Bingley PJ, Ellard S, Farmer AJ, McCarthy MI, Owen KR. Systematic assessment of etiology in adults with a clinical diagnosis of young-onset type 2 diabetes is a successful strategy for identifying maturity-onset diabetes of the young. Diabetes Care. 2012;35:1206-1212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 12. | Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia. 2010;53:2504-2508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 13. | Xu Y, Hu C, Yang T, Shi LX, Liu M, Hong TP, Zhou ZG, Wong JP, Ji LN, Zhu DL, Xu T, Li XY. Expert consensus on screening and treatment of maturity onset diabetes of the young. Zhonghua Tangniaobing Zazhi. 2022;14:423-432. |
| 14. | Fernandez-Zapico ME, van Velkinburgh JC, Gutiérrez-Aguilar R, Neve B, Froguel P, Urrutia R, Stein R. MODY7 gene, KLF11, is a novel p300-dependent regulator of Pdx-1 (MODY4) transcription in pancreatic islet beta cells. J Biol Chem. 2009;284:36482-36490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Lomberk G, Grzenda A, Mathison A, Escande C, Zhang JS, Calvo E, Miller LJ, Iovanna J, Chini EN, Fernandez-Zapico ME, Urrutia R. Krüppel-like factor 11 regulates the expression of metabolic genes via an evolutionarily conserved protein interaction domain functionally disrupted in maturity onset diabetes of the young. J Biol Chem. 2013;288:17745-17758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Wu S, Zhang G, Liu L, Wu W, Luo X. A novel KLF11 variant in a family with maturity-onset diabetes of the young. Pediatr Diabetes. 2022;23:597-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Sun Y, Qu J, Wang J, Zhao R, Wang C, Chen L, Hou X. Clinical and Functional Characteristics of a Novel KLF11 Cys354Phe Variant Involved in Maturity-Onset Diabetes of the Young. J Diabetes Res. 2021;2021:7136869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Jang KM. Maturity-onset diabetes of the young: update and perspectives on diagnosis and treatment. Yeungnam Univ J Med. 2020;37:13-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Urakami T. Maturity-onset diabetes of the young (MODY): current perspectives on diagnosis and treatment. Diabetes Metab Syndr Obes. 2019;12:1047-1056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |