Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.797
Peer-review started: October 17, 2022
First decision: November 11, 2022
Revised: December 2, 2022
Accepted: January 5, 2023
Article in press: January 5, 2023
Published online: February 6, 2023
Processing time: 112 Days and 3.5 Hours
Lactose intolerance (LI) is commonly seen in East Asian countries. Several studies showed that lactose or milk loading has been used as a treatment for lactose malabsorption (LM) in Western countries, but there have been no reports regarding this type of treatment in Japan. As lactose or milk loading requires ingestion of large amounts of lactose within a short period, this is considered to be too harsh for Japanese people because of their less habitual milk consumption (175 mL per day in average) than Western people. In this study, we demonstrated lactose tolerance acquisition in a suitable way for Japanese.
To examine the efficacy of lactose (cow’s milk) loading treatment in patients with LM.
Individuals with abdominal symptoms induced by milk or dairy products (LI symptoms) were identified with a questionnaire. A 20 g lactose hydrogen breath test (LHBT) was carried out to confirm LM diagnosis and to evaluate co-existence of small intestinal bacterial overgrowth (SIBO). Respondents diagnosed with LM were selected as study subjects and were treated with incremental loads of cow’s milk, starting from 30 mL and increasing up to 200 mL at 4-7 d intervals. After the treatment, changes in symptoms and LM diagnostic value of 20 g LHBT were investigated. Stool samples pre- and post-treatment were examined for changes in intestinal microbiota using 16S rRNA sequencing. Informed consent was obtained prior to each stage of the study.
In 46 subjects with LI symptoms (10-68 years old, mean age 34 years old) identified with the questionnaire, 35 (76.1%) were diagnosed with LM by 20 g LHBT, and 6 had co-existing SIBO. The treatment with incremental cow’s milk was carried out in 32 subjects diagnosed with LM (14-68 years old, median age 38.5 years old). The mean period of the treatment was 41 ± 8.6 d. Impro
LM was diagnosed in approximately 75% of the subjects who had LI. Incremental loads of cow’s milk is regarded as a useful treatment for LM without affecting everyday life.
Core Tip: The incidence of lactose malabsorption (LM) is high in East Asians such as Japan. Colonic adaptation by daily consumption of milk or lactose has been known as a method to treat LM, reducing symptoms of lactose intolerance (LI). However, reports regarding such treatment have not been found in Japan. In this study, we clarified the prevalence of LM diagnosed among the Japanese patients who had LI symptoms, and evaluated the efficacy of incremental loads of cow’s milk as a treatment for LM without affecting everyday life.
- Citation: Hasegawa M, Okada K, Nagata S, Sugihara S. Efficacy of incremental loads of cow’s milk as a treatment for lactose malabsorption in Japan. World J Clin Cases 2023; 11(4): 797-808
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/797.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.797
Self-reported lactose intolerance (LI) affects approximately 45% of the Japanese population, according to a survey in 2015[1]. The average daily milk consumption by Japanese people was found to be around 175 mL, indicating less habitual milk consumption than that of Western countries, in spite of the nutritional benefit[2]. Current adjuvant treatment for lactose intolerance is self-administration of commercialized lactose-degrading enzyme before consuming milk or dairy products, yet its effect has been limited. Literature from other countries reported that colonic adaptation by daily milk or lactose consumption reduced LI symptoms in patients who also suffered lactose malabsorption (LM)[3], but patients who underwent this treatment were required to ingest large volumes of milk within a short period, which is considered to be too harsh for Japanese.
On the contrary, the abdominal symptoms can also be induced by psychological conditions, which should be ruled out from the lactose-induced symptoms[4,5]. To resolve this issue, LM is diagnosed non-invasively by the lactose hydrogen breath test (LHBT). In order to distinguish psychogenic symptoms, a single-blind comparative study (SBCS) was conducted on subjects with self-reported LI, as well as LHBT to diagnose LM. For subjects diagnosed with LM, lactose tolerance acquisition treatment is conducted in a suitable way for Japanese, followed by the assessment of the treatment efficacy.
As other studies have reported intestinal microbiota changes when clinical symptoms are alleviated by daily milk intake[6], the analysis of the intestinal microbiota was also conducted to assess the changes before and after the treatment.
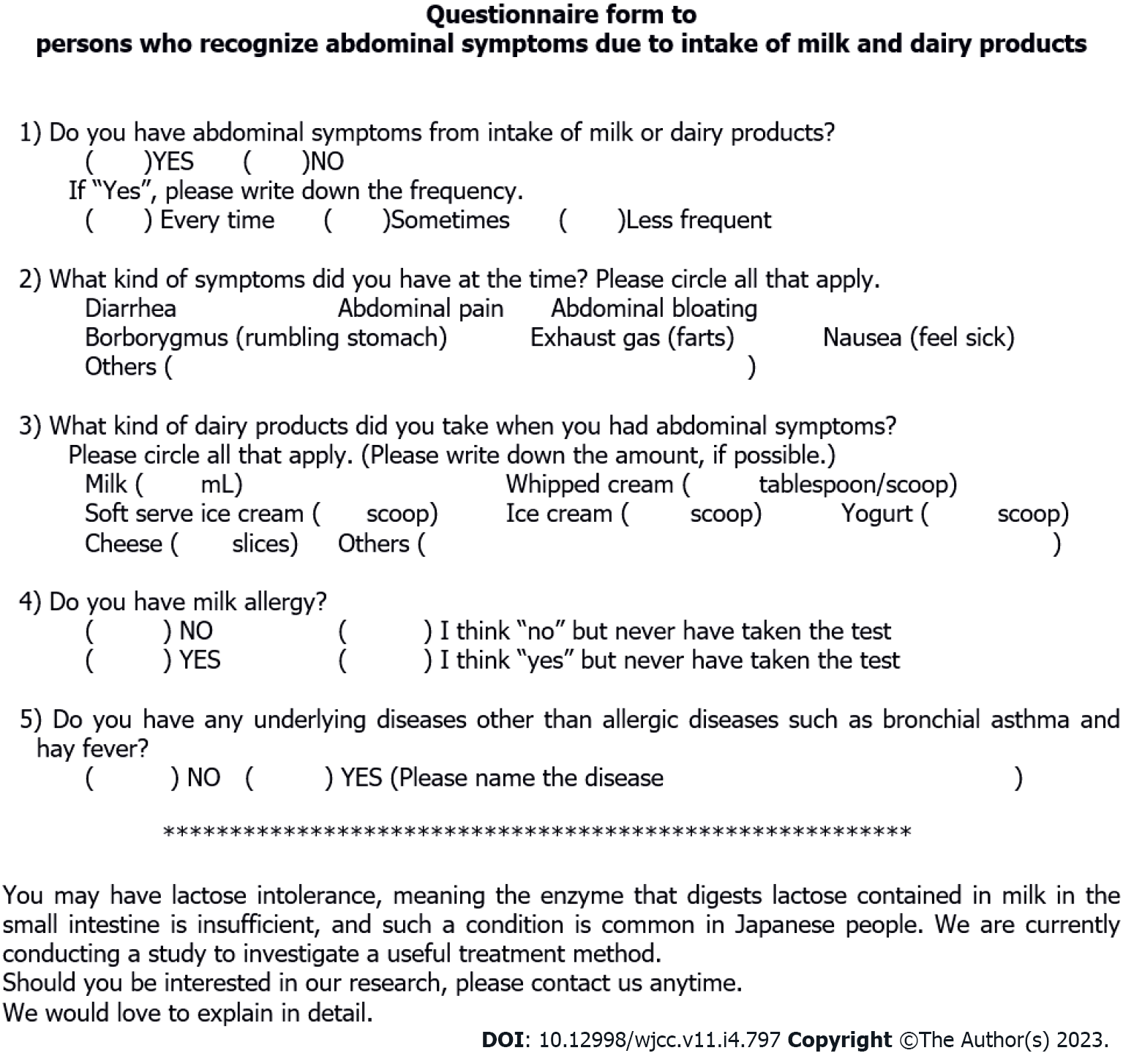
A questionnaire survey was undertaken by Japanese people aged between 10 and 70 years to identify subjects with abdominal symptoms due to cow’s milk and dairy products consumption. The questionnaire asked for the amount of milk and dairy products that caused abdominal symptoms and the severity of the symptoms, and people with milk allergy or other underlying diseases were excluded from the study (Figure 1).
This study was approved by the Tokyo Women's Medical University Ethics Committee. Informed consent was obtained from subjects prior to beginning each stage of the study.
Diagnostic studies: A 200 mL SBCS was conducted in order to identify abdominal symptoms caused by cow’s milk (Study A), and 20 g LHBT was performed to diagnose LM in these subjects (Study B). Study A and Study B were carried out separately, with a minimum 1-wk interval.
Study A (200 mL SBCS): Lactose-reduced milk (LRM) (containing approximately 1.9 g of lactose/200 mL) and general milk (GM) (unadjusted milk: Containing approximately 9.8 g of lactose/200 mL), were used as the test materials of the study. The subjects started from ingesting 200 mL of the test material (LRM or GM) after fasting, and abdominal symptoms, including bloating, abdominal pain, borborygmi, gas, and diarrhea, were recorded for up to 3 h after the intake. Symptom severity was recorded and classified into five grades, using visual analog scales (0: Absence; 1: Trivial; 2: Mild; 3: Moderate; 4: Severe).
These two trial tests were separately performed with an at least 1-wk interval. Outcomes of this study were evaluated and classified into three groups based on the characteristic of symptoms as follows: (1) More obvious symptoms induced by GM than with LRM; (2) symptoms induced by LRM or unclear difference between the two materials (unevaluable group); and (3) no symptoms induced by either material.
Study B (lactose challenge test: 20 g LHBT): The subjects were requested to fast overnight, at least 5 h prior to the lactose challenge. At the start of LHBT, the subject exhaled into a gas collection bag, followed by ingestion of 20 g lactose dissolved in approximately 150 mL of water. Breath samples were then collected at 30-min intervals for 3 h (7 times in total). Abdominal symptom severity was recorded during the test. The breath hydrogen concentration was measured by using MicroLyzer 12i (QuinTron Inst. Co. Inc., United States).
The diagnostic criterion for LM was set as 20 ppm or more hydrogen level from the baseline. In addition, diagnostic evaluation of small intestinal bacterial overgrowth (SIBO) was considered to indicate that the elevated breath hydrogen concentration and abdominal symptoms coexisted within 60 min from the start of the test.
Stool collection for analysis of intestinal microbiota: Stool samples were collected from the subjects before and after the treatment to evaluate changes in the intestinal microbiota. The stool samples were appropriately stored frozen until DNA extraction and microbiota profiling by sequencing the V4 region of the 16S rRNA[7], which was performed by Bioengineering Lab. Co., Ltd. An increase or decrease of intestinal microbiota population change before and after the treatment was evaluated by comparing each bacterium occupancy rate out of total bacteria.
The subjects identified with LM were requested to start the treatment immediately after completing the diagnostic studies. Subjects began taking 30 mL of general milk around the same time every day on an empty stomach, and the amount of milk was gradually increased by 30 mL after 4-7 d. If they were anxious about abdominal symptoms, they were allowed to maintain the same volume up to 7 d. During the treatment period, subjects were required to record their general conditions, amount of milk ingested, and symptoms. Subjects were instructed to avoid taking any other milk or dairy products on an empty stomach, except for the milk supplied for the study, otherwise dairy products were allowed in small amounts during or after meals. Throughout the treatment, subjects were also instructed to avoid taking confounding medicines such as antibiotics, probiotics, prebiotics, antidiarrheal agents, and intestinal regulators.
All subjects were informed about LM treatment protocol and consent was obtained prior to starting the treatment. Participants were also given the right to withdraw from the study at any time.
Doctors (authors) routinely monitored the progress of each subject fortnightly during the treatment period via phone or e-mail correspondence. Study participants were obliged to report any decline in their physical condition and follow care instructions from the physician where needed.
After the subjects succeeded in taking 200 mL of milk for more than 4 d, a final examination was conducted to evaluate the efficacy of the treatment, described as below.
The subjects were requested to return their completed questionnaire to their doctor after the completion of the treatment. Degree of symptom improvement after the treatment was rated as follows: 0: No symptoms; 1: Trivial symptoms; 2: Mild symptoms but improved; 3: Moderate symptoms but improved, and 4: No improvement. Capable volume of milk tolerated without anxiety about abdominal symptoms was also rated: 1: Up to 50 mL; 2: Up to 100 mL; 3: Up to 150 mL; 4: Up to 200 mL.
After completion of the treatment, 20 g LHBT was performed to examine changes in lactose tolerance before and after the treatment.
In addition, stool was also collected at the end of the study from participants, in order to identify changes in the intestinal microbiota by methods described previously.
Values were presented as mean ± standard deviation (SD). Fisher's exact test, paired t-test, or Wilcoxon test was applied wherever appropriate. A two-sided P value of < 0.05 was considered statistically significant. Logistic regression analysis was also applied to the 95% confidence interval (CI). All statistical analyses were performed using JMP.
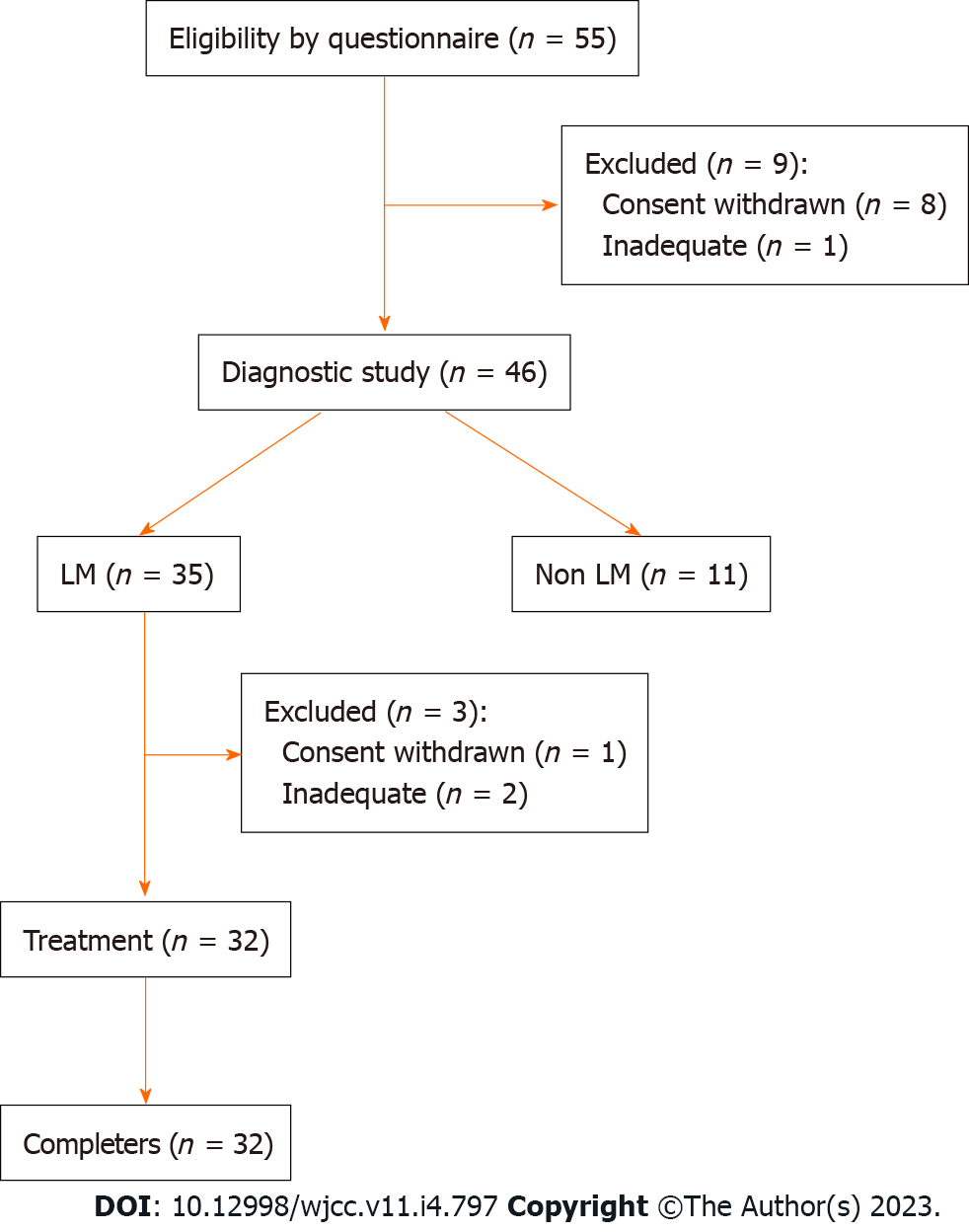
Following the questionnaire survey conducted between July 2017 and December 2019 regarding abdominal symptoms caused by lactose consumption, 55 subjects were recruited and 9 subjects were excluded according to the exclusion criteria, some of whom refused to participate to this study (Figure 2). Hence, 46 subjects aged 10-68 years (mean age: 34.0 years; males/females: 16/30) participated in the study upon informed consent.
The amount of milk at which the subjects recognized abdominal symptoms during their daily lives was found to be: 100 mL in 9 (19.6%) subjects, 150 mL in 4 (8.7%), 200 mL in 19 (41.3%), and 250 mL or more in 7 (15.2%). Five (10.9%) subjects did not answer as they were avoiding milk consumption. The remaining 2 (4.3%) subjects had abdominal symptoms induced by other dairy products, such as fresh cream.
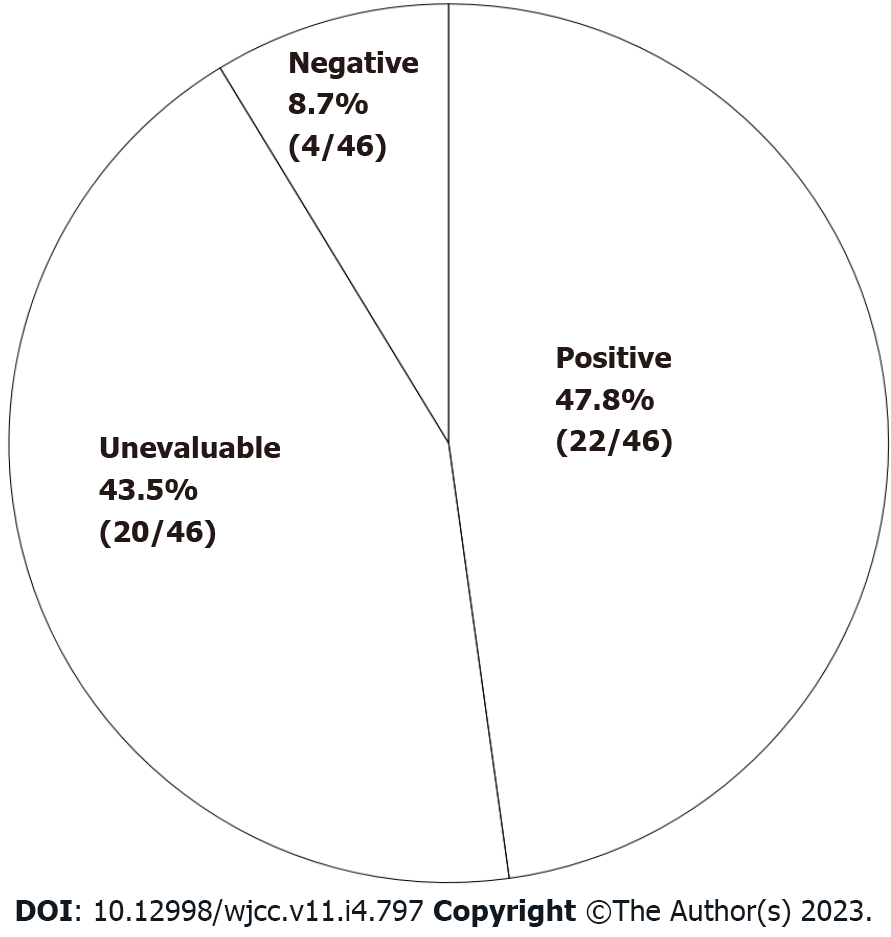
Diagnostic studies: For study A, namely, 200 mL single-blind comparative study (200 mL SBCS), the results consisted of: (1) More obvious symptoms induced by general milk than lactose-reduced milk (tested positive) in 22 (47.8%) subjects; (2) unevaluable symptoms in 20 (43.5%) subjects (symptoms induced by lactose-reduced milk in 16 subjects and unclear difference between two materials in 4); and (3) no symptoms induced by either material (tested negative) in 4 subjects (8.7%) (Figure 3). For study B (diagnosis with LM from 20 g LHBT and evaluation of SIBO), 35 (76.1%) out of 46 subjects were diagnosed with LM. Moreover, abdominal symptoms appeared at early stage (within 60 min from the start of the test) in 6 out of 35 subjects, suggesting that SIBO correlated with the rise of breath-hydrogen.
Furthermore, the reliability of the LM diagnosis by SBCS was also assessed. Setting the LM diagnosis by 20 g LHBT as the gold standard, the diagnosis precision by SBCS was 80.8% (sensitivity 86.4%, specificity 50.0%).
Characteristics seen in LHBT among the group of unevaluable subjects classified based on the result of SBCS: The onset of abdominal symptoms during the LHBT in the unevaluable group was investigated, and the results are summarized in Tables 1 and 2.
| SBCS | Time of abdominal symptom onset during LHBT (min) | ||||||||
| Result | n | 0 | 30 | 60 | 90 | 120 | 150 | 180 | No appearance |
| Positive | 19 | 1 | 7 | 6 | 1 | 1 | 3 | ||
| Unevaluable | 14 | 9 | 1 | 2 | 1 | 1 | |||
| Negative | 2 | 1 | 1 | ||||||
| Total | 35 | 1 | 17 | 7 | 3 | 2 | 3 | 2 | |
| SBCS | Time when symptoms appeared during LHBT (min) | ||||||||
| Result | n | 0 | 30 | 60 | 90 | 120 | 150 | 180 | No appearance |
| Positive | 3 | 1 | 1 | 1 | |||||
| Unevaluable | 6 | 5 | 1 | ||||||
| Negative | 2 | 1 | 1 | ||||||
| Total | 11 | 6 | 2 | 3 | |||||
Abdominal symptoms appeared within 30 min after lactose ingestion (early onset of symptoms) in 9 (64.3%) out of 14 unevaluable subjects diagnosed with LM (tested positive in LHBT) (Table 1). On the other hand, early onset of symptoms was found in 5 (83.3%) out of 6 unevaluable subjects diagnosed with non-LM (tested negative in LHBT) (Table 2). Overall, 14 (70.0%) out of 20 subjects in the unevaluable group had early onset of abdominal symptoms from LHBT.
The treatment study was conducted on 32 out of 35 subjects who received a definitive diagnosis of LM, after excluding 3 subjects: 2 subjects were regarded as inappropriate and 1 did not agree to the informed consent.
The age distribution was 14-68 years, with a median age of 38.5 years (males: females = 8:24). The treatment period was 29-66 d (mean 41 ± 8.6 d). All 32 subjects were compliant with the treatment regimen and completed the study schedule.
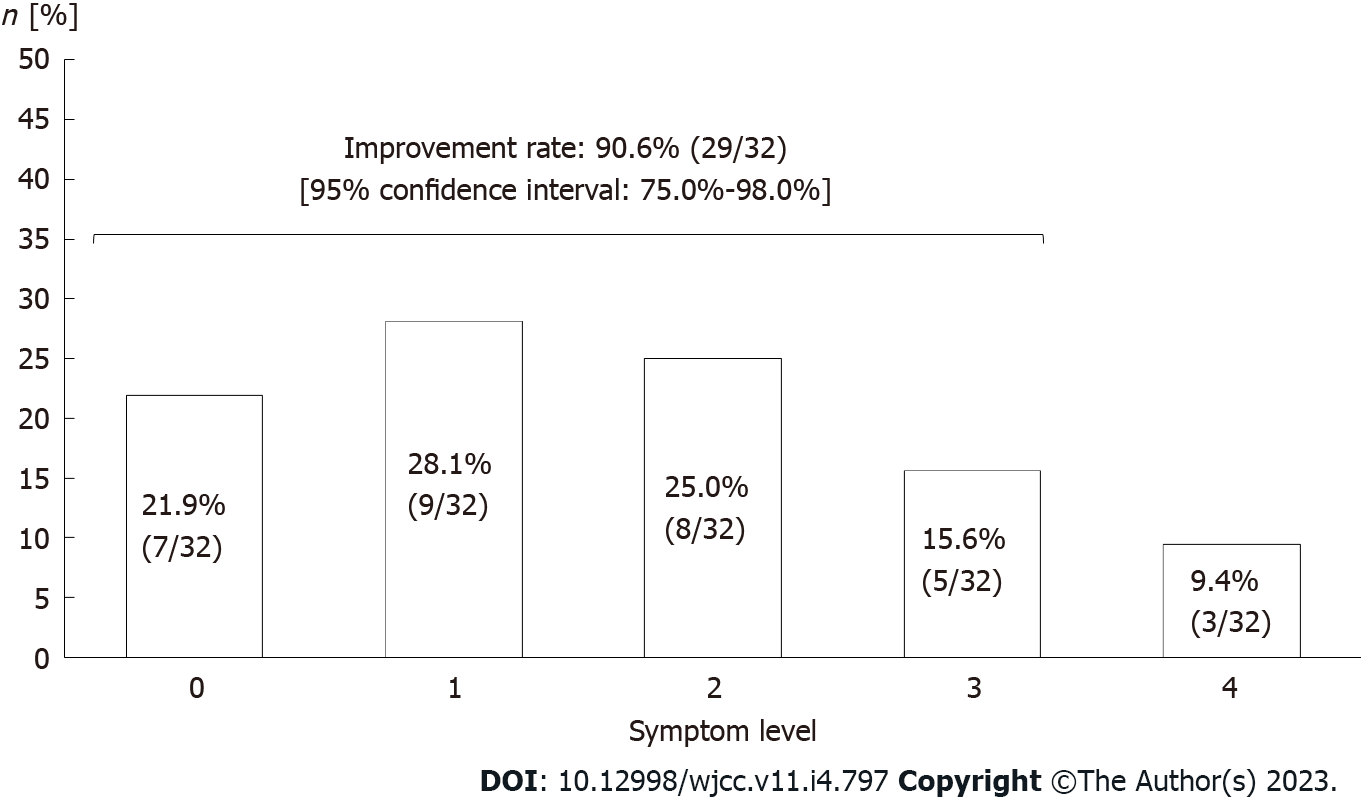
Evaluation of symptom improvement: After the treatment, "no symptoms", "trivial symptoms", "mild symptoms but improved", "moderate symptoms but improved", and "no improvement" indicated in 7 (21.9%), 9 (28.1%), 8 (25.0%), 5 (15.6%), and 3 subjects (9.4%), respectively (Figure 4). Thus, symptoms were estimated to have improved in 29 (90.6%; 95%CI: 75.0%-98.0%) out of 32 subjects in total.
Volume of milk which could be tolerated without anxiety of abdominal symptoms was classified into 3 capacity volumes: 200 mL in 15 (51.8%) subjects, 150 mL in 7 (24.1%), and 100 mL in 7 (24.1%).
Comparison of diagnostic values for LM by 20 g LHBT before and after the treatment: Therapeutic effect was also evaluated by using objective data of LHBT on 29 subjects who showed symptom improvement (Figure 5). Changes were defined based on 15 ppm difference in diagnostic value before and after the treatment.
A decrease of more than 15 ppm was seen in 10 (34.5%) subjects, indicative of an improvement after the treatment. An increase of more than 15 ppm was observed in 3 (10.3%) subjects, whereas a difference of 15 ppm or less, meaning no change, was seen in 16 (55.2%) subjects.
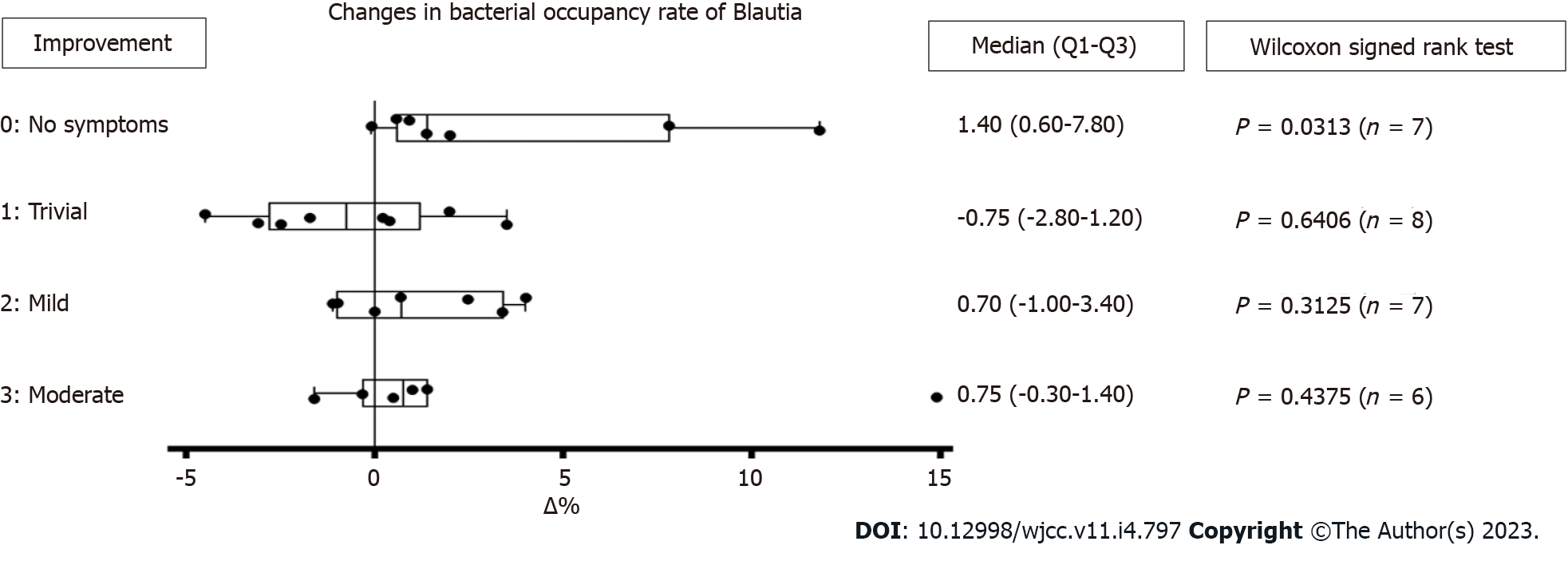
Fecal microbiota was assessed on 29 subjects who had therapeutic effects. There was no significant change in total bacterial occupancy before and after the treatment. However, there was a trending increase in Lachnospiraceae Blautia (median +0.65, P = 0.0789), and a trending decrease in Lachnospiraceae [Ruminococcus] (median -0.50, P = 0.0773). However, there was a significant change in bacterial occupancy rate based on the degree of symptom improvement. There was a significant increase of Blautia in 7 subjects who became symptom-free after the treatment (P = 0.0313) (Figure 6).
On the other hand, the change of diagnostic values of LHBT on the 7 subjects after the treatment varied: Decreased (improved) in 2 subjects, unchanged in 3, and increased in 2.
It has only been 50 years since LI was recognized and scientifically analyzed. Recently, LI was defined as a clinical syndrome characterized by abdominal symptoms after lactose consumption. However, LI needs to be distinguished from lactose maldigestion or malabsorption, which are also subclinical conditions, where LM can also be indicative of inefficient absorption of lactose caused by primary and secondary decrease of lactase activity or other intestinal conditions. Diagnosis of LI requires comparison with inert placebo, endorsed by a National Institute of Health conference[3,6,8,9].
LHBT is currently considered as the gold standard for diagnosing LM, and symptoms in this test are observed in a dosage-dependent manner. Recently, there have been many studies that apply a 20-25 g lactose dosage, as a more realistic dosage in LHBT for diagnosing LM[10]. Thus, 20 g of lactose was used in this study.
Our previous study showed that the prevalence of LM diagnosed by 20 g LHBT was 52% among 31 subjects (Japanese adults), regardless of the presence of subjective symptoms caused by milk or dairy product consumption[11]. Of all the subjects with self-reported LI symptoms, 76.1% were diagnosed with LM, suggesting that one quarter of the subjective symptoms may not be directly linked to LM. Furthermore, LM was distinguished from symptoms of self-reported LI by 200 mL SBCS. In our study, 43.5% of the subjects were found to be unevaluable, revealing that abdominal symptoms are often influenced by psychogenic conditions.
On diagnosing LM by LHBT in cases where oro-cecal transit time is within a normal range, the symptoms are believed to appear in 50-100 min after lactose ingestion. An increase in breath hydrogen is observed at least 60 min after lactose intake, peaking at around 120-150 min, indicating that breath hydrogen correlates with symptom onsets[12]. The “early onset of symptoms” was defined as appearance of abdominal symptoms within 30 min after lactose ingestion in LHBT, and accordingly, 70% of subjects in the unevaluable group tested by SBCS, had early onset of symptoms, suggesting a brain-gut interaction.
Moreover, this study showed that 6 out of 35 subjects diagnosed with LM were also suspected to have SIBO. Lactulose hydrogen breath test has been widely used to detect SIBO, while it does not have indicative criteria for SIBO. LHBT, on the other hand, can be useful for SIBO detection as an increase in breath hydrogen can be detected within 90 min after lactose ingestion. Thus, LM with SIBO can be distinguished from LM alone (by observing a peak of hydrogen after 90 min)[13]. However, a study of patients with chronic diarrhea in China, which applied hydrogen breath test with 10 g-lactulose loading and 20 g-lactose loading, reported that SIBO was more prevalent in patients with LI than those with LM. In this case, several overlapping pathological conditions were suspected[6,14].
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder classified by Rome IV. IBS is characterized by abdominal pain associated with abnormal bowel habit, but IBS patients can also suffer from other GI and non-GI symptoms, including psychological symptoms and psychiatric comorbidity[15]. Some studies in China reported that 80%-85% of the patients with diarrhea-predominant IBS also had LM[16,17].
Despite some limitations in evaluating IBS or SIBO, the LHBT provides many key pieces of information, such as transition of breath hydrogen and symptom onset during the test. Therefore, non-invasive 20 g LHBT is believed to be useful not only for diagnosing LM, but also for examining the cause of LI symptoms.
In Western countries, there are various methods of lactose load to treat LM, such as daily dose of 34 g lactose for 2 wk[5], incremental milk intake starting from 118 mL (4 oz) up to 708 mL (8 oz) in 6 d[18], and incremental lactose intake starting from 0.3-0.6 g/kg with adding 0.2 g/kg/d (max 1.0 g/kg) for 10-17 d[19]. In these studies, some participants refused to continue the treatments due to severe abdominal symptoms from lactose intake. In some reports from Europe, 12 g or less lactose was reported to be well tolerated with minimal or no symptoms[8,9,20], though even this low amount of lactose may still be intolerable for Japanese people. In our study, 9 out of 46 subjects had subjective symptoms caused by drinking 100 mL of milk (approximately 10 g of lactose), according to the questionnaire of self-reported LI symptoms. Hence, we started from 30 mL of milk intake and gradually increased the amount in every 4-7 d until 200 mL could be ingested successively. As a result, all subjects completed the treatment schedule without dropping out.
The mean treatment period was 41 d. After the treatment, 91% of the subjects showed improvement in their abdominal symptoms and 76% were able to drink 150-200 mL of milk at a time without anxiety of abdominal symptoms. These outcomes suggested that our original treatment for LM with ordinary milk was effective for Japanese patients without affecting quality of life. In addition, this treatment could be widely applied to Asian and African people suffering from LM[6].
Comparing the diagnostic values of LM by 20 g LHBT, undertaken before and after the treatment, abdominal symptoms improved only in one-third of the subjects and no change was seen in half of the subjects, suggesting that colonic adaptation was insufficient to see changes in diagnostic values regardless of improved symptoms. This could be due to limitations of this study such as lack of dietary restrictions except for milk, maximum amount of milk set at 200 mL, and insufficient sample size.
Some reports hypothesized that reduced symptoms were related to lactose adaptation of colonic bacteria, while other clinical studies reported that lactose induced growth of Bifidobacteria and Lactobacillues in intestinal microbiota[21,22]. Even though such bacteria were not observed in our study, it was interesting that there was a significant increase of fecal Blautia in 7 subjects who became symptom-free after the treatment. It is known that fecal Blautia is likely to decrease in patients who have obesity, liver diseases, and diabetes[23]. A fecal microbiota analysis in another study also had an interesting finding that Blautia significantly increased among subjects with LM after daily intake of 250 mL of whole milk for 4 wk[24]. Therefore, an increase of fecal Blautia found in our study indicated a favorable intestinal environment.
The treatment by incremental loads of ordinary cow’s milk was useful in treating LM without affecting quality of life. As three-fourths of the subjects with LI symptoms in our study were further diagnosed with LM and showed improved lactose tolerance post-treatment, this treatment may also benefit people with LI symptoms but unknown LM status.
Self-reported lactose intolerance (LI) has been known to have a high prevalence in Asian people. However, there has been no recent report in Japan regarding the prevalence of lactose malabsorption (LM). Some literature shows that colonic adaptation by daily milk or lactose ingestion reduces LI symptoms in patients with LM, but such treatment has not been reported in Japan.
According to the literature from Western countries, patients with LM who underwent milk or lactose loading therapy were required to ingest large volumes of milk within a short period. Applying the same treatment to Japanese people is considered to carry a high risk for abdominal symptoms during the treatment, due to less habitual consumption of milk than Western people. In this study, we implemented an original method of milk loading without affecting daily life of study subjects.
The aim of this study was to examine the efficacy of incremental cow’s milk loading for treating patients with LM.
We selected subjects with LI symptoms using a questionnaire, and the selected subjects underwent a 20 g lactose hydrogen breath test (LHBT) for diagnosis of LM. We then conducted the treatment of incremental loads of cow’s milk on the subjects diagnosed with LM, starting from 30 mL and increasing up to 200 mL at 4-7 d intervals. After the treatment, improvement of symptoms and LM diagnostic value of LHBT were investigated. Stool samples pre- and post-treatment were examined for changes in the intestinal microbiota using 16S rRNA sequencing.
By LHBT, LM was diagnosed in 35 (76%) out of 46 subjects with LI selected using the questionnaire. Improvement of abdominal symptoms after the treatment was seen in 29 (91%) out of 35 subjects with LM. The diagnostic value measured in LHBT before and after the treatment improved in 10 (35%) out of 29 subjects with reduced symptoms, and no change was observed in 16 (55%) subjects. Analysis of fecal microbiota showed a significant increase of Blautia in 7 subjects who became symptom-free after the treatment.
Incremental loads of cow’s milk that are commercially available is a useful treatment for LM without affecting daily lives of Japanese people.
The incremental loads of cow’s milk can be widely utilized for LM patients, as well as improve their quality of life. We would like to further verify the efficacy of the same treatment in a longer term study.
The authors thank Naoki Shimojo, Department of Pediatrics, Graduate School of Medicine, Chiba University, for his excellent advice. We also thank Sadako Nakamura, PhD, Jumonji University, and Yasushi Kawai, PhD, Nihon University, for their support in recruiting volunteers and for giving us useful comments throughout the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Pavlovic M, Serbia; Rocha R, Brazil; Zhang F, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | J-milk (Japan Dairy Association). The investigation about eating habit trend of milk, dairy products in 2015. March 31, 2016. Available from: https://www.j-milk.jp/report/trends/f13cn00000000x9t-att/hn0mvm0000000tv1.pdf. |
| 2. | The information of livestock: Investigation and Information Department, Agriculture & Livestock Industries Corporation. The investigation about consumption trend of milk, dairy products in 2015. 2016 May. Available from: https://www.alic.go.jp/content/000124597.pdf. |
| 3. | Szilagyi A. Adaptation to Lactose in Lactase Non Persistent People: Effects on Intolerance and the Relationship between Dairy Food Consumption and Evalution of Diseases. Nutrients. 2015;7:6751-6779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 4. | Zheng X, Chu H, Cong Y, Deng Y, Long Y, Zhu Y, Pohl D, Fried M, Dai N, Fox M. Self-reported lactose intolerance in clinic patients with functional gastrointestinal symptoms: prevalence, risk factors, and impact on food choices. Neurogastroenterol Motil. 2015;27:1138-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Briet F, Pochart P, Marteau P, Flourie B, Arrigoni E, Rambaud JC. Improved clinical tolerance to chronic lactose ingestion in subjects with lactose intolerance: a placebo effect? Gut. 1997;41:632-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 67] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Fassio F, Facioni MS, Guagnini F. Lactose Maldigestion, Malabsorption, and Intolerance: A Comprehensive Review with a Focus on Current Management and Future Perspectives. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 7. | von Ahsen U, Noller HF. Identification of bases in 16S rRNA essential for tRNA binding at the 30S ribosomal P site. Science. 1995;267:234-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Shaukat A, Levitt MD, Taylor BC, MacDonald R, Shamliyan TA, Kane RL, Wilt TJ. Systematic review: effective management strategies for lactose intolerance. Ann Intern Med. 2010;152:797-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Suchy FJ, Brannon PM, Carpenter TO, Fernandez JR, Gilsanz V, Gould JB, Hall K, Hui SL, Lupton J, Mennella J, Miller NJ, Osganian SK, Sellmeyer DE, Wolf MA. National Institutes of Health Consensus Development Conference: lactose intolerance and health. Ann Intern Med. 2010;152:792-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Gasbarrini A, Corazza GR, Gasbarrini G, Montalto M, Di Stefano M, Basilisco G, Parodi A, Usai-Satta P, Vernia P, Anania C, Astegiano M, Barbara G, Benini L, Bonazzi P, Capurso G, Certo M, Colecchia A, Cuoco L, Di Sario A, Festi D, Lauritano C, Miceli E, Nardone G, Perri F, Portincasa P, Risicato R, Sorge M, Tursi A; 1st Rome H2-Breath Testing Consensus Conference Working Group. Methodology and indications of H2-breath testing in gastrointestinal diseases: the Rome Consensus Conference. Aliment Pharmacol Ther. 2009;29 Suppl 1:1-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 11. | Kosuge N, Yoshimatsu M, Tsukada K. Investigation into lactose absorption in Japanese children and adults- Relation to intake of milk and dairy products-. J Jpn Pediatr Soc. 1998;102:1090-1097. |
| 12. | Law D, Conklin J, Pimentel M. Lactose intolerance and the role of the lactose breath test. Am J Gastroenterol. 2010;105:1726-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Pimentel M, Saad RJ, Long MD, Rao SSC. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am J Gastroenterol. 2020;115:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 253] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 14. | Zhao J, Fox M, Cong Y, Chu H, Shang Y, Fried M, Dai N. Lactose intolerance in patients with chronic functional diarrhoea: the role of small intestinal bacterial overgrowth. Aliment Pharmacol Ther. 2010;31:892-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Mearin F, Lacy BE, Chang L, Chey WD, Lembo AJ, Simren M, Spiller R. Bowel Disorders. Gastroenterology. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1781] [Cited by in RCA: 1898] [Article Influence: 210.9] [Reference Citation Analysis (3)] |
| 16. | Yang JF, Fox M, Chu H, Zheng X, Long YQ, Pohl D, Fried M, Dai N. Four-sample lactose hydrogen breath test for diagnosis of lactose malabsorption in irritable bowel syndrome patients with diarrhea. World J Gastroenterol. 2015;21:7563-7570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Wang Y, Xiong L, Gong X, Li W, Zhang X, Chen M. Small intestinal bacterial overgrowth as an uncommon cause of false positive lactose hydrogen breath test among patients with diarrhea-predominant irritable bowel syndrome in Asia. J Gastroenterol Hepatol. 2015;30:995-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Mummah S, Oelrich B, Hope J, Vu Q, Gardner CD. Effect of raw milk on lactose intolerance: a randomized controlled pilot study. Ann Fam Med. 2014;12:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Hertzler SR, Savaiano DA. Colonic adaptation to daily lactose feeding in lactose maldigesters reduces lactose intolerance. Am J Clin Nutr. 1996;64:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 152] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Misselwitz B, Butter M, Verbeke K, Fox MR. Update on lactose malabsorption and intolerance: pathogenesis, diagnosis and clinical management. Gut. 2019;68:2080-2091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 21. | Szilagyi A, Shrier I, Heilpern D, Je J, Park S, Chong G, Lalonde C, Cote LF, Lee B. Differential impact of lactose/Lactase phenotype on colonic microflora. Can J Gastroenterol. 2010;24:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Ito M, Kimura M. Influence of lactose on faecal microflora in lactose maldigestors. Microb Ecol Health Dis. 1993;6:73-76. [RCA] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, Zhao J, Zhang H, Chen W. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. 2021;13:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 828] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 24. | Li X, Yin J, Zhu Y, Wang X, Hu X, Bao W, Huang Y, Chen L, Chen S, Yang W, Shan Z, Liu L. Effects of Whole Milk Supplementation on Gut Microbiota and Cardiometabolic Biomarkers in Subjects with and without Lactose Malabsorption. Nutrients. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |