Published online Feb 16, 2023. doi: 10.12998/wjcc.v11.i5.1031
Peer-review started: December 14, 2022
First decision: December 26, 2022
Revised: December 28, 2022
Accepted: January 19, 2023
Article in press: January 19, 2023
Published online: February 16, 2023
Processing time: 61 Days and 17.3 Hours
No study on dual energy computed tomography (DECT) has been found in the literature to evaluate possibly fatal cardiac/myocardial problems in corona virus disease 2019 (COVID-19) patients. Myocardial perfusion deficits can be found in COVID-19 patients even without any significant coronary artery occlusion, and these deficits can be shown via DECT with a perfect interrater agreement.
To assess lung perfusion alterations in COVID-19 patients. To our knowledge, no study using DECT has been performed to evaluate possibly fatal cardiac/ myocardial problems in COVID-19 patients. The purpose of this study is to evaluate the role of DECT in the detection of COVID-19-related cardiac diseases.
Two blinded independent examiners evaluated CT images using the 17-segment model according to the American Heart Association’s classification of the segmentation of the left ventricular myocardium. Additionally, intraluminal diseases and abnormalities in the main coronary arteries and branches were investigated. Following segment-by-segment analysis, perfusion deficiencies identified on the iodine map pictures on DECT were identified.
The study enrolled a total of 87 patients. Forty-two of these individuals were classified as COVID-19 positive, and 45 were classified as controls. Perfusion deficits were identified in 66.6% (n = 30) of the cases. All control patients had a normal iodine distribution map. Perfusion deficits were found on DECT iodine map images with subepicardial (n = 12, 40%), intramyocardial (n = 8, 26.6%), or transmural (n = 10, 33.3%) anatomical locations within the left ventricular wall. There was no subendocardial involvement in any of the patients.
Myocardial perfusion deficits can be found in COVID-19 patients even without any significant coronary artery occlusion. These deficits can be shown via DECT with a perfect interrater agreement. Additionally, the presence of perfusion deficit is positively correlated with D-dimer levels.
Core Tip: To our knowledge, there has been no research on dual-energy computed tomography (DECT) to assess potentially fatal cardiac/myocardial issues in corona virus disease 2019 (COVID-19) patients. This investigation's goal is to assess DECT's contribution to the identification of cardiac conditions associated with COVID-19. Even in COVID-19 patients without any significant coronary artery occlusion, myocardial perfusion deficits can be identified, and these deficits can be demonstrated via DECT with perfect inter-observer agreement.
- Citation: Aydin F, Kantarci M, Aydın S, Karavaş E, Ceyhun G, Ogul H, Şahin ÇE, Eren S. COVID-19-related cardiomyopathy: Can dual-energy computed tomography be a diagnostic tool? World J Clin Cases 2023; 11(5): 1031-1039
- URL: https://www.wjgnet.com/2307-8960/full/v11/i5/1031.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i5.1031
Coronavirus disease 2019 (COVID-19) is an infectious disease that first surfaced in China in December 2019 and quickly spread throughout the world. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, the pathogen responsible for COVID-19, causes primarily respiratory symptoms[1]. Angiotensin-converting enzyme II (ACE2) is found in the respiratory system, intestinal enterocyte cells, cardiac muscle cells, and vascular endothelial cells, and is thought to be exploited by SARS-CoV-2 for cell entrance. The SARS-CoV-2 virus is hypothesized to initiate the inflammatory response by inhibiting ACE2. Subsequently, a systemic cytokine release begins, which can induce not only lung injury but can also cause systemic effects, potentially leading to multiorgan dysfunction. For example, due to its procoagulant nature, systemic inflammation can sensitize existing vascular plaques and as such raises the risk of cardiovascular disease[2].
Several different theories have been suggested to explain the pathophysiology of COVID-19 disease with respect to cardiac involvement. These pathophysiological processes uncovered have been demonstrated as potential sources of cardiac damage in COVID-19 patients, and this myocardial damage manifests itself in various clinical pictures. Among the most commonly documented cardiac problems in COVID-19 patients are acute myocarditis, acute myocardial infarction, arrhythmia, cardiomyopathy, and acute heart failure. All of these significantly increase morbidity and mortality in COVID-19 patients, underscoring the critical need for early detection and treatment of these cardiac sequelae[3,4].
Previous research has mostly focused on the significance of cardiac magnetic resonance (CMR) imaging in COVID-19 patients. CMR has been demonstrated to be useful in determining the mechanism, prevalence, and degree of myocardial damage. Myocarditis is the most prevalent imaging diagnostic, with mapping anomalies and myocardial edema on T2 being the most common imaging findings, followed by late gadolinium enhancement (LGE). Furthermore, in a study of individuals who had recently recovered from COVID-19 infection, CMR revealed cardiac involvement and myocardial inflammation independently of preexisting comorbidities, the severity and overall course of the acute illness, or the time from the original diagnosis[5,6]. Despite the fact that CMR was found to be effective in detecting COVID-19-related myocardial problems, it has some drawbacks, including a long scan time, the fact that it is not universally available, the high cost, potential patient claustrophobia, incompatibility with pacemakers, and incompatibility with prostheses[7,8].
Dual-energy computed tomography (DECT) is a developing technology that provides information about material composition via image acquisition by varying photon energy levels[9]. In the last decade, DECT has been increasingly utilized for cardiac imaging[10,11]. When different energy levels of X-ray spectra penetrate through iodine-based contrast medium, each reveals unique absorption characteristics. Iodine mapping (plotting of the distribution of iodine in the myocardium) can be used to precisely detect cardiac perfusion deficits; for example, dark areas in a map indicate a lack of iodine and correlate to areas of low myocardial perfusion[10,12,13].
Recently, DECT perfusion imaging was used to assess lung perfusion alterations in COVID-19[14,15]. To our knowledge, no study on DECT has been performed to evaluate possibly fatal cardiac/myoca
The local institutional review board approved this study, and as a result of retrospective nature, informed consent was waived.
This retrospective study includes data from patients from January 2021 to June 2022. The case group included hospitalized individuals with diagnosis of COVID-19 who had a cardiology consultation due to chest pain and underwent DECT on suspicion of heart abnormalities. COVID-19 was identified using real-time reverse transcriptase-polymerase chain reaction (RT-PCR) tests on nasopharyngeal swabs.
As a control group, we included patients who had both a DECT scan to evaluate chest pain and a negative COVID-19 nasopharyngeal swab RT-PCR assay. For patients in either the study or control group, an exclusion criterion was the presence of any comorbidity (e.g., coronary artery disease, hypertension, hyperlipidemia, diabetes, history of coronary stent or by-pass, arrhythmia, etc). Etiological factors that may increase D-dimers, such as deep venous thrombosis, pulmonary embolism, liver and renal failure were evaluated, and patients with these conditions were eliminated. Finally, DECT examinations with insufficient qualifications (e.g., poor image quality, numerous artifacts) were not included.
A five-point scale was utilized to evaluate and categorize the image quality of each coronary segment on DECT: There are no motion artifacts in category 5, minor artifacts (mild blurring) in category 4, moderate artifacts (moderate blurring without discontinuity) in category 3, severe artifacts (doubling or discontinuity along the coronary segments) in category 2, and unreadable artifacts (doubling or discontinuity along the coronary segments) in category 1 (vessel structures not differentiable). A category score of 4 was deemed good for image quality.
Forty-nine patients met the inclusion criteria; however 7 were omitted (insufficient image quality in 3 patients, history of diabetes in 1 patient, presence of a previously applied coronary stent in 1 patient, and presence of deep venous thrombosis in 2 patients). Forty-two patients were included.
The DECT images were created via a 64-slice dual-source multi-detector CT scanner (Somatom Definition Flash; Siemens Healthcare, Forchheim, Germany). One mL/kg body weight iopromide (Ultravist 370 mg/mL; Bayer Schering Pharma, Berlin, Germany) was administered into the right antecubital vein with a flow rate of 5 mL/s, followed by 60 mL saline. A bolus tracking technique was used to pinpoint the area of interest (ROI) in the left ventricle (CARE-bolus; Siemens Healthcare). The data collection procedure was initiated at a predetermined time interval specified by a single ROI system with a trigger threshold of 200 Hounsfield units (HU) in the left ventricular blood pool. Data collection commenced eight seconds after triggering, with arterial phase data being gathered. Retrospective ECG pulsing with a low-pitch ECG-gated scan was used as the scan mode (a prospective protocol could not be applied due to the artifacts during the dual-energy protocol). All patients in the retrospective procedure received ECG dosage modulation. The CT dose index volume and the dosage-length product of all the scans were recorded.
Patients were encouraged to adopt the deep-inspiration breath-hold technique during the procedure, and the scan was conducted craniocaudally from the subcarinal level to the diaphragm. The limits of the reconstruction window of the initial axial pictures were set at 75% (end of diastolic phase) and 45% for the cardiac cycle (end of systolic phase).
For the myocardial evaluation, the high- (140 kV) and low-voltage (80 kV) information was reconstructed via a dual-energy convolution core (D30f) with a temporal resolution of 140 milliseconds and a thickness of 1.5 mm, with 1 mm increments utilized to maximize the signal-to-noise ratio. Next, the final data were analyzed using a three-material decomposition software platform (Syngo Multimodality Workplace; Siemens, Erlangen, Germany).
Two blinded radiologists (18 years and 6 years of experience in cardiac CT) independently examined the CT images using the 17-segment model according to the American Heart Association classification of the segmentation of the left ventricular myocardium. If the two independent readers disagreed on AE diagnosis, a consensus reading was performed. Additionally, intraluminal diseases and abnormalities in the main coronary arteries and branches were investigated. Prior to analyzing the myocardium with DECT, the “DE normalize contrast” process was used on the workstation to provide consistency for the visual evaluation and eliminate any bias due to inter-observer variability. Myocardial assessment was performed using arterial phase pictures. The dark spots on the color-coded iodine map were identified as perfusion deficiencies for each subject and section.
Following segment-by-segment analysis, the perfusion deficiencies on the iodine map pictures on DECT were identified. Each segment was also counted in terms of the number of segments involved and its anatomic location (transmural, intramyocardial, subepicardial, subendocardial).
SPSS version 20.0 was utilized (SPSS Inc, Armonk, NY, United States). To evaluate the normally distributed data, the Kolmogorov-Smirnov test was utilized. Numerical variables with a normal distribution were expressed as mean ± standard deviation (SD), but those with an atypical distribution were expressed as median (minimum-maximum) values. Number (n) and percentage values were used to denote categorical variables (%). On DECT scan, patients were classified according to the presence or absence of coronary artery stenosis and myocardial perfusion deficit. The Mann-Whitney U test for age distribution and the Chi-square test for sex and percentage distribution were used to determine compatibility in both groups. The influence of D-dimer on predicting the presence of perfusion deficit was described using logistic regression analysis. The correlation between HU and D-dimer values was tested with Pearson’s correlation. Observers 1 and 2 independently assessed the CT images for the presence of perfusion deficits. Cohen's Kappa coefficient was used to determine whether Observer 1 and Observer 2 were in agreement. Accordingly, the degree of agreement was classified as slight if the coefficient was between 0 and 0.20, fair if the coefficient was between 0.21 and 0.40, moderate if the coefficient was between 0.41 and 0.60, substantial if the coefficient was between 0.61 and 0.80, and almost perfect if the coefficient was between 0.81 and 1.00[16]. A value of P < 0.05 was accepted as statistically significant.
The current study enrolled a total of 87 patients. Forty-two of these individuals were COVID-19 positive (case group), while 45 were classified as negative controls. Table 1 shows the age, troponin-I, and D-dimer distributions of the patients.
| Case group | mean ± SD | Median (min-max) |
| Age | 42.95 ± 17.53 | 43.00 (20-73) |
| Troponin-I | 103.77 ± 446.24 | 3.5 (0.3-2051) |
| D-dimer | 820.71 ± 1022.05 | 390 (105-4000) |
| Control group | mean ± SD | Median (min-max) |
| Age | 49.68 ± 10.71 | 52.00 (28-64) |
| Troponin- I | 3.46 ± 3.49 | 2.7 (0.01-13) |
| D-dimer | 273.9 ± 76.6 | 267 (105-426) |
In the case group, mild coronary artery stenosis was detected in 9.5% (n = 4) of patients while significant stenosis (i.e. any stenosis larger than or equal to 50%) was detected in 4.7% (n = 2) of patients. In the control group, 11.1 % (n = 5) of patients had mild coronary artery stenosis. The prevalence of mild or significant coronary artery stenosis was comparable between the study and control groups (P > 0.05). Additionally, no structural changes or myocardial bridging were found in either the study or control group subjects.
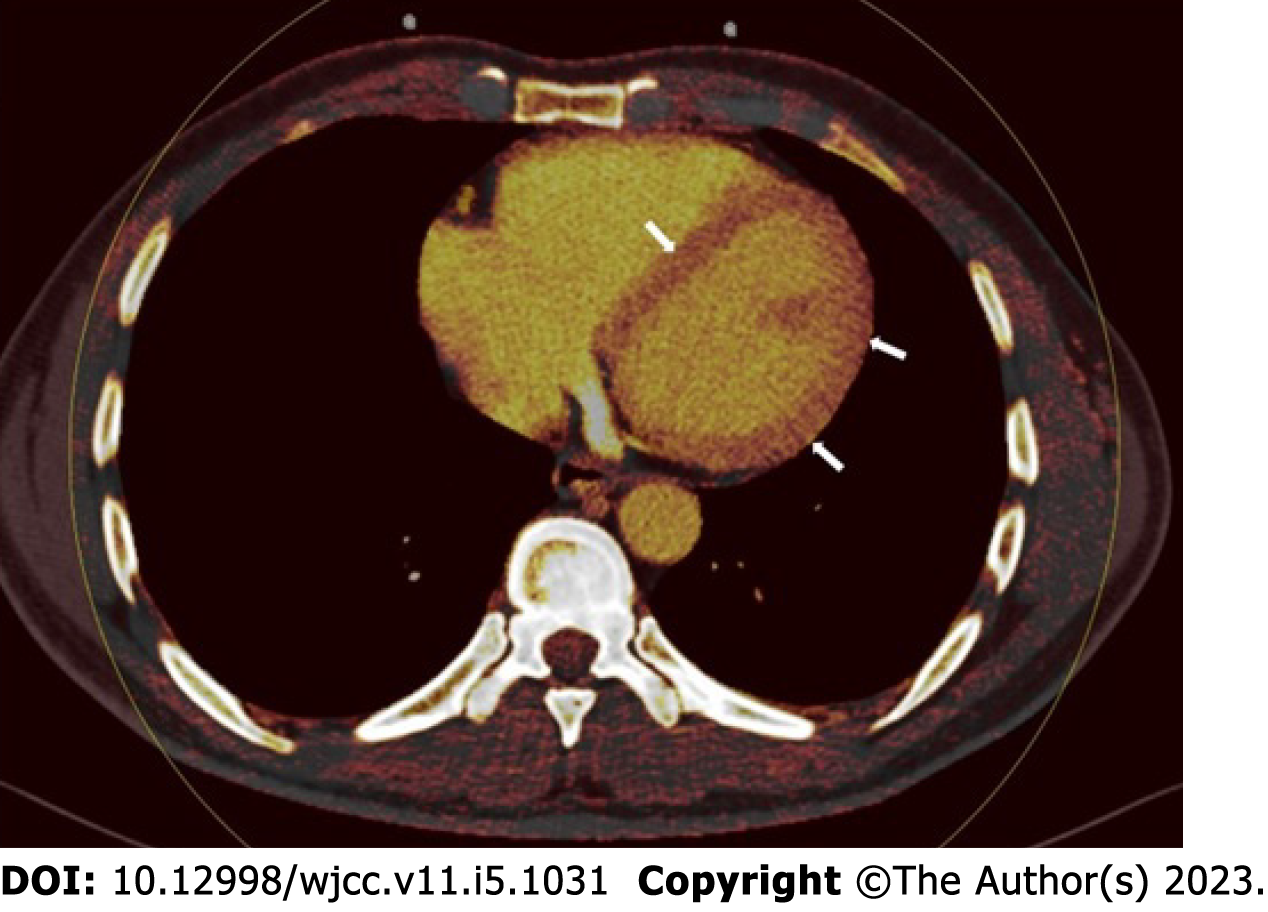
Perfusion deficit was identified in 66.6% (n = 30) of the case group in the myocardial perfusion imaging data analyzed with the iodine distribution map. On the other hand, no perfusion deficit was detected in the control group (Figure 1). The rate of myocardial perfusion deficit was significantly higher in the case group (P < 0.001).
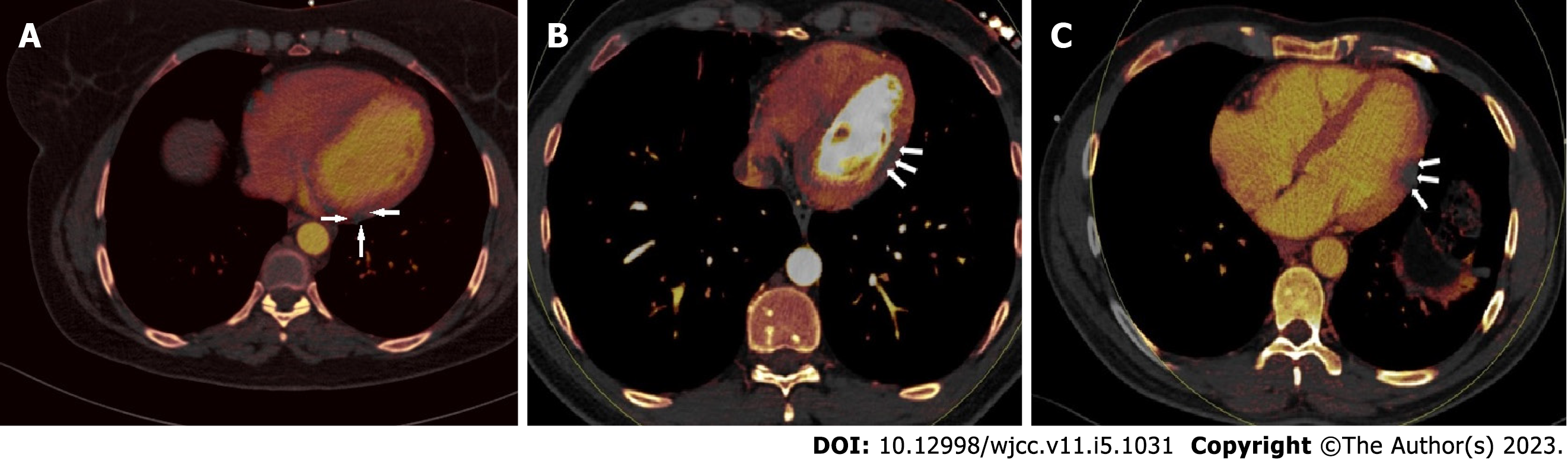
Perfusion deficits were found on DECT iodine map images with a subepicardial (n = 12, 40%), intramyocardial (n = 8, 26.6%), or transmural (n = 10, 33.3%) anatomical location within the left ventricular wall (Figure 2). There was no subendocardial involvement in any of the patients. Involvement patterns did not correspond to any coronary artery territories. ROI was used to measure the perfusion deficit and normal areas on the iodine map images on DECT. The mean value of the normal areas was 132.437 ± 31.11 HU (range, 60-217), while that of the perfusion deficits was 51.25 ± 17.19 HU (range, 28-86). Troponin-I levels had no significant correlation with HU values in perfusion deficit areas (P > 0.05). The HU levels of the perfusion deficits, on the other hand, increased in tandem with the D-dimer values (r = 0.765, P = 0.01).
In the case group, there was no significant correlation between troponin-I elevation and the occurrence of a myocardial perfusion deficit (P > 0.05). In this group, there was a significant connection between D-dimer increase and the occurrence of a perfusion deficit (i.e. higher D-dimer levels correlate with higher number of involved segments with perfusion deficit) (P = 0.012). Furthermore, it was discovered in the case group that an increase in D-dimer levels (upper limit of normal 500 ng/mL) increased the likelihood of the occurrence of perfusion deficits threefold (OR: 3, P < 0.001, 95%CI: 1.47-6.14).
Between Observer 1 and Observer 2, there was a substantial consistency in the detection of myocardial perfusion deficit across all case and control study group subjects. There was perfect agreement between the two observers (P < 0.001, kappa value = 0.896).
Using the dual-source CT angiography approach, we compared the COVID-19 positive case group to the control group comprising patients with clinical suspicion of coronary arterial disease. We did not find any significant difference in coronary luminal abnormalities between the groups. However, myocardial perfusion abnormalities were substantially more common in the COVID-19 case group compared to the controls. Furthermore, both observers revealed perfusion deficiencies with perfect agreement. These perfusion deficit locations were deemed to be substantial in terms of myocardial damage, correlating to the dark lesions in the iodine mapping method.
ACE2 is the host cell receptor for the SARS-CoV-2 spike protein, which facilitates viral infection of the heart, vascular tissues, and circulating cells. One of the frequent extrapulmonary signs of COVID-19 is acute cardiac injury, which can have long-term consequences. ACE2 can be detected in various tissues such as those of the heart, lung, intestines, kidney, testis, nose, and mouth. Nasal and pulmonary epithelial cells are thought to be the primary sites of the infection; however, following initial viral replication, myocardial cells can also exhibit necessary components for viral uptake. According to gene expression investigations, the ventricular myocardial cells express all of the essential mediators of SARS-CoV-2 binding and entrance[17,18].
Evidence of acute cardiac compromise is not rare in hospitalized COVID-19 patients; cardiac-related events include acute heart failure (3%-33%), cardiogenic shock (9%-17%), myocardial ischemia or infarction (0.9%-11%), left ventricular dysfunction (10%-41%), right ventricular dysfunction (33%-47%), biventricular dysfunction (3%-15%), stress cardiomyopathy (2%-5.6%), arrhythmia (9%-17%), venous thromboembolism (23%-27%), and arterial thrombosis secondary to viral-mediated coagulopathy[19]. It was discovered that D-dimer and fibrinogen degradation products can be raised in COVID-19 cases as a result of viral-mediated coagulopathy and microangiopathy. Additionally, D-dimer values were shown to be related to the presence and extent of lung perfusion anomalies discovered using DECT, as well as the presence of “long COVID” symptoms[14,15,20].
CMR abnormalities suggestive of damage have also been observed: T1 mapping anomalies (which may indicate extensive myocardial damage like fibrosis and/or edema); T2, short tau inversion recovery, or T2 mapping abnormalities (more specific findings of myocardial inflammation, similar to with acute myocarditis); LGE, as a sign of acute myocardial injury and/or fibrosis; and pericardial involvement. All of these findings can be considered COVID-19-associated cardiac problems[19,21].
While CMR manifestations of cardiac COVID-19 have been extensively explored, there is no study to our knowledge that describes COVID-19-related myocardial perfusion anomalies using DECT. We have demonstrated that DECT can accurately detect COVID-19-related cardiac perfusion abnormalities. Additionally, we reduced the effect of false positive results produced by ischemia episodes by utilizing a control group. We were unable to pinpoint the perfusion deficits to a vascular territory and did not discover any perfusion deficit in the subendocardial area when segmental and regional distributions were evaluated. Additionally, these data rule out the notion that the perfusion deficiencies were caused primarily by coronary artery obstruction and ischemia, rather than by COVID-19-associated cardiomyopathy. Moreover, these findings support the hypothesis of direct viral myocardial damage.
According to our findings, even in the absence of severe coronary artery obstruction, the myocardium does exhibit perfusion abnormalities in the setting of COVID-19 infection. Additionally, we demonstrated a favorable correlation between high D-dimer values and perfusion impairments. These findings are consistent with the findings that COVID-19 induces microangiopathy and that D-dimer levels serve as a marker for this microangiopathic process. As with lung perfusion deficits discovered using DECT, increased D-dimer values are associated with myocardial perfusion problems, which can be diagnosed with cardiac DECT.
Troponin levels are elevated in 20% to 30% of hospitalized individuals with COVID-19. According to these elevated troponin levels, acute myocardial injury occurs at an overall incidence of between 8% and 62%, with a higher prevalence of elevated troponin levels being associated with worse disease severity[22,23]. On the other hand, despite abnormal CMRs, three trials revealed normal troponin levels following COVID-19[23-25]. We could not detect any relation between troponin-I levels and cardiac perfusion deficits. This result might be explained with the phase or the severity of the viral myocardiopathy.
In cases of COVID-19, chest pain and thromboembolic cardiovascular complications are extremely common. Particularly in emergency situations, chest pain caused by COVID-19 pneumonia and other cardiovascular complications or diseases can easily coexist. Cardiac CT exams are increasingly utilized in the diagnostic evaluation of chest pain[14]. By using DECT to detect COVID-19-associated myocardial damage and distinguish this entity from other cardiovascular causes in a single session, we can provide rapid and effective diagnosis and treatment. In addition, CMR, the alternative diagnostic tool for defining COVID-19-associated myocardial damage, is more difficult to access and considerably more costly than DECT. In addition to more claustrophobic complaints, CMR also has longer examination periods[26]. Despite DECT’s advantages, there are a few significant limitations in this study, including the study’s retrospective approach and relatively small sample size. Additionally, our coronary arterial findings were not confirmed via a gold standard test (i.e. invasive angiography). Furthermore, CMR scans were not performed to confirm the identified perfusion anomalies. We are also unable to offer patients with clinical follow-up data.
In summary, myocardial perfusion deficits can be detected in COVID-19 patients (even in the absence of significant coronary artery occlusion) via DECT. Our study demonstrated a perfect interrater agreement in interpretation of DECT data, highlighting the potential ease of interpretation of this imaging modality. Lastly, the presence of myocardial perfusion deficits in COVID-19 patients is positively correlated with D-dimer levels, indicating this easily accessible test as a reasonable serum marker for this important cardiac sequela of SARS-CoV-2 viral infection.
Previous research has mostly focused on the importance of cardiac magnetic resonance (CMR) imaging in coronavirus disease 2019 (COVID-19) patients. Although CMR has been found to be effective in detecting myocardial problems associated with COVID-19, dual energy computed tomography (DECT) is increasingly used in favor of CMR due to CMR’s long scanning time, non-universal availability, and high cost.
To the best of our knowledge, there has been no study in the literature on DECT evaluating potentially fatal cardiac/myocardial problems in patients with COVID-19. The aim of the current study is to evaluate the role of DECT in detecting COVID-19-related heart disease.
To reveal that in COVID-19 patients without significant coronary artery occlusion, myocardial perfusion deficits can be demonstrated by DECT.
Data from this retrospective study include that gathered from patients between January 2021 and June 2022. The case group includes individuals hospitalized with a diagnosis of COVID-19 who had a cardiology consultation due to chest pain and underwent DECT for suspected heart abnormality. DECT images were generated using a 64-slice dual-source multidetector CT scanner after 1 mL/kg body weight iopromide was administered. Two blinded radiologists independently reviewed the CT images using the 17-segment model according to the American Heart Association classification system.
A total of 87 patients were included in the current study. Of these, 42 were COVID-19 positive (case group). The presence of myocardial perfusion deficit was significantly higher in the case group (P < 0.001). Involvement patterns did not correspond to any coronary artery region. HU levels of perfusion deficits increased in parallel with D-dimer values (r = 0.765, P = 0.01). There was a significant correlation between the increase in D-dimer and the incidence of perfusion deficit in the case group (P = 0.012). Moreover, it was discovered that the increase in D-dimer levels (upper limit of normal 500 ng/mL) in the case group increased the probability of perfusion deficit to occur threefold (OR: 3, P < 0.001, 95%CI: 1.47-6.14). There was excellent agreement between observers (P < 0.001, kappa value = 0.896).
One of the common extrapulmonary manifestations of COVID-19 is acute cardiac injury, which can have serious long-term consequences. According to gene expression investigations, ventricular myocardial cells express all of the essential mediators of SARS-CoV-2 binding and entrance. It has been demonstrated that D-dimer and fibrinogen degradation products may be elevated in COVID-19 cases as a result of viral-mediated coagulopathy and microangiopathy. In addition, D-dimer values have been shown to correlate with the presence and extent of lung perfusion abnormalities discovered using DECT, as well as with prolonged COVID symptoms.
Abnormalities suggestive of cardiac injury have been described previously, most frequently using CMR. However, a study describing myocardial perfusion anomalies using DECT does not exist to our knowledge. Here, we have demonstrated that DECT can accurately detect cardiac perfusion abnormalities associated with COVID-19. In addition, we demonstrated a positive correlation between high D-dimer values and perfusion disorders. Troponin levels are elevated in 20% to 30% of individuals hospitalized with COVID-19, however, we did not detect a relationship between troponin-I levels and cardiac perfusion deficits detected using DECT.
Myocardial perfusion defects may be present in patients with COVID-19, even in the absence of significant coronary artery occlusion. These defects can be demonstrated via DECT with excellent interoperator agreement. Additionally, COVID-19-associated myocardial perfusion defects are positively correlated with elevated D-dimer levels.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Su C, China; Velikova TV, Bulgaria S-Editor: Wang LL L-Editor: Filipodia P-Editor: Wang LL
| 1. | Jester JV, Steel D, Salz J, Miyashiro J, Rife L, Schanzlin DJ, Smith RE. Radial keratotomy in non-human primate eyes. Am J Ophthalmol. 1981;92:153-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18185] [Article Influence: 3637.0] [Reference Citation Analysis (0)] |
| 2. | Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1709] [Article Influence: 341.8] [Reference Citation Analysis (0)] |
| 3. | Tahir F, Bin Arif T, Ahmed J, Malik F, Khalid M. Cardiac Manifestations of Coronavirus Disease 2019 (COVID-19): A Comprehensive Review. Cureus. 2020;12:e8021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Long B, Brady WJ, Koyfman A, Gottlieb M. Cardiovascular complications in COVID-19. Am J Emerg Med. 2020;38:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 648] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 5. | Skakun NP, Vorontsov AA, Skakun GK, Shendevitskiĭ VI. [Fetal alcohol syndrome (a review of the literature)]. Vopr Okhr Materin Det. 1980;25:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1240] [Cited by in RCA: 1464] [Article Influence: 292.8] [Reference Citation Analysis (0)] |
| 6. | Kato S, Azuma M, Fukui K, Kodama S, Nakayama N, Kitamura H, Hagiwara E, Ogura T, Horita N, Namkoong H, Kimura K, Tamura K, Utsunomiya D. Cardiac involvement in coronavirus disease 2019 assessed by cardiac magnetic resonance imaging: a meta-analysis. Heart Vessels. 2022;37:1570-1582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Saeed M, Van TA, Krug R, Hetts SW, Wilson MW. Cardiac MR imaging: current status and future direction. Cardiovasc Diagn Ther. 2015;5:290-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 8. | Stokes MB, Nerlekar N, Moir S, Teo KS. The evolving role of cardiac magnetic resonance imaging in the assessment of cardiovascular disease. Aust Fam Physician. 2016;45:761-764. [PubMed] |
| 9. | Karçaaltıncaba M, Aktaş A. Dual-energy CT revisited with multidetector CT: review of principles and clinical applications. Diagn Interv Radiol. 2011;17:181-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Vliegenthart R, Pelgrim GJ, Ebersberger U, Rowe GW, Oudkerk M, Schoepf UJ. Dual-energy CT of the heart. AJR Am J Roentgenol. 2012;199:S54-S63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | McCollough CH, Leng S, Yu L, Fletcher JG. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology. 2015;276:637-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 1070] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 12. | Ruzsics B, Lee H, Zwerner PL, Gebregziabher M, Costello P, Schoepf UJ. Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur Radiol. 2008;18:2414-2424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 13. | Zhao RP, Hao ZR, Song ZJ. Diagnostic value of Flash dual-source CT coronary artery imaging combined with dual-energy myocardial perfusion imaging for coronary heart disease. Exp Ther Med. 2014;7:865-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Aydin S, Kantarci M, Karavas E, Unver E, Yalcin S, Aydin F. Lung perfusion changes in COVID-19 pneumonia: a dual energy computerized tomography study. Br J Radiol. 2021;94:20201380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Idilman IS, Telli Dizman G, Ardali Duzgun S, Irmak I, Karcaaltincaba M, Inkaya AC, Demirkazik F, Durhan G, Gulsun Akpinar M, Ariyurek OM, Akpinar E, Rello J, Akova M, Akata D. Lung and kidney perfusion deficits diagnosed by dual-energy computed tomography in patients with COVID-19-related systemic microangiopathy. Eur Radiol. 2021;31:1090-1099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 16. | McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22:276-282. [PubMed] |
| 17. | Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, Slavov D, Schwisow JA, Rodriguez EM, Carroll IA, Keuer TA, Buttrick PM, Kao DP. Dynamic Regulation of SARS-Cov-2 Binding and Cell Entry Mechanisms in Remodeled Human Ventricular Myocardium. JACC Basic Transl Sci. 2020;5:871-883. [PubMed] [DOI] [Full Text] |
| 18. | Lin H-B, Liu PP. COVID-19 and the Heart: ACE2 Level is Not Destiny, But the Company It Keeps May Hold the Fate. JACC Basic to Translational Science. 2020;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV 3rd, Kwon DH, Singh T, Tilton JC, Tsai EJ, Tucker NR, Barnard J, Loscalzo J. COVID-19 and Cardiovascular Disease: From Bench to Bedside. Circ Res. 2021;128:1214-1236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 226] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (2)] |
| 20. | Aydin S, Unver E, Karavas E, Yalcin S, Kantarci M. Computed tomography at every step: Long coronavirus disease. Respir Investig. 2021;59:622-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Ojha V, Verma M, Pandey NN, Mani A, Malhi AS, Kumar S, Jagia P, Roy A, Sharma S. Cardiac Magnetic Resonance Imaging in Coronavirus Disease 2019 (COVID-19): A Systematic Review of Cardiac Magnetic Resonance Imaging Findings in 199 Patients. J Thorac Imaging. 2021;36:73-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 22. | Smilowitz NR, Jethani N, Chen J, Aphinyanaphongs Y, Zhang R, Dogra S, Alviar CL, Keller N, Razzouk L, Quinones-Camacho A, Jung AS, Fishman GI, Hochman JS, Berger JS. Myocardial Injury in Adults Hospitalized With COVID-19. Circulation. 2020;142:2393-2395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3006] [Article Influence: 601.2] [Reference Citation Analysis (1)] |
| 24. | Rajpal S, Tong MS, Borchers J, Zareba KM, Obarski TP, Simonetti OP, Daniels CJ. Cardiovascular Magnetic Resonance Findings in Competitive Athletes Recovering From COVID-19 Infection. JAMA Cardiol. 2021;6:116-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 25. | Clark DE, Parikh A, Dendy JM, Diamond AB, George-Durrett K, Fish FA, Slaughter JC, Fitch W, Hughes SG, Soslow JH. COVID-19 Myocardial Pathology Evaluation in Athletes With Cardiac Magnetic Resonance (COMPETE CMR). Circulation. 2021;143:609-612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 26. | Papazoglou AS, Karagiannidis E, Moysidis DV, Sofidis G, Bompoti A, Stalikas N, Panteris E, Arvanitidis C, Herrmann MD, Michaelson JS, Sianos G. Current clinical applications and potential perspective of micro-computed tomography in cardiovascular imaging: A systematic scoping review. Hellenic J Cardiol. 2021;62:399-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |