Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1077
Peer-review started: September 20, 2022
First decision: November 30, 2022
Revised: December 12, 2022
Accepted: January 19, 2023
Article in press: January 19, 2023
Published online: February 16, 2023
Processing time: 147 Days and 0 Hours
Maple syrup urine disease (MSUD) is an autosomal recessive genetic disorder caused by defects in the catabolism of the branched-chain amino acids (BCAAs). However, the clinical and metabolic screening is limited in identifying all MSUD patients, especially those patients with mild phenotypes or are asymptomatic. This study aims to share the diagnostic experience of an intermediate MSUD case who was missed by metabolic profiling but identified by genetic analysis.
This study reports the diagnostic process of a boy with intermediate MSUD. The proband presented with psychomotor retardation and cerebral lesions on magnetic resonance imaging scans at 8 mo of age. Preliminary clinical and metabolic profiling did not support a specific disease. However, whole exome sequencing and subsequent Sanger sequencing at 1 year and 7 mo of age identified bi-allelic pathogenic variants of the BCKDHB gene, confirming the proband as having MSUD with non-classic mild phenotypes. His clinical and laboratory data were retrospectively analyzed. According to his disease course, he was classified into an intermediate form of MSUD. His management was then changed to BCAAs restriction and metabolic monitoring conforming to MSUD. In addition, genetic counseling and prenatal diagnosis were provided to his parents.
Our work provides diagnostic experience of an intermediate MSUD case, suggesting that a genetic analysis is important for ambiguous cases, and alerts clinicians to avoid missing patients with non-classic mild phenotypes of MSUD.
Core Tip: Clinical and metabolic screening is limited in identifying all patients with maple syrup urine disease (MSUD), especially those patients with mild phenotypes or are asymptomatic. Here we present the diagnostic process of an intermediate MSUD case with a mild phenotype, who was missed by metabolic profiling but identified by genetic analysis. This case suggests that genetic testing could contribute to the identification of non-classic mild MSUD cases and alerts clinicians to avoid missing these patients.
- Citation: Lin YT, Cai YN, Ting TH, Liu L, Zeng CH, Su L, Peng MZ, Li XZ. Diagnosis of an intermediate case of maple syrup urine disease: A case report. World J Clin Cases 2023; 11(5): 1077-1085
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1077.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1077
Maple syrup urine disease (MSUD), named for a maple syrup odor in urine, is a rare inborn error of metabolism, with an estimated prevalence of 1/185000 live births[1]. Also known as branched-chain α-ketoacid dehydrogenase (BCKD) deficiency, MSUD is caused by defects of the mitochondrial BCKD complex which leads to disruption in the catabolism of the branched-chain amino acids (BCAAs), namely leucine, isoleucine, and valine. To date, five different genes, BCKDHA, BCKDHB, DBT, DLD, and PPM1K, have been identified as genetic causes of MSUD, all of them having an autosomal recessive inheritance pattern[2-6].
MSUD is characterized by clinical manifestations of neurological impairments, feeding problems and metabolic decompensation, and metabolic profile of elevated BCAAs in the plasma and elevated branched-chain ketoacids (BCKAs) in the urine. According to the defective component of the BCKD complex and the severity of the phenotypes, MSUD can be divided into five forms, namely classic, intermediate, intermittent, thiamine-responsive, and E3-deficient[1,7].
Generally, MSUD is diagnosed by the presence of clinical features accompanied by characteristic plasma and urine metabolic profiles, and further confirmed by a genetic analysis. The management is comprised of BCAAs restriction and metabolic monitoring, which typically yields good outcomes. Moreover, domino liver transplantation provides an effective strategy to achieve a normal dietary intake[1,8-10].
Early diagnosis and treatment is important for MSUD as acute metabolic crisis is life-threatening. However, the clinical and metabolic screening is limited in identifying all MSUD patients. Those mild cases under the BCAAs criteria to be considered presumptive positive for MSUD are probably missed, as well as intermittent patients with normal development and BCAAs levels when asymptomatic[11-14]. Thus, a supplementary genetic testing could contribute to the identification of ambiguous cases.
To share the diagnostic experience and alert clinicians to avoid missing of diagnosis, here we present an intermediate case of MSUD who was missed by metabolic profiling but identified by genetic analysis. Genetic counseling and prenatal diagnosis were further provided to the family.
The proband is a boy aged 1 year and 7 mo with psychomotor retardation.
The proband presented to our clinic with psychomotor retardation at 8 mo of age as he was unable to sit. He achieved head control at 4 mo of age. Subsequently he began to sit at 11 mo of age, and was able to stand and walk independently at 1 year and 5 mo of age, but had a tendency to fall. He started babbling at 1 year and 5 mo of age, and was unable to say a word or recognize his parents at 1 year and 7 mo of age.
He had no past admission or known medical problems. His development was normal in the first 3 mo after birth.
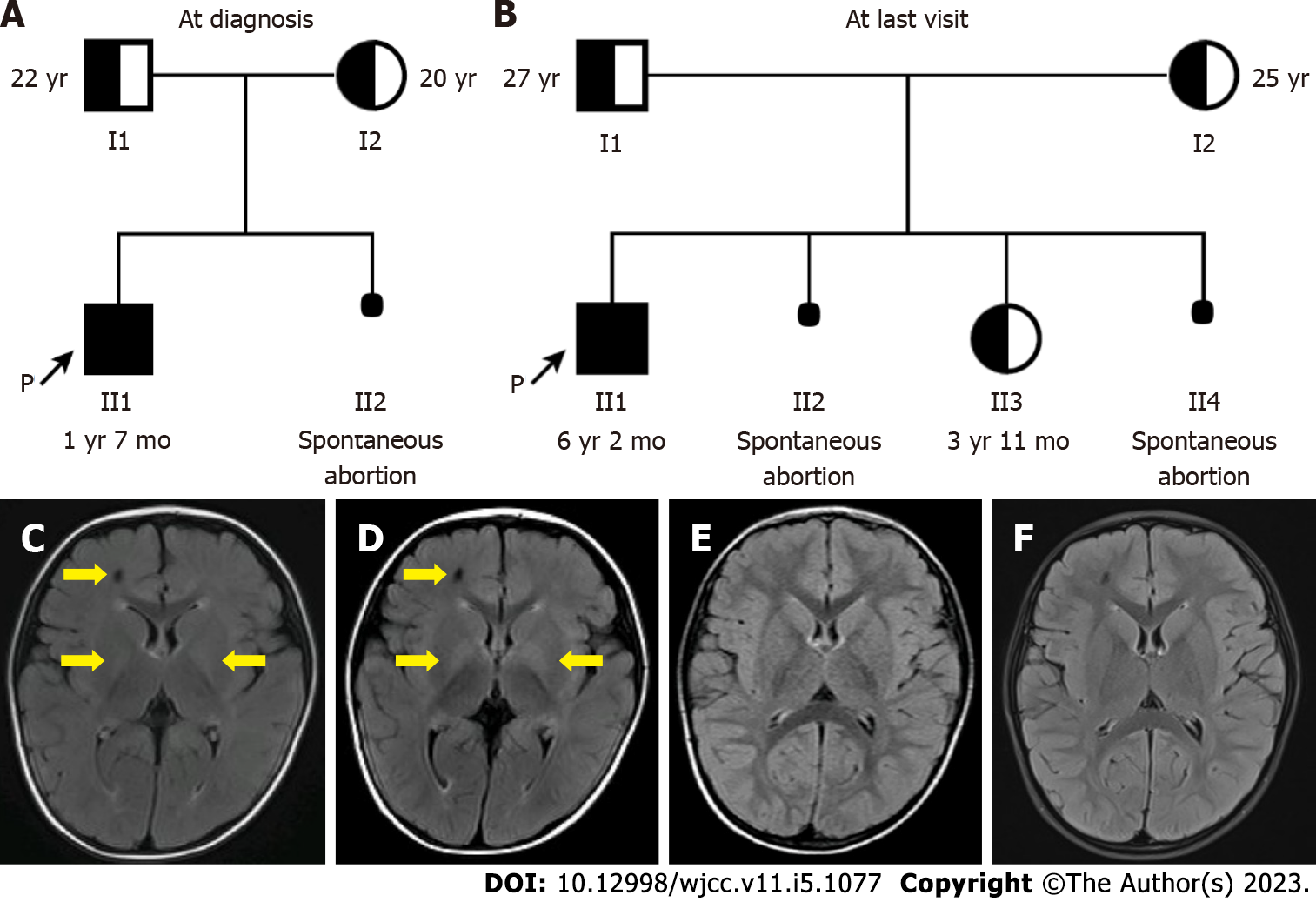
The family was of southern Chinese and Han ethnicity. The proband was the first child of the healthy parents (Figure 1A). He was delivered naturally at term with no perinatal issues. The pregnancy was normal and the neonatal period was uneventful. His parents were non-consanguineous, and there was no family history of psychomotor retardation or metabolic diseases. Spontaneous abortion of the second child of the parents occurred at 2 mo of pregnancy (Figure 1A).
The proband’s weight and height were normal at presentation (Table 1)[15]. He didn’t show facial dysmorphism. He had mild hypotonia without any pathological reflex. No obvious abnormalities were shown in his skeleton and viscera.
| Age | Height | Weight | ||
| cm | SD | kg | SD | |
| 8 mo | 70.0 | -0.5 | 8.8 | -0.5 |
| 1 yr 1 mo | 77.0 | 0.0 | 9.4 | -0.8 |
| 1 yr 7 mo | 82.5 | 0.2 | 11.6 | 0.1 |
| 1 yr 9 mo | 84.0 | -0.5 | 11.7 | -0.4 |
| 2 yr 5 mo | 92.0 | -0.7 | 12.4 | -0.4 |
| 3 yr 5 mo | 100.0 | 0.0 | 13.5 | -1.0 |
| 5 yr | 108.5 | -0.7 | 16.5 | -1.2 |
| 5 yr 8 mo | 113.7 | -0.5 | 17.6 | -1.3 |
| 6 yr 2 mo | 117.4 | -0.3 | 17.5 | -1.7 |
Routine biochemical test: Routine biochemical tests at 8 mo of age showed normal liver and renal function, blood glucose, blood ammonia, blood gas, and blood electrolyte (data not shown).
Enzymatic assay: The activities of sphingolipidoses-relevant enzymes at 8 mo of age were normal (data not shown).
Metabolic analysis: Quantitative plasma amino acid profiling by high-performance liquid chromatography and semi-quantitative urine organic acid analysis by gas chromatography-mass spectrometry were performed as described previously[16,17].
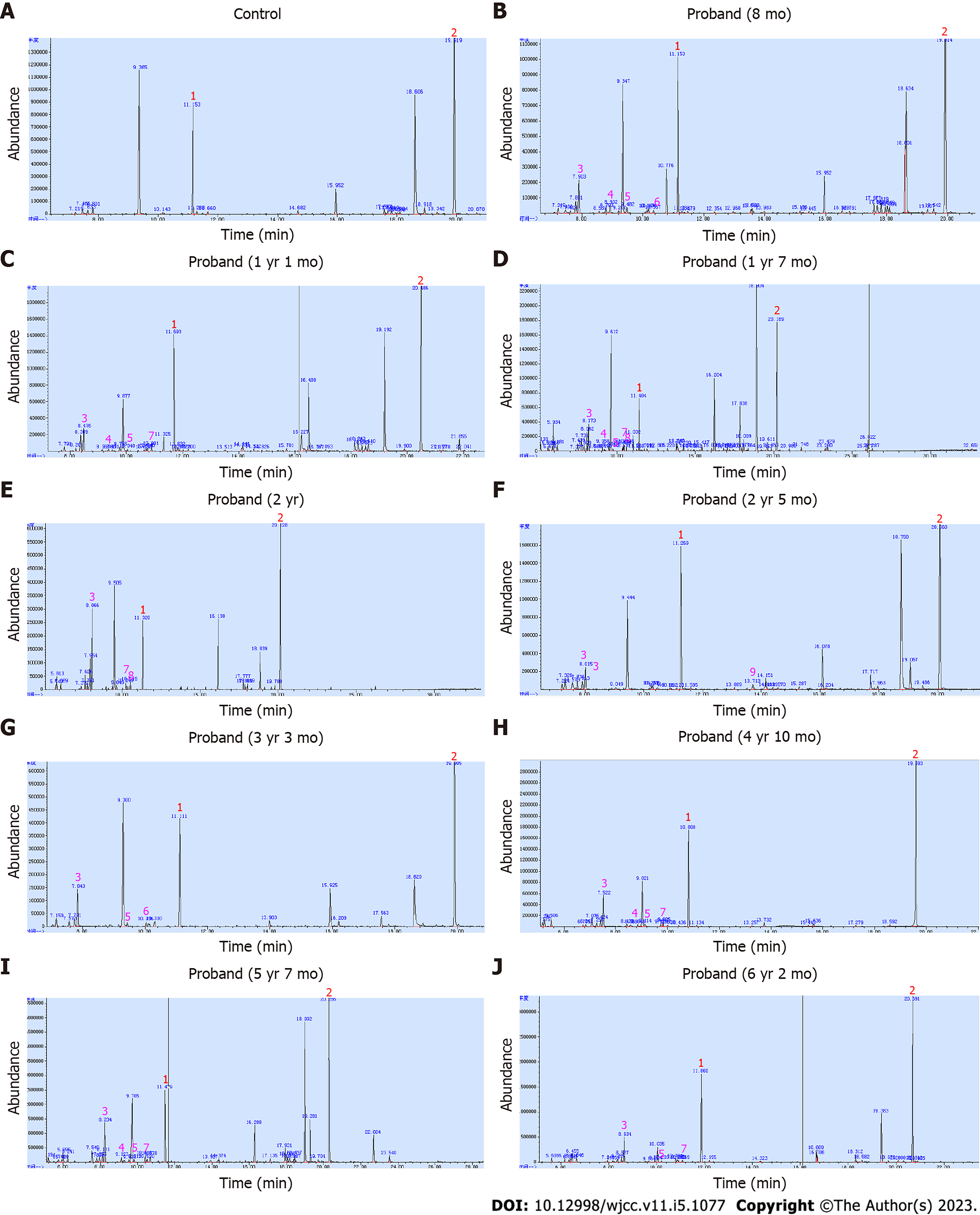
Plasma amino acid profiling at 8 mo of age showed elevations of leucine, isoleucine, valine, and leucine/alanine ratio (Table 2), which met the specific amino acid spectrum of MSUD[11]. However, no maple syrup odor was noticed in his urine. Urine organic acid analysis revealed a small amount of 2-OH-isovaleric acid (Figure 2B).
| Amino acid | Leucine (μmol/L) | Isoleucine (μmol/L) | Valine (μmol/L) | Alanine (μmol/L) | Leucine/alanine ratio |
| Reference range | 4.0-197.0 | 24.0-118.0 | 99.0-333.0 | 210.0-545.0 | |
| 8 mo | 468.6↑ | 258.8↑ | 574.2↑ | 229.0 | 2.12 |
| 1 yr 1 mo | 184.1 | 92.8 | 292.7 | 208.5↓ | 0.88 |
| 1 yr 7 mo | 327.0↑ | 165.8↑ | 460.7↑ | 252.3 | 1.29 |
| 2 yr | 258.3↑ | 199.4↑ | 406.8↑ | 150.1↓ | 1.72 |
| 2 yr 5 mo | 201.2↑ | 92.7 | 274.0 | 211.2 | 0.95 |
| 3 yr 3 mo | 288.1↑ | 157.4↑ | 311.9↑ | 186.0↓ | 1.55 |
| 4 yr 10 mo | 222.0↑ | 124.5↑ | 270.2 | 266.4 | 0.83 |
| 5 yr 7 mo | 258.5↑ | 156.1↑ | 295.3 | 171.2↓ | 1.51 |
| 6 yr 2 mo | 244.0↑ | 130.1↑ | 431.3↑ | 280.0 | 0.87 |
To exclude the impact of dietary factors and further define if this boy had MSUD, he had repeated sampling and analysis of plasma amino acids and urine organic acids. On the second examination at 1 year and 1 mo of age, the concentrations of plasma BCAAs returned to normal (Table 2), while the peak of urine 2-OH-isovaleric acid became lower (Figure 2C), which did not support the possible diagnosis of MSUD. The proband was then categorized as unexplained psychomotor retardation.
Genetic analysis: gDNA was extracted from whole blood samples using DNeasy Blood & Tissue Kit (QIAGEN). Whole exome sequencing (WES) was performed as described previously[18]. For Sanger sequencing, the exon sequences together with adjoining intron boundaries of the BCKDHB gene (NG_009775.2, NM_183050.3) were amplified and sequenced with an ABI 3730xl DNA Analyzer. The SNP databases were employed to exclude the polymorphic alleles, while HGMD (Professional 2022.3) and ClinVar were referenced for the confirmation of known pathogenic variants.
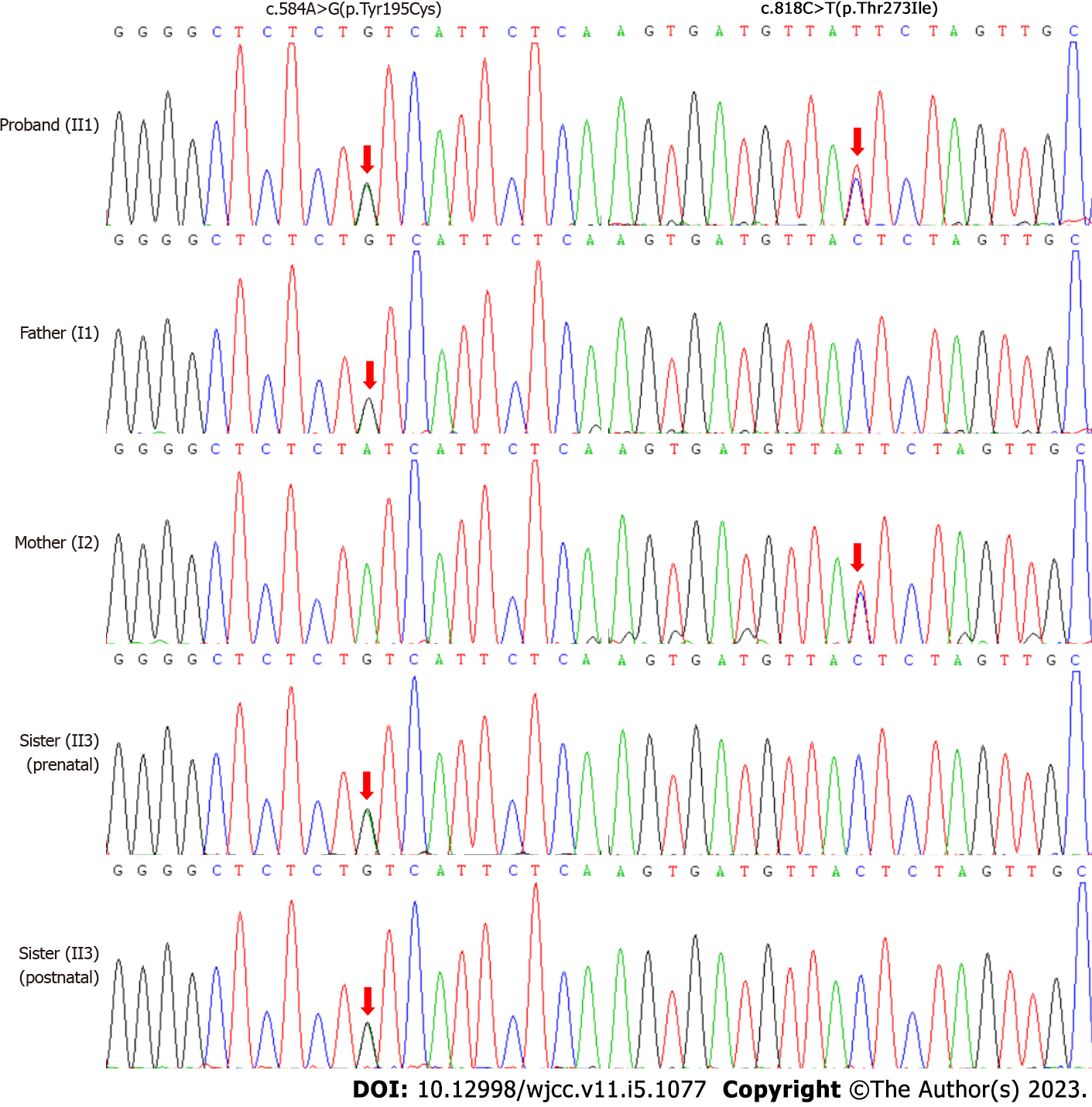
To identify the underlying genetic cause, WES of the proband-parents trio was performed when the proband was at age of 1 year and 7 mo. To our surprise, a compound heterozygous c.584A>G(p.T
He had brain magnetic resonance imaging (MRI) performed by another hospital at 8 mo of age (data not shown) and our hospital at 1 year and 3 mo of age (Figure 1C) and 1 year and 7 mo of age (Figure 1D), showing abnormal signal in the right frontal lobe, and increased T2/FLAIR signals symmetrically in the basal ganglia and trigone of lateral ventricles.
The proband’s semi-quantitative urine organic acid results were re-analyzed. In addition to a small amount of 2-OH-isovaleric acid, very small amounts of 3-OH-isovaleric acid, 2-OH-isocaproic acid, 2-keto-isocaproic acid, and 2-keto-3-methylvaleric acid were noted (Figure 2B and C). These small fluctuations were missed previously. Thus, a characteristic urine organic acid spectrum of MSUD was concluded, supporting the molecular findings.
After repeated questioning, the parents recalled that the proband once vomited after eating meat and he tended to refuse eating meat since then, but could tolerate milk. There was no severe acute illness that required hospitalization. They also concealed the medical history that the proband was supplemented with trivitamins B tablets (containing vitamin B1, B6, and B12) at another hospital between the first two analyses of plasma amino acids and urine organic acids, which directly interfered with the levels of BCAAs.
Based on the clinical phenotype, metabolic spectrum and molecular findings, the proband was confirmed to have MSUD with mild phenotypes at 1 year and 7 mo of age. According to his disease course, he was classified as an intermediate form of MSUD. Moreover, he was responsive to thiamine (vitamin B1) as trivitamins B tablets supplement changed his plasma BCAAs from mild elevation into normal level.
The proband’s management was then modified to BCAAs restriction and thiamine supplementation in conjunction with metabolic monitoring of plasma BCAAs and urine BCKAs.
During the follow-up until 6 years and 2 mo of age, the proband’s height and weight were persistently normal (Table 1). He could walk and run stably but slowly with mild hypotonia. His cognition and language were delayed for about 1 year compared with healthy peers, but his parents refused an intelligence test. No seizure was observed. Moreover, there was still no maple syrup odor in his urine. His plasma BCAAs and urine BCKAs levels were decreased in comparison with the first examination before treatment, but fluctuated (Table 2 and Figure 2). His brain MRI at 3 years and 5 mo of age (Figure 1E) and 5 years of age (Figure 1F) showed a substantial reduction of the cerebral lesions.
As the proband was diagnosed with MSUD and both of the parents carried a pathogenic heterozygous variant of the BCKDHB gene, a subsequent child of the parents has a recurrence risk of 25% to have MSUD. Prenatal diagnosis is offered to the parents to provide early diagnosis, which allows optimal management early at birth if the fetus is affected with MSUD. Preimplantation diagnosis is an alternative strategy to block the transmission of pathogenic variants. Moreover, members of the parental families can be offered screening genetic testing to identify potential carriers.
Prenatal diagnosis was provided for the mother’s third pregnancy (Figure 1B). The chorionic villi biopsy tissues were obtained at 13 wk of gestational age. Sanger sequencing showed that the fetus carried the c.584A>G(p.Tyr195Cys) heterozygous variant transmitted from the father, while the c.818C>T(p.Thr273Ile) variant of the mother was not delivered to her (Figure 3). Thus, the fetus was defined as a carrier. The postnatal genetic testing confirmed this result (Figure 3). Her liver and renal function, and levels of plasma amino acids and urine organic acids were normal at 2 mo of age (data not shown). On follow-up until 3 years and 11 mo of age, she remained healthy with normal mental and motor development.
In addition, spontaneous abortion of the fourth child of the parents occurred at 11 wk of pregnancy before the prenatal diagnosis appointment (Figure 1B). Unfortunately, abortus samples were not retained for further genetic testing.
In MSUD, the classic form with the most severe phenotype presenting early in the 1st week of life accounts for most cases, while about 20% of cases with non-specific symptoms and varying clinical severity are classified into non-classic variant forms, including intermediate, intermittent, thiamine-responsive, and E3-deficient[7,13]. The classic cases with significantly elevated plasma BCAAs and urine BCKAs are easy to be detected by specific metabolic screening, while non-classic variant cases might be missed sometimes, especially when they are asymptomatic or have normal plasma BCAAs and urine BCKAs[11-14,20].
In this work, we present the diagnostic process of an intermediate MSUD patient with a mild phenotype, who was missed by preliminary metabolic profiling but was later identified by genetic testing.
In retrospect, the proband could have been diagnosed during his first visit at 8 mo of age, because the first plasma amino acid profiling showed specific spectrum of MSUD. However, because the elevation of BCAAs was not as significantly high as in the classic form[20], a verification analysis was required to exclude the impact of dietary factors. The subsequent verification analysis did not support the diagnosis of MSUD.
At that time, the parents did not provide adequate information for our inquiry as they were both young and inexperienced parents. They were also not well educated. They thought the history of meat refusal was not important as they believed it was usual and normal for young children to have dietary preferences. They also did not report the use of vitamin B supplement initially as they thought vitamins were routinely used for general health care of infants and children.
In China, trivitamins B tablets, levocarnitine (L-carnitine), and coenzyme Q10 tablets with minimal side-effects are often prescribed to children with psychomotor retardation. These supplements are perceived by parents to improve the psychomotor retardation to some extent. However, these supplements may interfere with the results of biochemical testing for metabolic diseases and hence hampers the prompt diagnosis of a specific metabolic disease.
Moreover, the urine organic acid analysis is semi-quantitative in our laboratory, which limits the detection sensitivity and efficiency. Very low peaks on the chromatograms were missed sometimes. In contrast, a quantitative urine organic acid analysis is more sensitive to improve diagnostic efficiency and accuracy.
The above-mentioned reasons led to the missing of MSUD diagnosis in this case based on laboratory metabolic indicators. Fortunately, the case was identified by WES although delayed. As the proband’s unexplained psychomotor retardation was not a specific clinical feature of a metabolic disorder, it is difficult to determine a specific disease-causing gene to look for in genetic testing. Since traditional Sanger sequencing targeting a specific gene was unavailable, WES, one of the next-generation sequencing (NGS) techniques, was employed as an alternative. Currently, NGS has been widely used in molecular diagnostic testing, especially in unexplained disorders, because it enables screening for a large amount of candidate genes rapidly in one test[21,22].
In fact, the diagnosis of MSUD in the proband could have been confirmed much earlier if we had implemented genetic testing rather than re-examination of metabolites, when he was suspected to suffer from MSUD. Either Sanger sequencing or targeted NGS involving the five causative genes of MSUD could have confirmed his diagnosis earlier. An earlier definitive diagnosis of MSUD would be beneficial by enabling genetic counseling and prenatal diagnosis for the family.
Generally, a metabolic disease is diagnosed by a characteristic metabolic profile followed by a confirmatory genetic analysis. Early detection and intervention is important for favorable prognosis. However, for some cases with mild non-classic or normal phenotypes, metabolic profiling may fail to detect the disease, and they could only be identified when a metabolic crisis episode occurs, which is usually life-threatening.
In two previously reported intermittent patients of MSUD, maple syrup urine odor was noted during the episode of vomiting and diarrhea or high fever[13,14]. In our case, maple syrup odor was never present in his urine. The diagnosis of our case was achieved by a genetic analysis before acute metabolic crisis occurred, underlining the importance of genetic testing in early diagnosis of suspicious cases.
Our work adds to the current knowledge of the disease course of intermediate form of MSUD. Our experience on this intermediate MSUD case suggests that a genetic analysis is important for ambiguous cases suspected by metabolic profiling. Our study also alerts clinicians to be cautious of variable clinical phenotypes, metabolic spectrum and medical history to avoid the missing of the diagnosis in patients with non-classic mild phenotypes of MSUD.
The authors would like to thank the enrolled family for participation in this study. We also thank the medical imaging center and clinical laboratory center at Guangzhou Women and Children’s Medical Center for assistance with brain MRI and routine biochemical tests.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Genetics and heredity
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghimire R, Nepal; Lambrecht NW, United States; Samadder S, India S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang H
| 1. | Blackburn PR, Gass JM, Vairo FPE, Farnham KM, Atwal HK, Macklin S, Klee EW, Atwal PS. Maple syrup urine disease: mechanisms and management. Appl Clin Genet. 2017;10:57-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 2. | Chuang JL, Fisher CR, Cox RP, Chuang DT. Molecular basis of maple syrup urine disease: novel mutations at the E1 alpha locus that impair E1(alpha 2 beta 2) assembly or decrease steady-state E1 alpha mRNA levels of branched-chain alpha-keto acid dehydrogenase complex. Am J Hum Genet. 1994;55:297-304. [PubMed] |
| 3. | Edelmann L, Wasserstein MP, Kornreich R, Sansaricq C, Snyderman SE, Diaz GA. Maple syrup urine disease: identification and carrier-frequency determination of a novel founder mutation in the Ashkenazi Jewish population. Am J Hum Genet. 2001;69:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 4. | Fisher CW, Fisher CR, Chuang JL, Lau KS, Chuang DT, Cox RP. Occurrence of a 2-bp (AT) deletion allele and a nonsense (G-to-T) mutant allele at the E2 (DBT) locus of six patients with maple syrup urine disease: multiple-exon skipping as a secondary effect of the mutations. Am J Hum Genet. 1993;52:414-424. [PubMed] |
| 5. | Odièvre MH, Chretien D, Munnich A, Robinson BH, Dumoulin R, Masmoudi S, Kadhom N, Rötig A, Rustin P, Bonnefont JP. A novel mutation in the dihydrolipoamide dehydrogenase E3 subunit gene (DLD) resulting in an atypical form of alpha-ketoglutarate dehydrogenase deficiency. Hum Mutat. 2005;25:323-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Oyarzabal A, Martínez-Pardo M, Merinero B, Navarrete R, Desviat LR, Ugarte M, Rodríguez-Pombo P. A novel regulatory defect in the branched-chain α-keto acid dehydrogenase complex due to a mutation in the PPM1K gene causes a mild variant phenotype of maple syrup urine disease. Hum Mutat. 2013;34:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Simon E, Flaschker N, Schadewaldt P, Langenbeck U, Wendel U. Variant maple syrup urine disease (MSUD)--the entire spectrum. J Inherit Metab Dis. 2006;29:716-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Strauss KA, Carson VJ, Soltys K, Young ME, Bowser LE, Puffenberger EG, Brigatti KW, Williams KB, Robinson DL, Hendrickson C, Beiler K, Taylor CM, Haas-Givler B, Chopko S, Hailey J, Muelly ER, Shellmer DA, Radcliff Z, Rodrigues A, Loeven K, Heaps AD, Mazariegos GV, Morton DH. Branched-chain α-ketoacid dehydrogenase deficiency (maple syrup urine disease): Treatment, biomarkers, and outcomes. Mol Genet Metab. 2020;129:193-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Burrage LC, Nagamani SC, Campeau PM, Lee BH. Branched-chain amino acid metabolism: from rare Mendelian diseases to more common disorders. Hum Mol Genet. 2014;23:R1-R8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Spada M, Angelico R, Dionisi-Vici C. Maple Syrup Urine Disease and Domino Liver Transplantation: When and How? Liver Transpl. 2019;25:827-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Puckett RL, Lorey F, Rinaldo P, Lipson MH, Matern D, Sowa ME, Levine S, Chang R, Wang RY, Abdenur JE. Maple syrup urine disease: further evidence that newborn screening may fail to identify variant forms. Mol Genet Metab. 2010;100:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Sajeev M, Chin S, Ho G, Bennetts B, Sankaran BP, Gutierrez B, Devanapalli B, Tolun AA, Wiley V, Fletcher J, Fuller M, Balasubramaniam S. Challenges in Diagnosing Intermediate Maple Syrup Urine Disease by Newborn Screening and Functional Validation of Genomic Results Imperative for Reproductive Family Planning. Int J Neonatal Screen. 2021;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Pode-Shakked N, Korman SH, Pode-Shakked B, Landau Y, Kneller K, Abraham S, Shaag A, Ulanovsky I, Daas S, Saraf-Levy T, Reznik-Wolf H, Vivante A, Pras E, Almashanu S, Anikster Y. Clues and challenges in the diagnosis of intermittent maple syrup urine disease. Eur J Med Genet. 2020;63:103901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Axler O, Holmquist P. Intermittent maple syrup urine disease: two case reports. Pediatrics. 2014;133:e458-e460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Li H, Ji CY, Zong XN, Zhang YQ. [Height and weight standardized growth charts for Chinese children and adolescents aged 0 to 18 years]. Zhonghua Er Ke Za Zhi. 2009;47:487-492. [PubMed] |
| 16. | Yi P, Liu L, Mei H, Zeng F, Huang Z, Niu H. Establishment of reference range of plasma amino acids for younger Chinese children by reverse phase HPLC. J Pediatr Endocrinol Metab. 2011;24:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 17. | Jiang M, Liu L, Mei H, Li X, Cheng J, Cai Y. Detection of inborn errors of metabolism using GC-MS: over 3 years of experience in southern China. J Pediatr Endocrinol Metab. 2015;28:375-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Lin Y, Sheng H, Ting TH, Xu A, Yin X, Cheng J, Mei H, Shao Y, Zeng C, Zhang W, Rao M, Liu L, Li X. Molecular and clinical characteristics of monogenic diabetes mellitus in southern Chinese children with onset before 3 years of age. BMJ Open Diabetes Res Care. 2020;8:e001345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 19. | Ali EZ, Ngu LH. Fourteen new mutations of BCKDHA, BCKDHB and DBT genes associated with maple syrup urine disease (MSUD) in Malaysian population. Mol Genet Metab Rep. 2018;17:22-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Su L, Lu Z, Li F, Shao Y, Sheng H, Cai Y, Liu L. Two homozygous mutations in the exon 5 of BCKDHB gene that may cause the classic form of maple syrup urine disease. Metab Brain Dis. 2017;32:765-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Schuster SC. Next-generation sequencing transforms today's biology. Nat Methods. 2008;5:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1124] [Cited by in RCA: 955] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 22. | Najmabadi H, Hu H, Garshasbi M, Zemojtel T, Abedini SS, Chen W, Hosseini M, Behjati F, Haas S, Jamali P, Zecha A, Mohseni M, Püttmann L, Vahid LN, Jensen C, Moheb LA, Bienek M, Larti F, Mueller I, Weissmann R, Darvish H, Wrogemann K, Hadavi V, Lipkowitz B, Esmaeeli-Nieh S, Wieczorek D, Kariminejad R, Firouzabadi SG, Cohen M, Fattahi Z, Rost I, Mojahedi F, Hertzberg C, Dehghan A, Rajab A, Banavandi MJ, Hoffer J, Falah M, Musante L, Kalscheuer V, Ullmann R, Kuss AW, Tzschach A, Kahrizi K, Ropers HH. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011;478:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 667] [Cited by in RCA: 748] [Article Influence: 53.4] [Reference Citation Analysis (0)] |