Published online Feb 6, 2023. doi: 10.12998/wjcc.v11.i4.780
Peer-review started: October 15, 2022
First decision: November 16, 2022
Revised: November 30, 2022
Accepted: January 9, 2023
Article in press: January 9, 2023
Published online: February 6, 2023
Processing time: 113 Days and 16.2 Hours
Selective laser trabeculoplasty (SLT) is a relatively safe and effective therapy in lowering intraocular pressures (IOP) for glaucoma.
To study the long-term effects of SLT on IOP and number of glaucoma medica
This is a retrospective study in which 75 eyes of 70 patients with open-angle glaucoma (OAG, n =36) and eyes with prior glaucoma surgery (PGS, n =39) were included. Changes in mean IOP and number of glaucoma medications used evaluated at 1 d, 1 wk, 1 mo, 3 mo, 6 mo, 12 mo, and 36 mo after laser treatment.
All patients (33 male, 37 female) were Chinese. The mean age was 44.34 ± 16.14 years. Mean pre-SLT IOP was 22.75 ± 2.08 mmHg in OAG and 22.52 ± 2.62 mmHg in PGS. Mean IOP was significantly reduced 1 d, 1 wk, 1 mo and 3 mo after laser treatment (P < 0.05, respectively). Whereas, there were no significant differences between baseline and SLT treated groups at the 6th month both in OAG (P = 0.347, P > 0.05) and in PGS (P = 0.309, P > 0.05). Six months after SLT treatment, some patients received retreatment of SLT or were given more topical IOP-lowering medication to control the IOP. By the end of our study, the average IOP decreased to 20.73 ± 1.82 mmHg in OAG and 20.49 ± 1.53 mmHg in PGS groups. The number of glaucoma medications used was significantly reduced until the end of 3 years compared to baseline.
SLT could reduce IOP as adjunctive treatment both in OAG and PGS groups. SLT significantly reduced the number of glaucoma medications used 3-years following treatment in glaucoma patients.
Core Tip: Selective laser trabeculoplasty (SLT) could reduce intraocular pressure as adjunctive treatment both in open-angle glaucoma and prior glaucoma surgery groups of patients. SLT significantly reduced the number of glaucoma medications used 3-years following treatment in glaucoma patients.
- Citation: Zhu J, Guo J. Selective laser trabeculoplasty as adjunctive treatment for open-angle glaucoma vs following incisional glaucoma surgery in Chinese eyes. World J Clin Cases 2023; 11(4): 780-787
- URL: https://www.wjgnet.com/2307-8960/full/v11/i4/780.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i4.780
Selective laser trabeculoplasty (SLT) is a relatively safe and effective therapy in lowering intraocular pressures (IOP) for glaucoma which was first described in 1995 by Latina and Park[1]. This is an Nd: YAG laser improves aqueous outflow by selectively targeting the pigmented trabecular meshwork (TM) cells and presumably does not produce any damage to the microstructure of the TM[2].
SLT could be offered as initial treatment at decreasing IOP for open-angle glaucoma (OAG) and ocular hypertension. The Laser in Glaucoma and ocular Hyper Tension (LiGHT) study demonstrated that SLT could be offered as a first-line treatment of decreasing IOP without the adverse effects and costs of long-term medication use[3]. However, in the LiGHT study, nearly 15% of patients treated with SLT required additional IOP lowering interventions within 1 year, and some patients require retreatment to maintain IOP lowering[3]. SLT treatment can be particularly helpful for patients with poor compliance and drug intolerance[3]. A randomized clinical trial reported that there was no statistically significant difference between the SLT treated group and the drug therapy group after 1 year[4]. Ang et al[5] demonstrated that the rate of successful IOP reduction was higher in the medication group compared with SLT group at 24 mo. However, SLT did not cause any changes affecting ocular blood flow as does tropic intraocular pressure drugs[3]. It has been reported that repeated treatment of SLT safely provides significant IOP reductions in OAG through nearly 8 years of follow-up[6].
SLT could be used to reduce the IOP of patients who failed to achieve target IOP after receiving maximally tolerated medical therapy (MTMT). Previous studies have demonstrated significant reductions in mean IOP and the number of glaucoma medications used in patients who received SLT as a secondary treatment after receiving MTM[7]. A 5-year study showed that although the long-term decrease of IOP after SLT treatment may not be obvious, the numbers of drug use were reduced[8].
SLT could reduce the uncontrolled intraocular pressure after glaucoma surgery. There are many anti-glaucoma surgeries including trabeculectomy, Ahmed glaucoma drainage valve, trabeculectomy combined with cataract extraction and other surgical methods[9]. In patients with prior glaucoma surgery (PGS), the IOP rises again due to glaucoma filtering bleb scar, inflammatory reaction, and other reasons. A prospective study reported that the IOP of 18 patients with uncontrolled IOP after trabeculectomy decreased by 24% treated with SLT for 9 mo[10]. Previous studies have also demonstrated that two groups of patients with PGS (n =53) and without operation history (n =53), the IOP reduction rates were 7.3% and 10.8% respectively after SLT treatment at 6 mo of follow-up (P = 0.42, P > 0.05)[11].
Therefore, our aim was to determine the efficacy of SLT as adjunctive treatment in patients with previous incisional glaucoma surgery whose IOP remains or becomes uncontrolled, and the number of medications used up to 3 years.
Outpatients who underwent SLT were reviewed retrospectively at the Department of Ophthalmology, Third People's Hospital of Chengdu, Affiliated Hospital of Southwest Jiao Tong University from September 2016 to January 2020. This is a single-center study. During this research, the ethical use of human subjects was approved by the Ethics and Research Committee of Chengdu Third People's hospital (Approval No. [2022]-S-5). All the patients signed written informed consent.
In our study, 75 consecutive eyes of 70 patients (33 male, 37 female) with OAG (n = 36), and PGS (n = 39) were enrolled. The information of patients is shown in Table 1.
| Characteristics | |
| Age (yr, mean ± SD) | 44.34 ± 16.14 |
| Gender | |
| Male (n) | 33 (3 both eyes) |
| Female (n) | 37 (2 both eyes) |
| Diagnosis | |
| OAG (n) | 36 |
| PGS (n) | 39 |
| Total | 75 |
Inclusion criteria: Age ≥ 18 years, an increased IOP (> 21 mmHg), open atrial angle and scleral process can be seen by gonioscopy, OAG diagnostic criteria are met. OAG diagnostic criteria: Glaucomatous optic nerve damage (cup-to-disc ratio, C/D > 0.5, or difference in the C/D > 0.2), visual field defect, mean defect of visual field < -1.00 DB[12]. Inclusion criteria of PGS group: Those diagnosed as OAG, who have undergone trabeculectomy, drainage nail implantation, Ahmed glaucoma valve implantation or trabeculectomy combined with cataract extraction (Table 2); These patients have been treated with MTMT, but fail to reach the target IOP[11]. Exclusion criteria consisted of patients who were, unable to have SLT successfully performed, history of uveitis, or lost to follow up before 1 mo. Also, patients with other prior major incisional eye surgeries were excluded apart from cataract surgery alone.
| Type of PGS | Trabeculectomy | Drainage nail implantation | Ahmed glaucoma valve implantation | Trabeculectomy combined with cataract extraction |
| Number of patients | 18 | 2 | 4 | 15 |
All patients received SLT more than 1 year after anti-glaucoma surgery. These patients agreed to SLT treatment and signed the consent form for laser surgery.
Eyes were pretreated with topical anesthesia of 0.4% obucaine hydrochloride (Benoxil). And Latina anterior chamber gonioscope was placed after anesthesia. SLT was performed with a q-switched, frequency-doubled 532 nm Nd: YAG laser (selecta duet, Lumenis, Israel) which has a spot size of 400 μm and pulse duration of 3ns. Nonoverlapping 100 ± 10 Laser spots were applied to 360 degrees of the TM. The initial energy level was set at 0.8 mJ. The energy was increased or decreased until cavitation bubbles within the TM were just noted. In this study, the therapeutic energy was 0.6-1.2 mJ. Pranoprofen eye drops were used as postoperative medications.
Detailed ophthalmic examinations including visual acuity, intraocular pressure, slit lamp microscope, gonioscopy, visual field, Optical Coherence tomography for retinal nerve fiber layer thickness, funduscopic examination was conducted both before SLT. After SLT, the patients were followed up regularly (1 d, 1 wk, 1 mo, 3 mo, 6 mo, 1 year and 3 year). Complications, IOP and C/D were observed at each follow-up time point. The IOP was measured by Goldmann applanation in all patients. The 3 years follow-up were monitored by National Institute for Health and Care Excellence guidance to avoid bias in clinical decision-making.
SPSS 23.0 (version 23.0; IBM Corporation, Armonk, NY, USA) statistical software was used for statistical analysis. The means and standard deviation of IOP at different time points before and after SLT treatment was calculated. The paired t-test of two independent samples was used to compare the IOP between baseline and post treatment at different time points for statistical analysis; The statistical analysis of the number of medications used were analyzed using Wilcoxon signed rank test and tested using Generalized Estimating Equations and Poisson regression models. The variables that did not meet the normal distribution were analyzed using the Mann Whitney U test. The comparative evaluation of treatment effects between and within different types of glaucoma after SLT was tested by analysis of variance. The success rate was calculated with Kaplan-Meier survival curve analysis. P < 0.05 was considered statistically significant.
In this study, SLT was performed on 75 eyes from 70 OAG patients (33 males and 37 females) included. Before SLT treatment, all eyes were given glaucoma medications (1 to 4 drugs). The average age of patients receiving SLT was 44.34 ± 16.14 yr (Table 1). The mean IOP before treatment was 22.75 ± 2.08 mmHg in OAG and 22.52 ± 2.62 mmHg in PGS (Table 3).
| Groups | Baseline | 1 d | 1 wk | 1 mo | 3 mo | 6 mo | 12 mo | 36 mo |
| Eyes (n) | 36 | 36 | 36 | 36 | 36 | 36 | 35 | 28 |
| OAG | 22.75 ± 2.08 | 16.29 ± 3.62 | 18.97 ± 5.42 | 18.62 ± 4.39 | 21.32 ± 2.19 | 22.59 ± 2.26 | 20.13 ± 1.8 | 20.73 ± 1.82 |
| P1 value | < 0.0001 | 0.0005 | 0.0005 | 0.0020 | 0.3465 | < 0.0001 | 0.0009 | |
| Cumulative proportion of SLT success (%) | 87.13 | 76.81 | 68.27 | 47.66 | 24.96 | |||
| Eyes (n) | 39 | 39 | 39 | 39 | 39 | 39 | 33 | 29 |
| PGS | 22.52 ± 2.62 | 16.45 ± 4.16 | 18.51 ± 5.09 | 18.80 ± 4.99 | 19.21 ± 3.82 | 21.94 ± 2.11 | 20.28 ± 1.61 | 20.49 ± 1.53 |
| P1 value | < 0.0001 | 0.0002 | 0.0002 | 0.0002 | 0.3086 | < 0.0001 | 0.0002 | |
| Cumulative proportion of SLT success (%) | 84.70 | 67.40 | 59.39 | 42.15 | 27.61 | |||
| P2 value | 0.517 | 0.785 | 0.517 | 0.696 | 0.006 | 0.433 | 0.987 | 0.594 |
The average number of medications used before SLT treatment was 3.39 ± 0.69 in OAG and 2.97 ± 0.74 in PGS group (Table 4). The course of IOP over the 36 mo of the study is shown in Table 3. 75 eyes were followed up for longer than 1 year, and 58 eyes were followed up for more than 3 years.
| Number of medications | Baseline | 1 d | 1 wk | 1 mo | 3 mo | 6 mo | 12 mo | 36 mo |
| OAG | 3.39 ± 0.69 | 3.31 ± 0.82 | 2.75 ± 0.5 | 2.06 ± 0.79 | 2.19 ± 0.71 | 2.39 ± 0.64 | 2.34 ± 0.64 | 2.5 ± 0.69 |
| P1 value | 0.083 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| PGS | 2.97 ± 0.74 | 2.85 ± 0.67 | 2.51 ± 0.56 | 1.33 ± 1.28 | 1.44 ± 1.23 | 1.77 ± 0.99 | 1.79 ± 1.02 | 1.9 ± 0.82 |
| P1 value | 0.025 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| P2 value | 0.015 | 0.005 | 0.048 | 0.004 | 0.003 | 0.006 | 0.008 | 0.007 |
In the OAG group, IOP began to rise at the 6th month (P = 0.3465, P > 0.05). The mean IOP was 22.59 ± 2.26 mmHg at the 6th month and there was no significant difference compared to baseline.
Six months after SLT treatment, 6 eyes undertook retreatment of SLT, 4 eyes were given more topical IOP-lowering medications to control the IOP in the OAG group.
In the PGS group, there was a statistically significant lower IOP in the study compared with pretreatment levels after SLT treatment at all points (P < 0.001; Table 3) except for the 6th month (P =0.309, P > 0.05). The average IOP was 21.94 ± 2.11 mmHg at 6 mo and there was no significant difference compared to the baseline. At the 6th month, 4 eyes undertook retreatment of SLT and 2 eyes were given more topical IOP-lowering medications to control the IOP.
In this retrospective study, there was no statistically significant difference between the OAG and PGS groups at all time intervals (P > 0.05; Table 3) except for the 3rd month (P = 0.0039, P < 0.05; Table 3). During the first 3 mo of follow-up, the IOP in the PGS group was 18.76 ± 3.92 mmHg and that in the OAG group was 21.32 ± 2.19 mmHg. The PGS group had a better and longer effect on IOP than the OAG group.
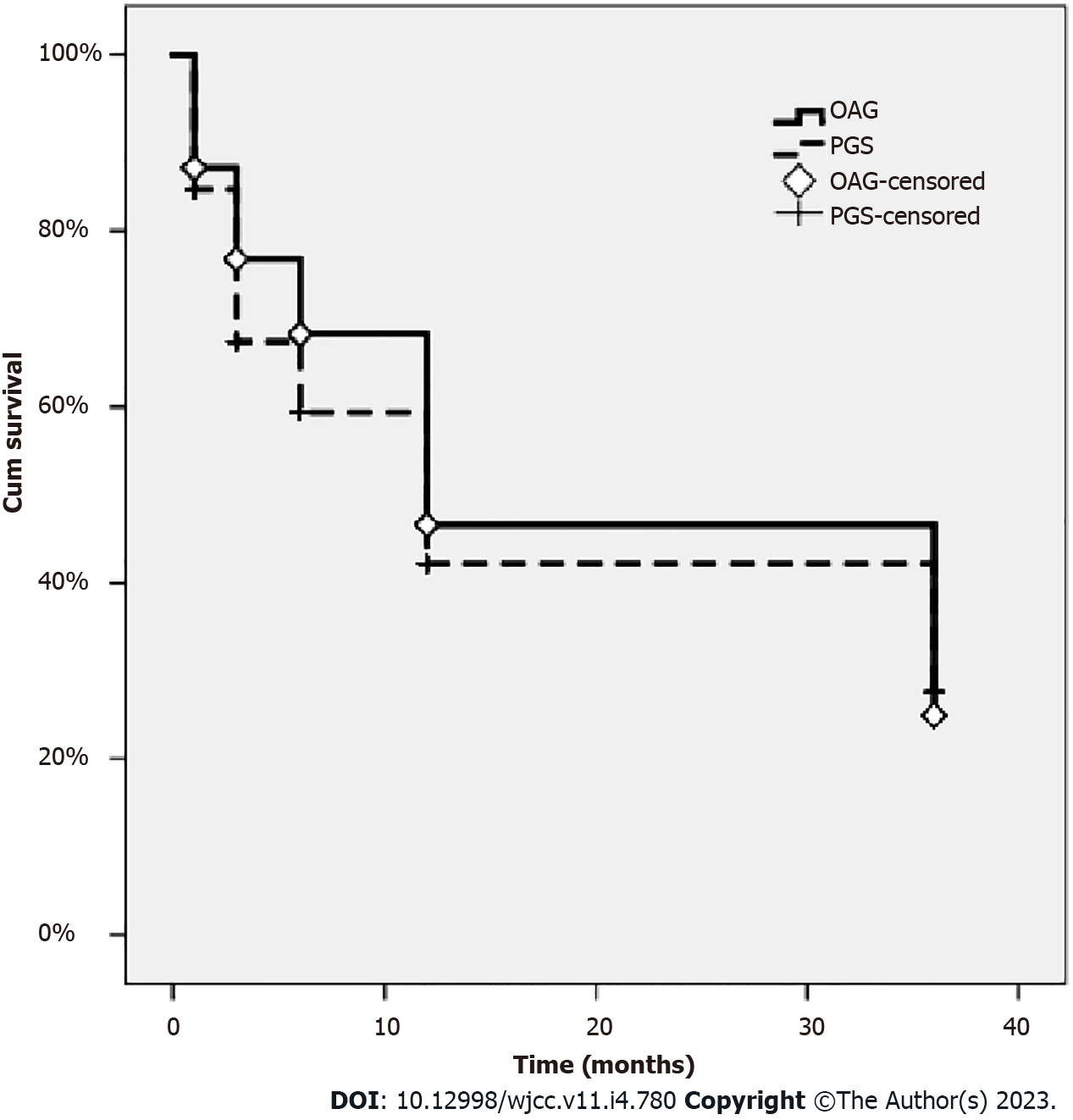
On the Kaplan-Meier survival analysis, the success rates after 1, 3, 6, 12, and 36 mo were 87.13%, 76.81%, 68.27%, 47.66% and 24.96% in the OAG group, and 84.70%, 67.40%, 59.39%, 42.15% and 27.61% in the PGS group, respectively (P = 0.320; Log-rank test) (Figure 1 and Table 3).
Table 4 presented the number of glaucoma medications used. There were no statistical differences on the 1st day after SLT treatment compared with the baseline (P = 0.083, P > 0.05). As the ocular spike was observed in part of patients at the 1st day after laser treatment, the number of anti-glaucoma drugs were not changed. The number of drugs was gradually reduced 1 wk after treated by laser and lasted for 36 mo.
Of the 70 patients, 5 had both eyes treated. Six eyes experienced IOP spikes on the 1st day after SLT. In these eyes, the IOP returned to baseline after the appropriate intervention. No other complications after SLT therapy occurred in any eye. In the span of 3 years, 10 eyes (12.99%) underwent a repeat SLT, 5 eyes (6.49%) underwent glaucoma surgery. At the 6th months point, two patients in the PGS group had been excluded because of the progression of visual field, and they underwent glaucoma surgery again.
Conjunctival hyperemia occurred in 56 eyes (72.7%), which disappeared after 1 d. There was an anterior chamber reaction in 49 eyes (50.51%). Tyndall (±) ~ (+) in the anterior chamber was observed in 1 ~ 2 h after SLT and disappeared after 1 wk. A transient IOP elevation (spike) was observed 1 h after SLT, IOP ≥ 5 mmHg in 6 eyes (7.79%) and IOP ≥ 1 ~ 4 mmHg in 15 eyes (19.48%).
There were no severe complications in these patients, such as ocular inflammation, hyphemia, choroidal effusion, or retinal detachment.
Due to the IOP spike after treatment, the number of drugs used on the 1st day after SLT treatment did not decrease. Whereas, on the 1st day after SLT treatment, the IOP was greatly reduced. The difference was statistically significant at different time points until the 6th month after SLT treatment. Two prospective studies have reported that SLT can effectively reduce IOP 4 years and 6 years after treatment in patients who have used the maximum dose of IOP reducing drugs. 44% and 59% of patients have at least 20% of an IOP reduction, respectively[6,13]. However, our results showed that the action time of SLT lasted about 6 mo. After the 6th month, the IOP of some patients increased again. Therefore, at the 6th month after SLT treatment, some patients underwent SLT treatment again or added the numbers of anti-glaucoma drugs to control IOP. This result is not consistent with the published results, which may be related to race, trabecular meshwork structure or the distribution of trabecular meshwork pigment. The effectiveness and safety of SLT was affected by pigmentation of trabecular meshwork and laser energy[14].
In our study, the PGS group was included. These patients had a history of previous glaucoma surgery, 39 eyes, including 18 eyes after trabeculectomy, 2 eyes after drainage nail implantation, 4 eyes after glaucoma valve implantation and 15 eyes after trabeculectomy combined with cataract extraction. The chamber angular structure was not changed except for the surgical site in the PGS group, which provided the conditions for the SLT treatment. As there was no significant difference in IOP after SLT treatment between OAG and PGS groups, the history of glaucoma surgery might have little impact on the results of SLT laser treatment. The residual function in the trabecular meshwork pathway still has potential to be modulated in a post-surgical eye, which provided the conditions for the SLT treatment.
The energy used was between 0.6-1.2 mJ in our study. High energy laser could cause transient high intraocular pressure. For patients with heavy pigmentation in trabecular meshwork, high energy laser may lead to continuous IOP[15]. It was reported that SLT with appropriate high energy (1.2-1.5 mJ) can effectively reduce IOP in patients with steroid-induced glaucoma[16]. It is also reported that low-energy SLT treatment is also effective. The results show that low-energy laser could also effectively reduce IOP in OAG patients for 2 years after 360° SLT with initial energy of 0.3 mJ[17]. In the future research, we could further analyze the impact on IOP with different laser energy.
Our study shows that SLT could reduce the number of drugs as an adjunctive therapy. Juzych reported that in another study of OAG, SLT could effectively reduce IOP and reduce the number of drugs used after 5 years[18]. In a prospective randomized controlled study, the number of drugs decreased in varying degrees 1, 3 and 5 years after SLT treatment[19]. These results suggest that SLT can be used in the treatment of glaucoma patients with poor drug or surgical control.
A limitation of this study is a retrospective study which may have selection bias. It would be a better control for this potential selection bias in a prospective study. There are more types of glaucoma that could be treated by SLT, such as glaucoma secondary to silicone oil eye after vitrectomy, steroid-induced glaucoma and so on[16].
In summary, SLT may be efficacious in eyes with prior incisional glaucoma surgery and it provides an effective treatment option to lower IOP to avoid or postpone subsequent incisional glaucoma procedures.
Selective laser trabeculoplasty (SLT) is a relatively safe and effective therapy in lowering intraocular pressures (IOP) for glaucoma. SLT could be offered as an initial treatment at decreasing IOP for open-angle glaucoma (OAG) and ocular hypertension. SLT could be used to reduce the IOP of patients who failed to achieve target IOP after receiving maximally tolerated medical therapy. SLT could reduce the uncontrolled intraocular pressure after glaucoma surgery.
To find out whether SLT could reduce IOP in patients with prior glaucoma surgery.
Our aim was to determine the efficacy of SLT as adjunctive treatment in patients with previous incisional glaucoma surgery whose IOP remains or becomes uncontrolled, and the number of medications used up to 3 years.
Outpatients who underwent SLT were reviewed retrospectively at the Department of Ophthalmology, Third People's Hospital of Chengdu, Affiliated Hospital of Southwest Jiao Tong University from September 2016 to January 2020. 75 consecutive eyes of 70 patients (33 male, 37 female) with OAG (n = 36), and PGS (n = 39) were enrolled. The IOP was measured both before and after SLT and followed up to 3 years.
The means and standard deviations of IOP at different time points before and after SLT treatment was calculated. The statistical analysis of the number of medications used were analyzed using the Wilcoxon signed rank test. The comparative evaluation of treatment effects between and within different types of glaucoma after SLT was tested by the analysis of variance. The success rate was calculated with the Kaplan-Meier survival curve analysis.
The average age of patients receiving SLT was 44.34 ± 16.14 yr (Table 1). The mean IOP before treatment was 22.75 ± 2.08 mmHg in the OAG group and 22.52 ± 2.62 mmHg in the PGS group (Table 3). The average number of medications used before SLT treatment was 3.39 ± 0.69 in the OAG group and 2.97 ± 0.74 in the PGS group (Table 4). 75 eyes were followed up for longer than 1 year, and 58 eyes were followed up for more than 3 years. There was no statistically significant difference between the OAG and PGS groups. The success rates after 1, 3, 6, 12, and 36 mo were 87.13%, 76.81%, 68.27%, 47.66% and 24.96% in the OAG group, and 84.70%, 67.40%, 59.39%, 42.15% and 27.61% in the PGS group, respectively. The number of drugs was gradually reduced 1 wk after being treated by laser and lasted for 36 mo.
SLT could reduce IOP as an adjunctive treatment both in the OAG and PGS groups. The residual function in the trabecular meshwork pathway still has potential to be modulated in a post-surgical eye, which provided the conditions for the SLT treatment. SLT significantly reduced the number of glaucoma medications used 3-years following treatment in glaucoma patients.
SLT may be efficacious in eyes with prior incisional glaucoma surgery and it provides an effective treatment option to lower IOP to avoid or postpone subsequent incisional glaucoma procedures.
We thank all patients for their participation and trust in our study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bravetti GE, Switzerland; Liu Q, China S-Editor: Liu GL L-Editor: Filipodia P-Editor: Liu GL
| 1. | Latina MA, Park C. Selective targeting of trabecular meshwork cells: in vitro studies of pulsed and CW laser interactions. Exp Eye Res. 1995;60:359-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 248] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 2. | Latina MA, Tumbocon JA. Selective laser trabeculoplasty: a new treatment option for open angle glaucoma. Curr Opin Ophthalmol. 2002;13:94-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 3. | Gazzard G, Konstantakopoulou E, Garway-Heath D, Garg A, Vickerstaff V, Hunter R, Ambler G, Bunce C, Wormald R, Nathwani N, Barton K, Rubin G, Morris S, Buszewicz M. Selective laser trabeculoplasty versus drops for newly diagnosed ocular hypertension and glaucoma: the LiGHT RCT. Health Technol Assess. 2019;23:1-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 4. | Apolostolski S, Susaković N, Lavrnić S, Stolić I, Trikić R. HLA antigens and immunoglobulin allotypes in myasthenia gravis. Neurologija. 1987;36:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 5. | Ang GS, Fenwick EK, Constantinou M, Gan ATL, Man REK, Casson RJ, Finkelstein EA, Goldberg I, Healey PR, Pesudovs K, Sanmugasundram S, Xie J, McIntosh R, Jackson J, Wells AP, White A, Martin K, Walland MJ, Crowston JG, Lamoureux EL. Selective laser trabeculoplasty versus topical medication as initial glaucoma treatment: the glaucoma initial treatment study randomised clinical trial. Br J Ophthalmol. 2020;104:813-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Weinand FS, Althen F. Long-term clinical results of selective laser trabeculoplasty in the treatment of primary open angle glaucoma. Eur J Ophthalmol. 2006;16:100-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Sayin N, Alkin Z, Ozkaya A, Demir A, Yazici AT, Bozkurt E, Demirok A. Efficacy of selective laser trabeculoplasty in medically uncontrolled glaucoma. ISRN Ophthalmol. 2013;2013:975281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Patel V, El Hawy E, Waisbourd M, Zangalli C, Shapiro DM, Gupta L, Hsieh M, Kasprenski A, Katz LJ, Spaeth GL. Long-term outcomes in patients initially responsive to selective laser trabeculoplasty. Int J Ophthalmol. 2015;8:960-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Stein JD, Khawaja AP, Weizer JS. Glaucoma in Adults-Screening, Diagnosis, and Management: A Review. JAMA. 2021;325:164-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 358] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Yang Y, Xu J, Yu M. Selective laser trabeculoplasty in treating post-trabeculectomy advanced primary open-angle glaucoma. Exp Ther Med. 2016;11:1090-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Sharpe RA, Kammerdiener LL, Williams DB, Das SK, Nutaitis MJ. Efficacy of selective laser trabeculoplasty following incisional glaucoma surgery. Int J Ophthalmol. 2018;11:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Francis BA, Loewen N, Hong B, Dustin L, Kaplowitz K, Kinast R, Bacharach J, Radhakrishnan S, Iwach A, Rudavska L, Ichhpujani P, Katz LJ. Repeatability of selective laser trabeculoplasty for open-angle glaucoma. BMC Ophthalmol. 2016;16:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Gracner T, Falez M, Gracner B, Pahor D. [Long-term follow-up of selective laser trabeculoplasty in primary open-angle glaucoma]. Klin Monbl Augenheilkd. 2006;223:743-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Kinori M, Hostovsky A, Skaat A, Schwartsman J, Melamed S. A novel method for quantifying the amount of trabecular meshwork pigment in glaucomatous and nonglaucomatous eyes. J Glaucoma. 2014;23:e13-e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Leahy KE, White AJ. Selective laser trabeculoplasty: current perspectives. Clin Ophthalmol. 2015;9:833-841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Xiao J, Zhao C, Liang A, Zhang M, Cheng G. Efficacy and Safety of High-Energy Selective Laser Trabeculoplasty for Steroid-Induced Glaucoma in Patients with Quiescent Uveitis. Ocul Immunol Inflamm. 2021;29:766-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Xu L, Yu RJ, Ding XM, Li M, Wu Y, Zhu L, Chen D, Peng C, Zeng CJ, Guo WY. Efficacy of low-energy selective laser trabeculoplasty on the treatment of primary open angle glaucoma. Int J Ophthalmol. 2019;12:1432-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Juzych MS, Chopra V, Banitt MR, Hughes BA, Kim C, Goulas MT, Shin DH. Comparison of long-term outcomes of selective laser trabeculoplasty versus argon laser trabeculoplasty in open-angle glaucoma. Ophthalmology. 2004;111:1853-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 19. | Bovell AM, Damji KF, Hodge WG, Rock WJ, Buhrmann RR, Pan YI. Long term effects on the lowering of intraocular pressure: selective laser or argon laser trabeculoplasty? Can J Ophthalmol. 2011;46:408-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |