Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8535
Peer-review started: September 6, 2023
First decision: November 1, 2023
Revised: November 14, 2023
Accepted: December 12, 2023
Article in press: December 12, 2023
Published online: December 26, 2023
Processing time: 107 Days and 5.7 Hours
Clear cell renal cell carcinoma (ccRCC) is a common type of tumor that can metastasize to any organs and sites. However, it is extremely rare for ccRCC to metastasize to the iris. Here, we describe a rare case of iris metastasis from ccRCC with a history of left nephrectomy in 2010.
A 62-year-old male was admitted to the hospital due to blurred vision and red eyes, and a mass was found on the iris in the right eye. B-scan ultrasonography revealed a well-bounded high-density lesion at the corner of the anterior chamber at the 3-4 o’clock position. Phacoemulsification with simultaneous intraocular lens implantation and iridocyclectomy was performed in the right eye. The lesion was confirmed to be metastatic ccRCC by histological and immunohistochemical analyses. The patient was still alive at 9 mo after surgical treatment. Ocular metastasis can be an initial sign with a poor prognosis. Timely detection and treatment may improve survival. Clinicians should pay attention to similar metastatic diseases to prevent misdiagnosis leading to missed treatment oppor
This report of the characteristics and successful management of a rare case of iris metastasis from ccRCC highlights the importance of a comprehensive medical history, histopathology, immunohistochemistry, and clinical manifestation for successful disease diagnosis.
Core Tip: Here, we report a rare case of iris metastasis from clear cell renal cell carcinoma. We found that a complete medical history, histopathology, and immunohistochemistry combined with clinical manifestations are crucial for the successful diagnosis of this disease. In addition, a total of 11 cases of iris metastasis from renal tumors were identified in the literature and are reviewed in this report.
- Citation: Wang TT, Chen XY, Min QY, Han YZ, Zhao HF. Iris metastasis from clear cell renal cell carcinoma: A case report. World J Clin Cases 2023; 11(36): 8535-8541
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8535.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8535
Renal cell carcinoma (RCC) is the most common type of renal tumor (approximately 90%), and clear cell RCC (ccRCC) is the most common subtype of RCC, accounting for approximately 75% of cases. More than 50% of patients with ccRCC are asymptomatic and rely on computed tomography (CT) for diagnosis[1]. While up to half of ccRCCs are confined to the kidney at presentation, approximately 30% develop metastases[2]. CcRCC can metastasize to any organs and sites including the eyes, but the most common sites of metastases are lung (50%) and bone (33%)[3]. It is very unusual for ccRCC to metastasize to the iris.
The iris is a rare location for cancer to metastasize and spread, and most instances occur with breast and lung cancers[4]. Iris metastasis is usually unilateral and unifocal; bilateral and multifocal involvement are considerably less common. Due to its rarity, iris metastasis is often misdiagnosed. In patients with iris lesions and a history of RCC, the possibility of kidney cancer metastasis should be considered. The clinician should perform an iridocyclectomy promptly and confirm the diagnosis by pathology.
In this article, we report a 62-year-old male who was diagnosed with iris metastasis from ccRCC. We hope this case will raise awareness of this rare disease, as clinical suspicion of this condition will lead to early diagnosis and treatment.
A 62-year-old male was admitted to the hospital due to the discovery of a mass in his right eye 1 mo prior with no history of eye disease.
The patient had a medical history of blurred vision and red eyes.
A previous left nephrectomy with the diagnosis of ccRCC [World Health Organisation/International Society of Urological Pathology (WHO/ISUP) nuclear grading was not mentioned].
The patient denied any family medical history.
The visual acuity of the left eye was 0.6 and that of the right eye was 0.4. The conjunctiva of the right eye was slightly congested, and the vessels of the nasal conjunctiva were tortuous and dilated. The cornea was clear. A keratic precipitate was visible behind the cornea and a red mass with a diameter of approximately 5 mm was visible at 2-5 o’clock in the front of the cornea. The depth of the anterior chamber was moderate. The iris texture was not clear and was partially posterior synechiae. The pupil was round, but slow to light reflex. Pigment was seen in the precrystalline capsule. There was no obvious abnormality in the left eye except the phacoscotasmus. The intraocular pressure was 14 mmHg in the left eye and 21 mmHg in the right eye.
Complete blood counts, aspartate aminotransferase, alkaline phosphatase, bilirubin, serum electrolytes, creatinine, and urea were all within normal ranges.
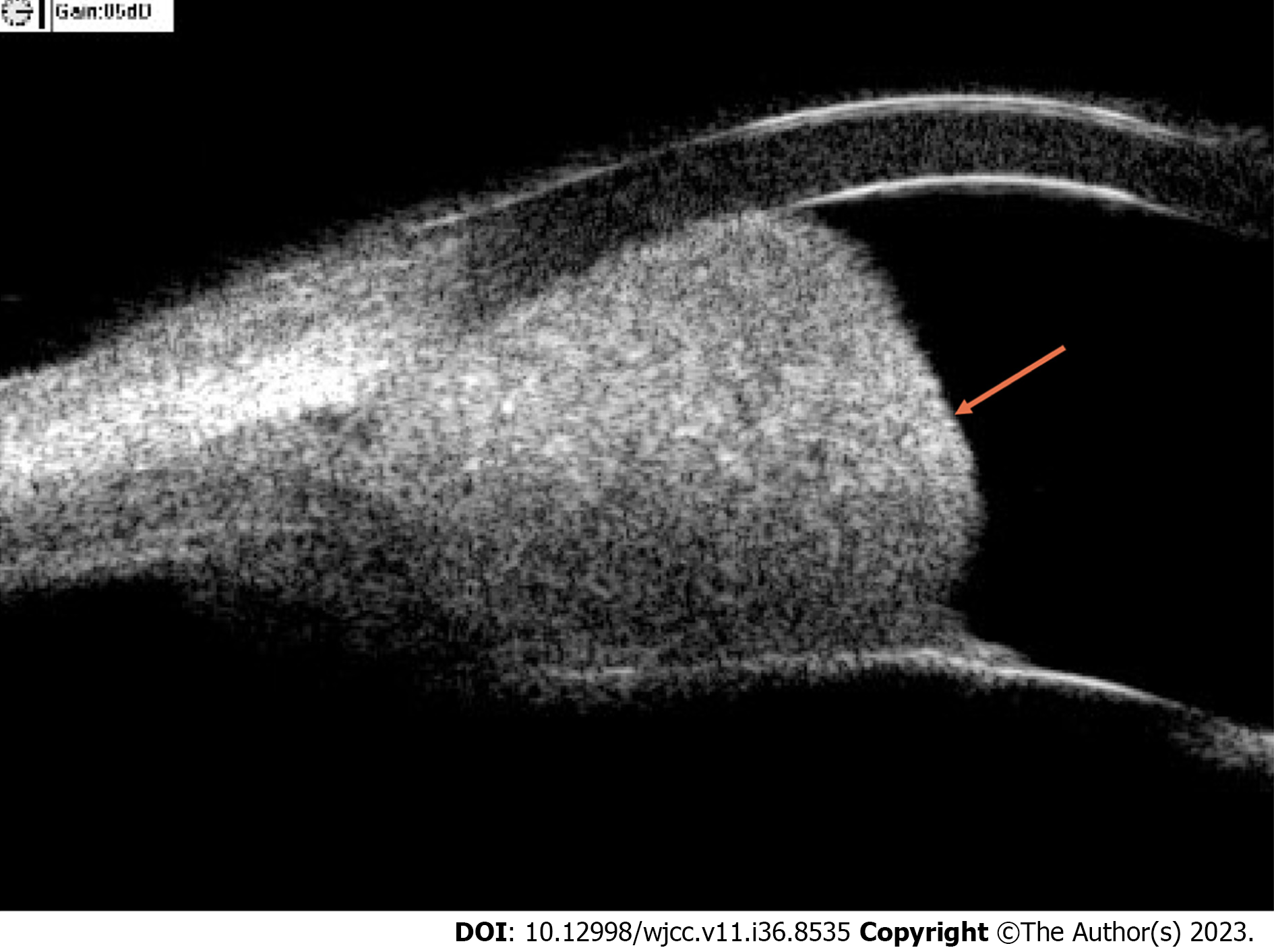
B-scan ultrasonography revealed that the central depth of the anterior chamber was approximately 3.14 mm and a well-bounded high-density lesion could be seen at the corner of the anterior chamber at 3-4 o’clock (Figure 1). Chest CT showed a lobulated uneven enhanced mass at the anterior basal segment of the lower lobe of the right lung, with clear boundaries and small streaks at the margin, indicating the high possibility of malignancy. Abdominal CT showed that the left kidney was absent and there was no obvious mass in the right kidney.
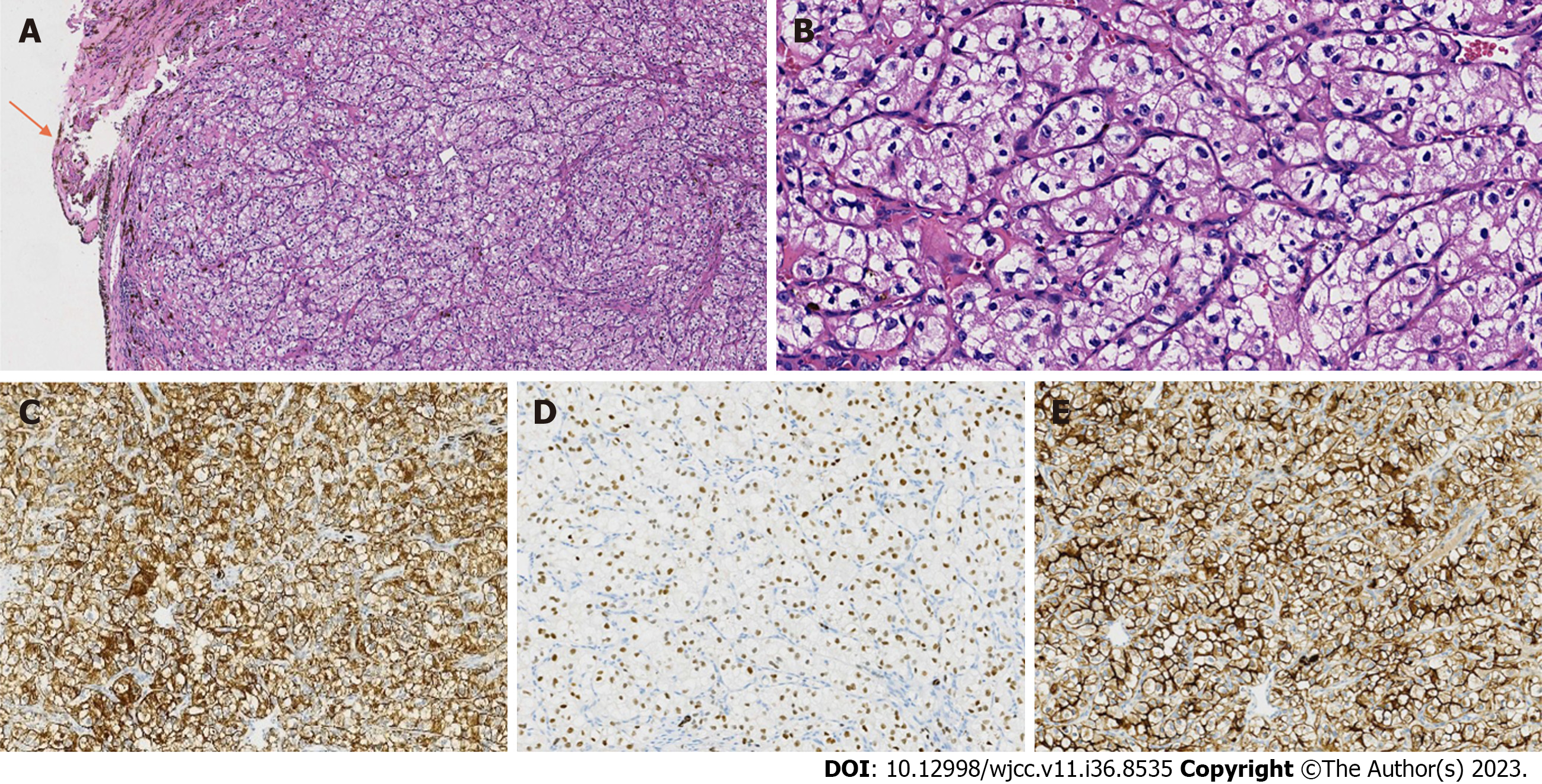
The mass was received in several small pieces with a total diameter of 1 cm. Histologically, normal iris tissue could be seen around the tumor (Figure 2A). There was no fibrous envelope separation between the tumor and iris tissue. The tumor cells were large, cube-shaped, and in a solid nest-like arrangement. The cytoplasm was clear because it contained a lot of glycogen and lipids. The nuclei were round or ovate, and the nucleoli were visible (hematoxylin and eosin staining, 400 ×) (Figure 2B). There were abundant capillaries in the mesenchyme. Tumor cells were immunoreactive for cytokeratin pan (Figure 2C), vimentin, paired box gene 8 (Figure 2D), cluster of differentiation 10 (CD10) (Figure 2E), and carbonic anhydrase IX, but were negative for cytokeratin 7, CD117, CK20, and thyroid transcription factor-1. The Ki-67 proliferation index was 10%.
The lesion was confirmed to be metastatic ccRCC (WHO/ISUP nuclear grade 2).
To restore the patient’s vision and make a clear diagnosis, phacoemulsification with simultaneous intraocular lens implantation and iridocyclectomy was performed after the clinician assessed the patient’s condition in the right eye. During the operation, a lesion about the size of a peanut could be clearly seen on the surface of the iris. The lesion was confirmed to be metastatic ccRCC. After the diagnosis was confirmed, the patient refused targeted drug therapy and transbronchial lung biopsy (TBLB) and discharged himself from the hospital against the advice of doctors.
The patient was still alive, with no recurrence noted at 9 mo post-surgery.
RCC includes many subtypes, with the predominant ones being ccRCC, papillary renal tumors, and chromophobe renal tumors[5]. Of these three subtypes, ccRCC is the most common and has the worst prognosis because it is usually found at an advanced stage[4]. CcRCC may have no specific symptoms in the early stage, whereas symptoms such as hematuresis may appear in the advanced stage. However, a diagnosis cannot be made only by urine examination and should be assisted by imaging examination. More than 90% of kidney tumors can be detected by CT and such imaging can distinguish between benign or malignant tumors, as well as their relationship to surrounding tissue[1].
The preferred treatment for any nonmetastatic, solid, or Bosniak III or IV complex cystic kidney mass is surgical excision, preferably using a minimally invasive approach[1]. After local nephrectomy, tumors often recur near the surgical scar, and approximately 30% of nonmetastatic kidney tumors will metastasize to other organs after surgery[6]. Metastasis of RCC is hematogenous and lymphatic. The lung is the most common location of distant metastasis, followed by the bone and liver. Ocular metastasis is rare, and iris metastasis has only been recorded in a few cases[3,4,6-8]. Both primary and metastatic renal tumors have von Hippel-Lindau (VHL) mutations, while primary tumors also have the telomerase reverse transcriptase (TERT) promoter C228T mutation, but abnormal deletion of TERT appears in metastatic tumors[2]. The absence of TERT abnormality in metastasis supports the notion that an aberrant VHL protein is sufficient to confer metastatic capacity in ccRCC, as proposed in tumors with low-grade histology. Metastasis of ccRCC to the iris may originate from low-grade clones in high-grade primary tumors[2].
Iris metastasis is extremely rare. In previous reports, the most common site of uveal metastases is the choroid, followed by the iris and ciliary body. This may be due to the posterior ciliary artery supplying a large amount of blood to the posterior choroid. Iris metastasis accounts for 7.8% of uveal metastasis[8]. The most common primary tumors with iris metastasis are breast cancer in women and lung cancer in men[3]. In addition, iris metastasis can also occur with esophageal squamous cell carcinoma[9], prostate carcinoma[10], kidney cancer[1-4,6,7], gastrointestinal malignancies[9], sarcoma and melanoma[11]. Nephrogenic iris metastasis accounts for < 1% of ocular metastasis and < 5% of iris metastasis[12]. After diagnosis of uveal metastatic cancer, more than half of patients have associated systemic metastasis, with the lungs being the most common site, followed by bones, liver, and central nervous system[2]. Our patient had suspected lung metastasis, but the patient refused TBLB, and we were unable to make a definitive diagnosis.
In the domestic and international literature, we found only 12 cases of iris metastasis from renal tumors, including our report (Table 1). This further confirms the rarity of nephrogenic iris metastases. The 12 patients were all male, aged between 54-years-old and 70-years-old. There were eight cases of blurred vision and decreased vision. The iris masses were 2-10 mm in size. Among them, 8 cases had ccRCC as the primary tumor and 1 case had renal adenocarcinoma. The time from diagnosis of RCC, ccRCC, or renal adenocarcinoma to metastasis to the iris ranged from 1 mo to 13 years, and 4 cases were detected by iris metastasis as the first manifestation. There were 4 cases of ipsilateral metastasis and five cases of contralateral metastasis, and only one case of bilateral iris metastasis. There were 3 cases that only metastasized to the iris, compared with 5 cases that simultaneously metastasized to the iris and lungs and 7 cases with metastasis to other organs. Four patients died between 2 and 9 mo after the diagnosis of iris metastasis due to multiple site metastasis or cerebral infarction. Only 5 patients remained alive during the follow-up (6-18 mo).
| Ref. | Sex | Age in year | Presenting symptoms | Tumor size | Type of RCC | Time to detection of iris metastasis after diagnosis of primary RCC | Simultaneous lung metastasis | Other metastatic sites | Systemic outcome |
| Lopes Abath Neto et al[2] | Male | 56 | Painless blurred vision in right eye | 3.5 mm × 2.2 mm | ccRCC (right) | Iris metastasis is the first manifestation | NA | Nothing | Alive |
| Ware et al[3] | Male | 70 | Iris mass in left eye | 3 mm × 2 mm | RCC (left) | Iris metastasis is the first manifestation | NA | Conjunctiva | NA |
| Shome et al[4] | Male | 67 | Progressive painless vision loss in left eye for 3 mo | 2.8 mm × 1.5 mm | ccRCC (right) | 14 mo | Yes | Bone, lymph node | Alive |
| Ikeda et al[7] | Male | 55 | Decreased vision in the right eye | 8 mm × 4 mm | ccRCC (right) | 21 mo | Yes | Brain, liver, bone, lymph nodes | Deceased |
| Marie-Louise et al[12] | Male | NA | Ocular tumor, ocular hypertension | NA | ccRCC | NA | NA | Cerebellar | Alive |
| Wyzinski et al[13] | Male | 60 | Sudden and painless loss of vision in right eye (bilateral mass) | 2.8 mm | RCC (left) | Iris metastasis is the first manifestation | NA | Nothing | Deceased |
| Mello et al[14] | Male | 61 | Blurred vision and iris lesion in right eye | 7 mm × 4 mm | Renal adenocarcinoma | 5 yr | Yes | Cerebrum, liver | Alive |
| Ramskold et al[15] | Male | 63 | Pain in right eye, photophobia | 8.1 mm × 5.7 mm × 4.6 mm | RCC (left) | 2 yr | Yes | Mediastinum, paratracheal lymph nodes | NA |
| Lou and Zhang[16] | Male | 54 | Foreign body sensation in left eye | Mung bean size | ccRCC (left) | 1 mo | Yes | Nothing | NA |
| Xing[17] | Male | 59 | Blurred vision in left eye; pain and exophthalmia in right eye | 4 mm × 4 mm | ccRCC (right) | 2 mo | NA | Brain, skin, rib | Deceased |
| Lu et al[18] | Male | 59 | Left eye secretions, foreign body sensation, red eye, swelling pain, vision loss, ipsilomatous headache, nausea, vomiting | 4.5 mm × 3 mm × 1.5 mm | ccRCC (right) | Iris metastasis is the first manifestation | NA | Nothing | Deceased |
| This study | Male | 62 | Blurred vision, red eyes, mass on iris in right eye | 10 mm | ccRCC (left) | 13 yr | NA | Nothing | Alive |
The main clinical manifestations of iris metastasis are blurred vision, ocular pain, redness, photophobia[8], diplopia, exophthalmos, and periorbital swelling[9]. Visual acuity is usually poor when patients develop ipsilateral choroidal metastasis associated with retinal detachment, or with severe anterior chamber tumor implantation with secondary glaucoma[7]. When tumor cells spread to the anterior chamber, secondary glaucoma can occur owing to the spread of inflammation. Metastatic tumors may manifest as stromal nodules or ill-defined iris thickening. Only 10% of the iris mass is visible, with the mass being 1-12 mm in size and isolated, fragile, yellowish-white, pink, or red[8]. The pink or red color may be due to the abundance of capillaries in the tumor.
When a patient is known to have a primary tumor and a significant iris mass, the possibility of iris metastasis from the primary tumor should be considered and a timely biopsy should be performed to make a definitive diagnosis. However, when a patient has no exact primary tumor and there is no obvious iris mass, it is easy to miss the diagnosis of iris metastasis. Slit lamp examination, fluorescein angiography, and ocular ultrasound are highly warranted. In addition, early systemic examination is crucial to determine whether there are tumors in other organs. At this time, attention should be paid to the identification of other diseases of the eyes. Differential diagnoses of nonpigmented iris masses include iris choristoma, acquired iris cysts, unpigmented melanoma, sphincter-leiomyomas, granulomatous iridocyclitis, and xanthogranulomas[13].
When an ophthalmologist is unable to make an immediate diagnosis, slit lamp examination, fluorescein angiography, and ocular ultrasound should be performed first, even if these tests do not provide additional assistance in diagnosis. Positron emission tomography/CT can help identify benign or malignant lesions and indicate potential primary sites[9]. Ultimately, diagnostic tests for an iris mass include fine-needle aspiration biopsy and mass excision biopsy. When tumor cells are seen under the microscope, the corresponding immunohistochemistry is performed in combination with the patient’s clinical symptoms, medical history, and imaging examination to obtain a final diagnosis and determine the primary tumor site.
Treatments for an iris mass include radiotherapy, laser therapy, chemotherapy, anti-vascular endothelial growth factor (VEGF), and ophthalmectomy. The current recommended treatment option is external beam radiation, which can damage the DNA of rapidly growing tumor cells and is effective in reducing the size of the lesion and improving or stabilizing visual acuity. Laser treatment-including transpupillary thermotherapy, laser photocoagulation with the use of argon or krypton, and photodynamic therapy-has significant curative effect. Chemotherapy, which uses different cytotoxic agents depending on the type of cancer, can lead to tumor shrinkage and sometimes even complete regression. VEGF-targeted treatment, such as bevacizumab, has been shown to be beneficial in patients with different types of cancers and has been used in clinical practice with significant efficacy[11]. In addition, systemic interferon therapy is effective for nephrogenic iris metastases, with a report showing that a tumor began to resolve after 3 wk of treatment and had completely resolved at 16 wk[7]. Therefore, conservative interferon therapy may be considered when a patient does not have visual loss, secondary glaucoma, or multiple organ metastasis.
Ocular metastasis is extremely rare and can be an initial sign with a poor prognosis of primary tumor. The prognosis of ocular mass is good after treatment, but systemic prognosis remains poor. The overall mean survival of iris metastasis is 20 mo and the median survival is 13 mo[8]. Timely detection and treatment may improve survival.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mubarak M, Pakistan S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Gray RE, Harris GT. Renal Cell Carcinoma: Diagnosis and Management. Am Fam Physician. 2019;99:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 3.4] [Reference Citation Analysis (1)] |
| 2. | Lopes Abath Neto O, Koretz ZA, Wald AI, Rath PP, Nikiforova M, Chu CT. Molecular profiling of renal cell carcinoma presenting as iris metastasis. Am J Ophthalmol Case Rep. 2022;27:101599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Ware GT, Haik BG, Morris WR. Renal cell carcinoma with involvement of iris and conjunctiva. Am J Ophthalmol. 1999;127:460-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Shome D, Honavar SG, Gupta P, Vemuganti GK, Reddy PV. Metastasis to the eye and orbit from renal cell carcinoma--a report of three cases and review of literature. Surv Ophthalmol. 2007;52:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Mohanty SK, Lobo A, Cheng L. The 2022 revision of the World Health Organization classification of tumors of the urinary system and male genital organs: advances and challenges. Hum Pathol. 2023;136:123-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (1)] |
| 6. | Gkolfinopoulos S, Psyrri A, Bamias A. Clear-cell renal cell carcinoma - A comprehensive review of agents used in the contemporary management of advanced/metastatic disease. Oncol Rev. 2021;15:530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Ikeda T, Sato K, Tokuyama T. Interferon alfa therapy against metastatic iris tumor of renal cell carcinoma. Arch Ophthalmol. 2000;118:846-847. [PubMed] |
| 8. | Shields JA, Shields CL, Kiratli H, de Potter P. Metastatic tumors to the iris in 40 patients. Am J Ophthalmol. 1995;119:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 115] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Lv D, Hu Z, Wang C, Gao S, Xu J. Iris metastasis from esophageal squamous cell carcinoma: A case report. Oncol Lett. 2015;10:790-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Sarenac TS, Janicijevic-Petrovic MA, Sreckovic SB, Radovanovic MR, Vulovic DD, Janicijevic KM. Prostatic carcinoma bilateral iris metastases. Bosn J Basic Med Sci. 2012;12:134-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Giuliari GP, Sadaka A. Uveal metastatic disease: current and new treatment options (review). Oncol Rep. 2012;27:603-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Marie-Louise J, Merle H, Donnio A, Ayeboua L, Jean-Charles A, Guyomarc'h J, Richer R. [Diagnosis of a clear cell renal carcinoma by biopsy of an iris metastasis associated with ocular hypertension in a black man from Martinique: Case report and literature review]. J Fr Ophtalmol. 2015;38:e247-e248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Wyzinski P, Rootman J, Wood W. Simultaneous bilateral iris metastases from renal cell carcinoma. Am J Ophthalmol. 1981;92:206-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Mello PC, Brasil OF, Vidoris A, Morales MC, Belfort RN. Iris metastases from systemic cancer: a report of three cases. Arq Bras Oftalmol. 2016;79:407-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Ramskold L, Lemaître S, Arora A. Iris metastasis from renal cell carcinoma. J Fr Ophtalmol. 2021;44:1278-1280. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Lou SX, Zhang SZ. [Iris metastasis of renal carcinoma: a case report]. Zhongguo Miniao Waike Zazhi. 1994;15:69. |
| 17. | Xing LQ. [Iris metastasis of renal clear cell carcinoma: a case report]. Shiyong Zhongliu Zazhi. 1997;136. |
| 18. | Lu PY, Gao RY, Meng YC. [Iris metastasis treated by surgery: a case report]. Taishan Yixueyuan Xuebao. 1998;92-93. |