Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.931
Peer-review started: October 1, 2022
First decision: November 30, 2022
Revised: December 13, 2022
Accepted: January 10, 2023
Article in press: January 10, 2023
Published online: February 6, 2023
Processing time: 128 Days and 0.2 Hours
Benign lymphoepithelial cyst (BLEC) of the parotid gland is a rare benign embryonic-dysplastic cystic tumor in the anterolateral neck that occurs most commonly in human immunodeficiency virus (HIV)-positive adults and rarely in non-acquired immune deficiency syndrome patients. The main presentation is a slow-growing, painless mass, and secondary infection may cause acute inflammatory symptoms.
A 44-year-old Chinese male patient presented with a 1-year history of a mass in the left side of the neck. On physical examination, a mass similar in size and shape to a quail egg was found in the left parotid gland. The mass was tough, without tenderness, and easily moveable. The results of HIV tests, including antibody and nucleic acid tests and CD4+ T cell examination, were negative. Imaging exam
The detailed characteristics of a BLEC in a patient without HIV infection contri
Core Tip: Benign lymphoepithelial cyst (BLEC) of the parotid gland is a rare lesion seldom found in non-acquired immune deficiency syndrome patients. BLECs are benign embryonic dysplastic cystic tumors that typically occur in the anterolateral part of the neck. We present a case of BLEC of the parotid gland in a non-human immunodeficiency virus -infected patient in order to improve clinicians’ understanding of the disease.
- Citation: Liao Y, Li YJ, Hu XW, Wen R, Wang P. Benign lymphoepithelial cyst of parotid gland without human immunodeficiency virus infection: A case report . World J Clin Cases 2023; 11(4): 931-937
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/931.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.931
Benign lymphoepithelial cyst (BLEC) of the parotid gland, which is also known as branchial cleft cyst, is a rare benign cystic neoplasm of embryonic dysplasia. It usually occurs in the anterolateral region of the neck but has been reported in the oral cavity or parotid gland in rare cases[1,2]. This disease mainly presents as a slow-growing tumor and is not associated with recurrence or metastasis. These tumors usually occur in patients with human immunodeficiency virus (HIV) infection and are rarely encountered in non-HIV-infected patients[3]. To improve clinicians’ understanding of this rare disease, the present report describes the imaging, histopathological, and diagnostic characteristics of a parotid gland BLEC found in a 44-year-old, non-HIV-infected patient. The case description is followed by a review of the relevant literature.
A 44-year-old Chinese man was admitted to the hospital on September 1, 2020, due to a painless mass on the left side of the neck that had been present for 1 year.
One year previously, the patient incidentally noticed a painless mass on the left side of his neck approximately 2.0 cm × 2.0 cm in size. The patient had neck swelling occasionally, and the severity of these symptoms gradually increased over the course of the disease. The patient reported no tenderness, redness, swelling, or heat pain at the mass; no earache, swelling, or pain around the ear; and no cheek pain, numbness or difficulty when opening the mouth.
The patient had a history of hypertension for 1 month and was regularly taking nifedipine sustained release tablets and Irbesartan tablets as antihypertensive treatment.
The patient had no personal or family history of benign or malignant tumors.
Physical examination on admission revealed a temperature of 36.5°C, resting respiratory rate of 20 breaths/min, heart rate of 80 bpm, and blood pressure of 117/70 mmHg. On physical examination, a mass similar in size and shape to a quail egg was found in the left parotid gland, measuring about 2.0 cm × 2.0 cm in size. The mass felt tough, without tenderness and was easily movable with no local redness, swelling, heat or pain. No abnormalities were observed in the contralateral parotid gland.
The laboratory test results showed a white blood cell count of 6.16 × 109/L, with 74% neutrophils, 135 g/L hemoglobin, and a platelet count of 277 × 109/L. The erythrocyte sedimentation rate was 2 mm/h, and all results for routine urine, stool, liver and kidney function tests as well as electrolyte and blood biochemical index levels were within normal ranges. The results of HIV tests, including antibody and nucleic acid tests as well as CD4+ T cell examination, were negative. Furthermore, all results of testing for carcinoembryonic antigen, neuron-specific enolase, cytokeratin 19 fragment, squamous cell carcinoma antigen, blood coagulation, and immune indexes were negative.
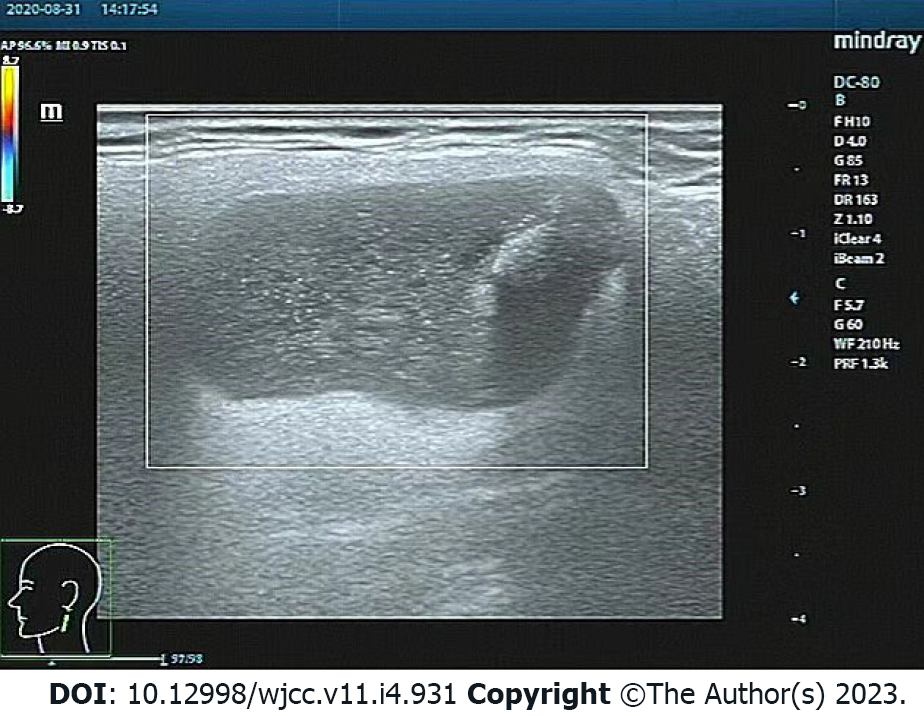
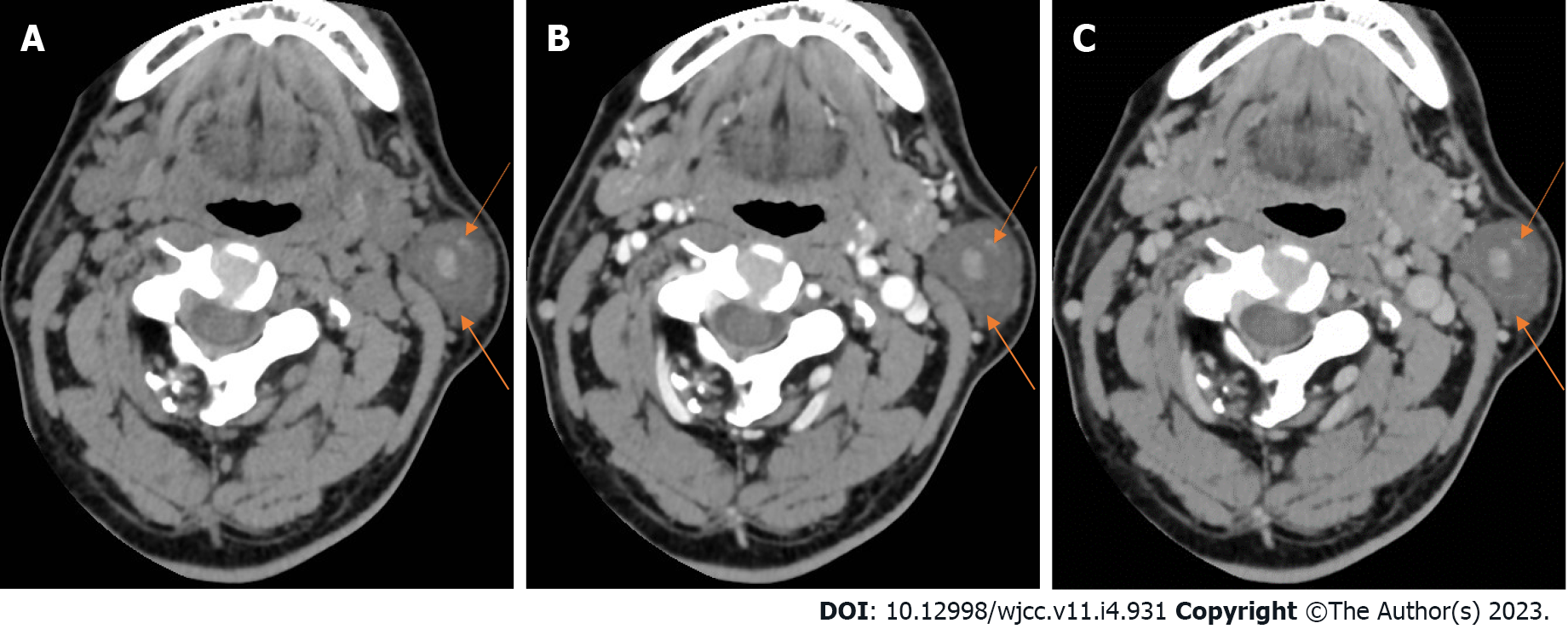
On August 31, 2020, ultrasound of the neck recognized a 2.2 cm × 2.2 cm hypoechoic mass in the left parotid gland, with a clear boundary, coarse calcification foci, and no blood flow signal (Figure 1). On September 2, 2020, neck computed tomography (CT) showed a round cystic lesion in the left parotid gland, with a maximum cross-sectional area of approximately 2.3 cm × 2.2 cm, uneven density, small patches with slightly high-density shadows, and clear boundaries (Figure 2A). On contrast-enhanced CT, the cyst wall was slightly enhanced in the arterial phase, but no obvious enhancement was observed in the cyst (Figure 2B). The enhancement degree in the venous phase was similar to that in the arterial phase (Figure 2C). No abnormality was found in the right parotid gland.
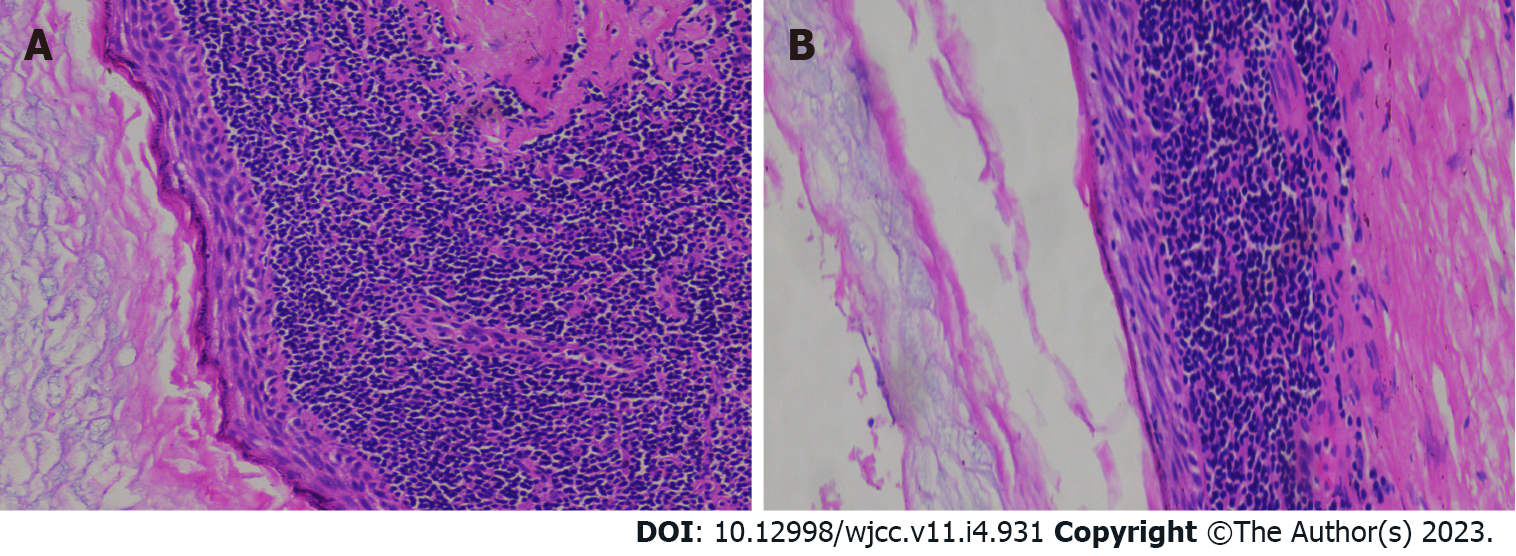
In general, the lesion was a pale round nodule with a complete capsule and small volume, accompanied by a small amount of parotid tissue attachment. The size was about 2.2 cm × 2.0 cm × 1.8 cm. The surface of the tumor was smooth and soft, and the surface skin mucosa was not abnormal, and the color was not special. The section was cystic, and the cyst was soybean dreg-like material with a wall thickness of 0.1 cm. Under light microscopy, the cyst wall was laminated squamous epithelium without epithelial nail process and the surface layer was mostly incomplete keratosis. The epithelium was surrounded by a large number of lymphoid stromata with lymphoid follicular formation and a center of occurrence (Figure 3). The histological features of the tumor were consistent with a diagnose of BLEC. The clinical and pathological data of this case are presented in Table 1.
| Finding | |
| Age, sex | 44 years, male |
| Family history | None |
| Personal history | None |
| Chief complaint | Left neck painless mass present for 1 year |
| Physical examination | A quail egg-shaped mass on the left parotid gland, about 2.0 cm × 2.0 cm in size, tough, without tenderness, easily moveable, no local redness, swelling, heat or pain, and no abnormalities in the contralateral parotid gland |
| Laboratory examinations | HIV tests, including antibody and nucleic acid tests and CD4+ T cell examination, were negative |
| Ultrasound of the neck | A 2.2 cm × 2.2 cm hypoechoic mass in the left parotid gland, with a clear boundary, coarse calcification foci, and no blood flow signal (Figure 1) |
| Neck CT | A round cystic lesion in the left parotid gland, with a maximum cross-sectional area of 2.3 cm × 2.2 cm, uneven density, small patches with slightly high-density shadows, and clear boundaries (Figure 2A). On enhanced scanning, the cyst wall was slightly enhanced in the arterial phase, but no obvious enhancement was observed in the cyst (Figure 2B). The enhancement degree in the venous phase was similar to that in the arterial phase (Figure 2C) |
| Pathology | The cyst wall was laminated squamous epithelium without epithelial nail process and the surface layer was mostly incomplete keratosis (Figure 3A); The epithelium was surrounded by a large number of lymphoid stromata with lymphoid follicular formation and a center of occurrence (Figure 3B) |
| Final diagnosis | Non-HIV-infected BLEC of parotid gland |
| Treatment | Surgical treatment (specific surgical method was termed left parotid gland tumor + partial superficial lobectomy + facial nerve exploration and protection + fascia flap plasty) |
| Follow-up | No recurrence of symptoms in 2-year clinical follow-up |
The patient was diagnosed with a non-HIV-infected BLEC of the parotid gland on the basis of history, clinical features, and ancillary examination (imaging and histopathology).
Because BLECs are benign, most cases of BLEC have a good prognosis. The principles of treatment are early diagnosis, infection control, and complete resection of the lesion without facial nerve injury. Treatment options include observation, repeat aspiration, sclerotherapy, radiotherapy, and surgery.
During the treatment period, the patient in the present case did not stop Nifedipine and Irbesartan for treatment of hypertension. Blood pressure and electrocardiography were normal at admission, and surgical treatment was possible under close monitoring of blood pressure. During surgery, care must be taken to identify the internal and external carotid arteries and vagus nerve, hypoglossal nerve, glossopharyngeal nerve, and superior laryngeal nerve to avoid injury to these structures. Finally, the patient and his family members chose surgical treatment (the specific surgical method was termed left parotid gland tumor + partial superficial lobectomy + facial nerve exploration and protection + fascia flap plasty). The operation was successful, and the patient returned to the ward safely after surgery.
The patient was followed up for 2 years. CT/magnetic resonance imaging (MRI) and color ultrasound were not performed during follow-up, because the patient felt no obvious symptoms.
BLEC is a rare cystic neoplasm with benign embryonic dysplasia. The first case of BLEC was reported by Hildebrandt in 1895, but only 21 cases were published by researchers through 1981. With the emergence of the HIV epidemic, the incidence of BLEC in the parotid gland gradually increased, and researchers found that BLECs were closely related to HIV infection in most cases, with BLEC as one of the early clinical manifestations. Around 3%-6% of HIV-positive adults and 1%-10% of HIV-positive children experience BLEC symptoms. In sharp contrast, BLECs are rarely found in non-HIV-infected patients, and the exact prevalence of BLEC in this population has not been reported[1-4]. In the present case, the results of HIV tests, including antibody and nucleic acid tests as well as CD4+ T cell examination, were negative. In previous reports, the disease was also known as branchial cleft cyst and usually observed in either the mandibular angle at the bottom of the outer regions or in the anterior portion of the sternocleidomastoid. It was found to occur less frequently in the mouth or parotid gland. In addition, its distribution in men and women is uniform. Although catheter jam seems to be a cause, the source of the blockage is often not obvious. Furthermore, its pathogenesis remains unclear. Currently, the majority of researchers believe that extraglandular lymphoid infiltration and/or intra-glandular lymphoid hyperplasia with duct obstruction and/or epithelial embedding is the main cause.
BLEC is a slow-growing tumor not associated with recurrence or metastasis[5-8]. The main symptom of this disease is a slowly growing, painless swollen mass, often combined with local symptoms such as dysphagia, dysphagia, dyspnea and stridor, and secondary infection can manifest as acute inflammatory symptoms, such as redness, swelling and fever[3,8]. The diagnosis of BLEC mainly depends on history, clinical manifestations, and preoperative auxiliary examination. Preoperative auxiliary diagnostic procedures include CT, MRI, ultrasound and fine needle aspiration (FNA)[9]. To date, imaging characteristics of this disease have been rarely reported. By reviewing the relevant literature[1,9], we found that the CT manifestations of a typical BLEC are a single, thin-walled round or oval cystic foci in the parotid gland, with clear boundaries and low density in the cyst. On enhanced scanning, only the cyst wall shows mild to moderate enhancement, and no enhancement is seen in the cyst. In the present case, CT showed that the small patches with slightly high-density in the parotid gland were caused by viscous fluid in the capsule or lesions complicated with infection. MRI mainly showed a single thin-walled cystic lesion in the parotid gland, which was round or oval in shape, with a clear boundary, and the cyst wall showed a low signal. Intracystic T1 weighted imaging showed low signal, while T2 weighted imaging showed high signal. Moreover, the cyst wall was significantly enhanced on enhanced scanning, but no enhancement was found in the cyst[2]. Ultrasonic features of the lesions included a circular shape with a complete capsule and smooth lining, and cystic wall blood flow signals were not rich. The epithelial cells in a BLEC may contain keratin, and shed inflammatory cells can form a viscous gel-like yellowish-white liquid[10,11]. The internal echo of the cyst can generally appear as one of three types, including coarse granular hyperecho and cloud weak echo mixed signal, clear cystic echo through sound, and cluster weak echo and cystic echo mixed. In this case, ultrasound showed typical mixed signals including coarse granular hyperechoic signal and cloud weak echo signal. FNA can be used as an important auxiliary method for the clinical diagnosis of lateral cervical lesions. The criteria for FNA cytological diagnosis of BLEC include thick, yellow, pustular fluid; non-nucleated cells; keratinocytes; and squamous epithelial cells of varying maturity[1]. Histologically, the walls of BLEC cysts are usually covered with squamous epithelium and/or, in some cases, columnar or cuboidal cells. Lymphoid tissue with or without germinal centers in subepithelial connective tissue is the most prominent morphological feature[12]. Histological examination of the mass case showed that the cyst wall was laminated squamous epithelium without epithelial nail process and the surface layer was mostly incomplete keratosis, surrounded by a large number of lymphoid stromata with lymphoid follicular formation and a center of occurrence. Differential diagnoses include: (1) Warthin tumor[2], which is more common in middle-aged and elderly patients and more likely to occur in the posterior lower pole of the parotid gland, with uneven internal density; (2) Intramuscular benign hemangioma[13], which is a fast-flowing intramuscular mass with uneven density and visible fat density in children that requires pathological examination for diagnosis; (3) Lymphoma[14], which is more common in men over 50 years old and appears as irregular soft tissue masses with a large range and uniform density on CT, without obvious calcification, cystic degeneration or necrosis, with diffuse growth to the surrounding area and mostly without adjacent bone destruction, and mild to moderate enhancement on enhanced scanning; (4) Thyroglossal duct cysts[15], which present as a painless mass in the front of the neck and are usually dumbbell shaped and movable when the tongue is extended or swallowed; CT shows a low-density, usually monocular parenchyma lesion during embryonic thyroid migration, mostly located in the midline and associated with the hyoid bone; (5) Lymphocele[16], which typically manifest as a cystic density focus with uniform density, clear boundary, thin cyst wall, no obvious exudation and calcification, and after enhancement, the cyst wall can present slightly uniform enhancement, with no enhancement in the cyst; (6) Metastatic lymph nodes[17], which are accompanied by a history of primary tumor, and on CT exhibit uneven density, calcification, cystic or necrotizing changes, uneven edges, adhesion to surrounding tissues, and obvious annular or peripheral enhancement; and (7) Lymphadenitis[18], which is more common in children and present with local redness, swelling, heat and pain while appearing mostly oval with a thick wall and ring and uniform enhancement without obvious wall nodules and calcification, but with a blurred surrounding fat space. The current treatment principles for this disease include early diagnosis, infection control, and cyst removal, and the treatment options include observation, repeated aspiration, sclerotherapy, radiotherapy, and surgical treatment[4]. Observation in asymptomatic patients shows that repeated aspiration therapy is generally ineffective, and cysts will recur within weeks to months. Sclerotherapy is limited in scope and is generally only used for cases in which the cystic fluid can be aspirated, while radiotherapy is mostly used for HIV-infected BLEC[3]. For our patient, surgery was the best treatment at present, and attention was paid to identify and avoid injury to adjacent blood vessels and nerves during surgery. In this case, surgical treatment was chosen by the patient, and the specific surgical procedure was termed left parotid gland tumor + partial superficial lobectomy + facial nerve exploration and protection + fascia flap plasty. The potential complications of surgical treatment include recurrence, the formation of persistent fistula, and cranial nerve injury, which require close follow-up. In this case, the patient survived well with no related discomfort after 2 years of follow-up.
BLEC in the absence of HIV infection is a rare benign cystic tumor of embryonic dysplasia, known as a branchial cleft cyst. The disease progresses slowly. Because the imaging, histopathology, and diagnosis and treatment characteristics of non-HIV-infected BLEC are poorly understood, the present case is reported to increase readers’ awareness of the disease.
We thank Medjaden Inc. for scientific editing of this manuscript.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: ISHIDA T, Japan; liu H, United States S-Editor: Liu GL L-Editor: A P-Editor: Liu GL
| 1. | Najib Z, Berrada O, Lahjaouj M, Oukessou Y, Rouadi S, Abada RA, Roubal M, Mahtar M. Cervical lymphoepithelial cyst: Case report and literature review. Ann Med Surg (Lond). 2021;61:185-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 2. | Joshi J, Shah S, Agarwal D, Khasgiwal A. Benign lymphoepithelial cyst of parotid gland: Review and case report. J Oral Maxillofac Pathol. 2018;22:S91-S97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Pillai S, Agarwal AC, Mangalore AB, Ramaswamy B, Shetty S. Benign Lymphoepithelial Cyst of the Parotid in HIV Negative Patient. J Clin Diagn Res. 2016;10:MD05-MD06. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Dave SP, Pernas FG, Roy S. The benign lymphoepithelial cyst and a classification system for lymphocytic parotid gland enlargement in the pediatric HIV population. Laryngoscope. 2007;117:106-113. [PubMed] [DOI] [Full Text] |
| 5. | Nahata V. Branchial Cleft Cyst. Indian J Dermatol. 2016;61:701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Zimmerman KO, Hupp SR, Bourguet-Vincent A, Bressler EA, Raynor EM, Turner DA, Rehder KJ. Acute upper-airway obstruction by a lingual thyroglossal duct cyst and implications for advanced airway management. Respir Care. 2014;59:e98-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Fujibayashi T, Itoh H. Lymphoepithelial (so-called branchial) cyst within the parotid gland. Report of a case and review of the literature. Int J Oral Surg. 1981;10:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Chavan S, Deshmukh R, Karande P, Ingale Y. Branchial cleft cyst: A case report and review of literature. J Oral Maxillofac Pathol. 2014;18:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Glosser JW, Pires CA, Feinberg SE. Branchial cleft or cervical lymphoepithelial cysts: etiology and management. J Am Dent Assoc. 2003;134:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | da Silva KD, Coelho LV, do Couto AM, de Aguiar MCF, Tarquínio SBC, Gomes APN, Mendonça EF, Batista AC, Nonaka CFW, de Sena LSB, Alves PM, Libório-Kimura TN, Louredo BVR, Câmara J, Caldeira PC. Clinicopathological and immunohistochemical features of the oral lymphoepithelial cyst: A multicenter study. J Oral Pathol Med. 2020;49:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Reynolds JH, Wolinski AP. Sonographic appearance of branchial cysts. Clin Radiol. 1993;48:109-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Hirota J, Maeda Y, Ueta E, Osaki T. Immunohistochemical and histologic study of cervical lymphoepithelial cysts. J Oral Pathol Med. 1989;18:202-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Wee SJ, Park MC, Chung CM, Tak SW. Intramuscular hemangioma in the zygomaticus minor muscle: a case report and literature review. Arch Craniofac Surg. 2021;22:115-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Thanarajasingam G, Bennani-Baiti N, Thompson CA. PET-CT in Staging, Response Evaluation, and Surveillance of Lymphoma. Curr Treat Options Oncol. 2016;17:24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Corvino A, Pignata S, Campanino MR, Corvino F, Giurazza F, Tafuri D, Pinto F, Catalano O. Thyroglossal duct cysts and site-specific differential diagnoses: imaging findings with emphasis on ultrasound assessment. J Ultrasound. 2020;23:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Hekiert A, Newman J, Sargent R, Weinstein G. Spontaneous cervical lymphocele. Head Neck. 2007;29:77-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Suh CH, Baek JH, Choi YJ, Lee JH. Performance of CT in the Preoperative Diagnosis of Cervical Lymph Node Metastasis in Patients with Papillary Thyroid Cancer: A Systematic Review and Meta-Analysis. AJNR Am J Neuroradiol. 2017;38:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 18. | Desai S, Shah SS, Hall M, Richardson TE, Thomson JE; Pediatric Research in Inpatient Settings (PRIS) Network. Imaging Strategies and Outcomes in Children Hospitalized with Cervical Lymphadenitis. J Hosp Med. 2020;15:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |