Published online Dec 26, 2023. doi: 10.12998/wjcc.v11.i36.8475
Peer-review started: October 1, 2023
First decision: November 16, 2023
Revised: November 10, 2023
Accepted: December 7, 2023
Article in press: December 7, 2023
Published online: December 26, 2023
Processing time: 81 Days and 17.4 Hours
Atrial fibrillation (AF) is one of the most common persistent arrhythmias among adult cardiovascular diseases. It is important to identify potential risk factors for AF. Members of the insulin-like growth factor (IGF) family exert a variety of effects on various cell types in the context of the pathogenesis of cardiovascular diseases, and previous population-based studies indicate associations between IGF family members and AF. However, the causal effects of IGF family members in AF have not been evaluated.
In the current study two-sample Mendelian Randomization (MR) was used to assess genetic relationships between IGF family members and AF.
MR was performed based on genome-wide association study (GWAS) datasets, and concentration levels of 14 IGF family members were retrieved. An initial MR analysis was conducted to identify single nucleotide polymorphisms potentially associated with IGF serum concentrations. A GWAS meta-analysis including 60620 AF cases and 970216 control participants of European ancestry was then conducted to identify AF causal effects. Two-sample MR packages were used to perform MR analysis in R. MR-Egger, weighted median (WM), and inverse va
In two-sample MR assessments there were lower levels of circulating IGF binding protein 3 in both WM [odds ratio (OR) 0.964, 95% confidence interval (CI) 0.940–0.960, P = 0.006] and IVW (OR 0.968, 95%CI: 0.947–0.987, P = 0.001) analyses. Higher serum levels of IGF2 receptor were associated with AF (OR 1.045, 95%CI: 1.016–1.076, P = 0.039). In reverse MR analysis conducted to investigate casual effects, elevated levels of circulating CYR61 were associated with AF (OR 1.060, 95%CI: 1.005–1.119, P = 0.031).
The results of the present study provide novel insights into the pathogenesis of AF, and the implications of serum IGF family member concentrations when assessing the risk of AF. The study generated evidence on the potential roles of developmental pathological effects in the pathogenesis of AF. Further observational and experimental studies are critically needed.
Core Tip: Due to the high prevalence of atrial fibrillation (AF), and adverse outcomes related to it, it is important to identify risk factors associated with development of the condition. Insulin-like growth factor (IGF) family members exert a variety of effects on various cell types in the context of the pathogenesis of cardiovascular diseases, and previous population-based studies indicate associations between IGF family members and AF. However, the causal effects of IGF family members in AF have not been evaluated. The results of the current study provide novel insights on the pathogenesis of AF, and implications of serum IGF family member concentrations when assessing the risk of AF. The study generated evidence on the potential roles of developmental pathological effects in the pathogenesis of AF. Further observational and experimental studies are critically needed.
- Citation: Lin S, Tang J, Li X, Wu G, Lin YF, Li YF. Mendelian randomization provides evidence for a causal effect of serum insulin-like growth factor family concentration on risk of atrial fibrillation. World J Clin Cases 2023; 11(36): 8475-8485
- URL: https://www.wjgnet.com/2307-8960/full/v11/i36/8475.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i36.8475
Atrial fibrillation (AF) is one of the most common arrhythmias in clinical practice worldwide. It recently ranked as the persistent arrhythmia with the highest prevalence in the elderly population. The risk of AF increases with age, with a sharp increase between the ages of 60 and 69 years, and progressive increases from 70–79 years and 80–89 years. The phenotypes of AF are heterogeneous[1], and it is a major public health problem in both developing and developed countries.
Heart failure and metabolic disorders such as diabetes and obesity have been identified as contributing to the pathogenesis of AF, and these associations have been extensively documented. With regard to the exact mechanisms underlying AF, predominant theories center on structural remodeling induced by external stressors, including hemo
Developmental aspects of AF have been documented in clinical and translational studies. Early myocardium development and its interaction with large vessels, particularly pulmonary veins, has been considered a major con
The peptidic hormone insulin-like growth factor (IGF) family comprises two ligands (IGF1 and IGF2), two receptors (IGF1R and IGF2R), seven high-affinity binding proteins (IGFBPs 1–7), a substantial group of IGFBP proteases, and a novel category of proteins known as low-affinity IGFBP-related proteins (IGFBP-rPs). It has been well established that the family plays pivotal roles in growth and development, regulating processes such as proliferation, differentiation, metabolism, and cell survival in various tissues. It is also associated with metabolic disorders, including hypertension, obesity, and stroke. Recent research indicates that reduced IGF1 Levels are linked to an elevated risk of cardiovascular disease-associated mortality. To date no population-based study has investigated associations between AF morbidity and members of the IGF family, such as IGF1 and IGFBP3[10]. In experimental rat models Wang et al[3] demonstrated that IGF1 was associated with atrial fibrosis and participated in AF. It has also been reported that IGF1 and IGFBPs are involved in diabetes, which exacerbates interstitial fibrosis in the atria. These associations have been seen in both animal studies[11,12] and human studies[13].
The above-described associative observations were primarily derived from conventional observational studies, which are susceptible to sample size limitations, reverse causation bias, and confounding factors[14]. It is often challenging to draw definitive conclusions given these considerations and the inherent heterogeneity between different studies, rendering it difficult to conduct causal effect analyses based on these prior studies. Additionally, conventional studies often have limitations with respect to the number of variables that can be observed. Consequently, investigations into the causal effects of all members of the IGF family on the risk of AF are limited.
To mitigate the influences of reverse causality and potential confounding factors from environmental and social sources, the current study used a Mendelian randomization (MR) methodology. The approach relies on genetic variants strongly and exclusively associated with the phenomenon of interest, so-called instrumental variables, to establish causal associations. Two-sample MR analysis was conducted to investigate genetic relationships between IGF family members and AF. The aim was to determine whether IGF family members could be considered contributors to AF.
This study was design to assess the causal effects of IGF family members in the risk of AF. The related traits of IGF family members had been identified, and fourteen IGF family members traits included: IGF1, IGF1-sR, IGF-IIR, IGFBP1, IGFBP2, IGFBP3, IGFBP4, IGFBP5, IGFBP6, IGFBP7, IGF-LR1, CTGF, WISP1 and CYR61. Besides, three traits had been retrived for genetic association of AF, including ebi-a-GCST006414, UKB-b-536, and finn-b-I9_AF. First, the effects of fourteen IGF family members and their serum concentration were evaluated to identify the potential single nucleotide polymorphisms (SNPs) as one sample MR analysis. Then two-sample MR analysis had been completed among AF traits to measure the causa effects of IGF family members in AF pathogensis in the largest sample size trait (ebi-a-GCST006414). Then, further confirmation had been performed among three AF traits to validate the results. After that, the reverse MR analysis to rule out the bias in analysis to evaluate the causal effects of AFs in regulating the expression of circulating IGF family members’ proteins. And there was no existed protocol.
We acquired the genome-wide association studies (GWAS) summary data for AF from a comprehensive combination of sources, including the Nord-Trøndelag Health Study, the deCODE cohort, the MGI cohort, the DiscovEHR collaboration cohort, the AFGen Consortium, and the United Kingdom Biobank resource[15]. This dataset encompassed a total of 60620 AF cases and 970216 control participants. The identification of atrial fibrillation events within the summary dataset was based on diagnostic codes, self-reports, operation codes, or causes of death. Additionally, we utilized GWAS summary datasets from the FinnGen Biobank and the UK Biobank as duplications.
To identify SNPs associated with IGF family members, we extracted and selected data from the latest and largest genome-wide association studies (GWAS) available in the UK Biobank resource, the KORA cohorts[16], and the IN
| Trait | GWAS id | Sample size | Number of SNPs |
| Atrial Fibrillation | ebi-a-GCST006414 | 1030836 | 33519037 |
| Atrial Fibrillation | ukb-b-536 | 337199 | 10894596 |
| Atrial Fibrillation | finn-b-I9_AF | - | 16379794 |
| IGF-1 | prot-c-2952_75_2 | - | 501428 |
| IGF-I sR | prot-c-4232_19_2 | - | 501428 |
| IGF-IIR | prot-c-3676_15_3 | - | 501428 |
| IGFBP-1 | prot-c-2771_35_2 | - | 501428 |
| IGFBP-2 | prot-c-2570_72_5 | - | 501428 |
| IGFBP-3 | prot-c-2571_12_3 | - | 501428 |
| IGFBP-4 | prot-c-2950_57_2 | - | 501428 |
| IGFBP-5 | prot-c-2685_21_2 | - | 501428 |
| IGFBP-6 | prot-c-2686_67_2 | - | 501428 |
| IGFBP-7 | prot-c-3320_49_2 | - | 501428 |
| IGF-LR1 | prot-a-1455 | 3301 | 10534735 |
| CTGF | prot-c-2975_19_2 | - | 501428 |
| WISP-1 | prot-c-3057_55_1 | - | 501428 |
| CYR61 | prot-a-758 | 3301 | 10534735 |
We utilized LDSC (v1.0.1, https://github.com/bulik/Ldsc) software to assess the genetic correlations between AF and each member of the IGF family. LDSC is a robust approach for conducting genetic correlation analyses of complex diseases or traits. It allows for the discrimination between true polygenetic effects and potential mixed biases, encom
In the present study, we employed MR analysis to assess the potential causal relationship between each member of the IGF family and AF. We conducted the analysis using the inverse variance weighted (IVW) method and initially identified significant IGF family members through ldSC analysis, which were subsequently included in further analyses. For each IGF family member, we selected SNPs strongly predictive of exposure at the genome-wide significance level (P < 5 × 10-8). To minimize potential pleiotropy, we excluded SNPs associated with multiple cytokines. Additionally, we retained SNPs with low linkage disequilibrium (r2 < 0.1) to avoid the confounding effects of correlated SNPs. However, it should be noted that despite these efforts, none of the SNPs associated with IGF family members showed significant associations with AF in the harmonized GWAS datasets. Consequently, we adopted a more stringent cutoff (P < 1 × 10-5) to select SNPs predicting IGF family members. We reported the number of included SNPs, along with effect estimates, confidence intervals, and P values.
MR estimates were derived using the IVW method and the MR-Egger method, both implemented under a random-effects model. To assess the robustness of our IVW results, we conducted tests for heterogeneity, multiple validity tests, and sensitivity analyses using weighted median estimation and MR–Egger regression. The TwoSampleMR packages[19] (version 0.5.6) in R (version 4.0.4) were utilized for performing the MR analysis. The statistical significance level was set at P < 0.05.
Fourteen molecules were included in the first one-sample MR to identify SNPs potentially influencing their serum concentrations; IGF1 (prot-c-2952_75_2), IGF1-sR (prot-c-4232_19_2), IGF2R (prot-c-3676_15_3), IGFBP1 (prot-c-2771_35_2), IGFBP2 (prot-c-2570_72_5), IGFBP3 (prot-c-2571_12_3), IGFBP4 (prot-c-2950_57_2), IGFBP5 (prot-c-2685_21_2), IGFBP6 (prot-c-2686_67_2), IGFBP7 (prot-c-3320_49_2), IGF-LR1 (prot-a-1455), CTGF (prot-c-2975_19_2), WISP1 (prot-c-3057_55_1), and CYR61 (prot-a-758). Of the 14 IGF family members with serum concentrations reported in published studies, 13 were associated with more than one genomewide significant SNP site. Detailed information after clumping of LD-independent SNPs as exposure are presented in Supplementary material. All F-statistics were above 10, indicating that the results were less likely to be affected by weak instrument bias.
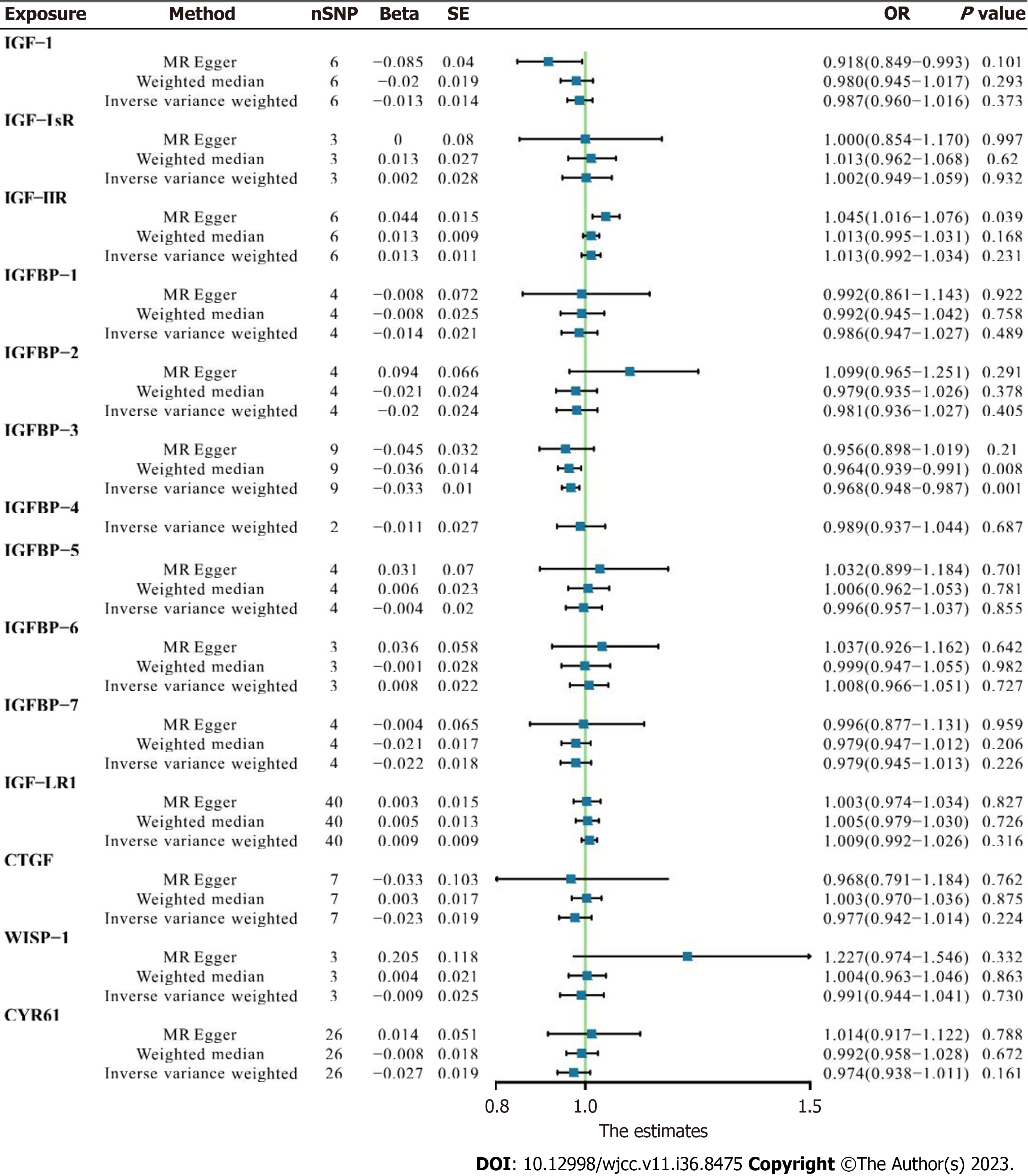
In the first one-step MR analysis the MR-Egger method and the IVW method were used. More than one significant SNP was identified at the genome-wide level (P < 0.001), and these SNPs were used to calculate causal associations with AF. In pooled data analysis three molecules were associated with AF. Lower levels of circulating IGF1 were negatively associated with AF onset [odds ratio (OR) 0.918, 95% confidence interval (CI) 0.849–0.993, MR-Egger analysis]. IGFBP3 was negatively correlated with AF prevalence in both WM analysis (OR 0.964, 95%CI: 0.940–0.960, P = 0.006) and IVW analysis (OR 0.968, 95%CI: 0.947–0.987, P = 0.001). Higher serum IGF2R was positively correlated with AF pathogenesis in MR-Egger analysis (OR 1.045, 95%CI: 1.016–1.076, P = 0.039). Other IGF family members were not significantly associated with the risk of AF (Figure 1).
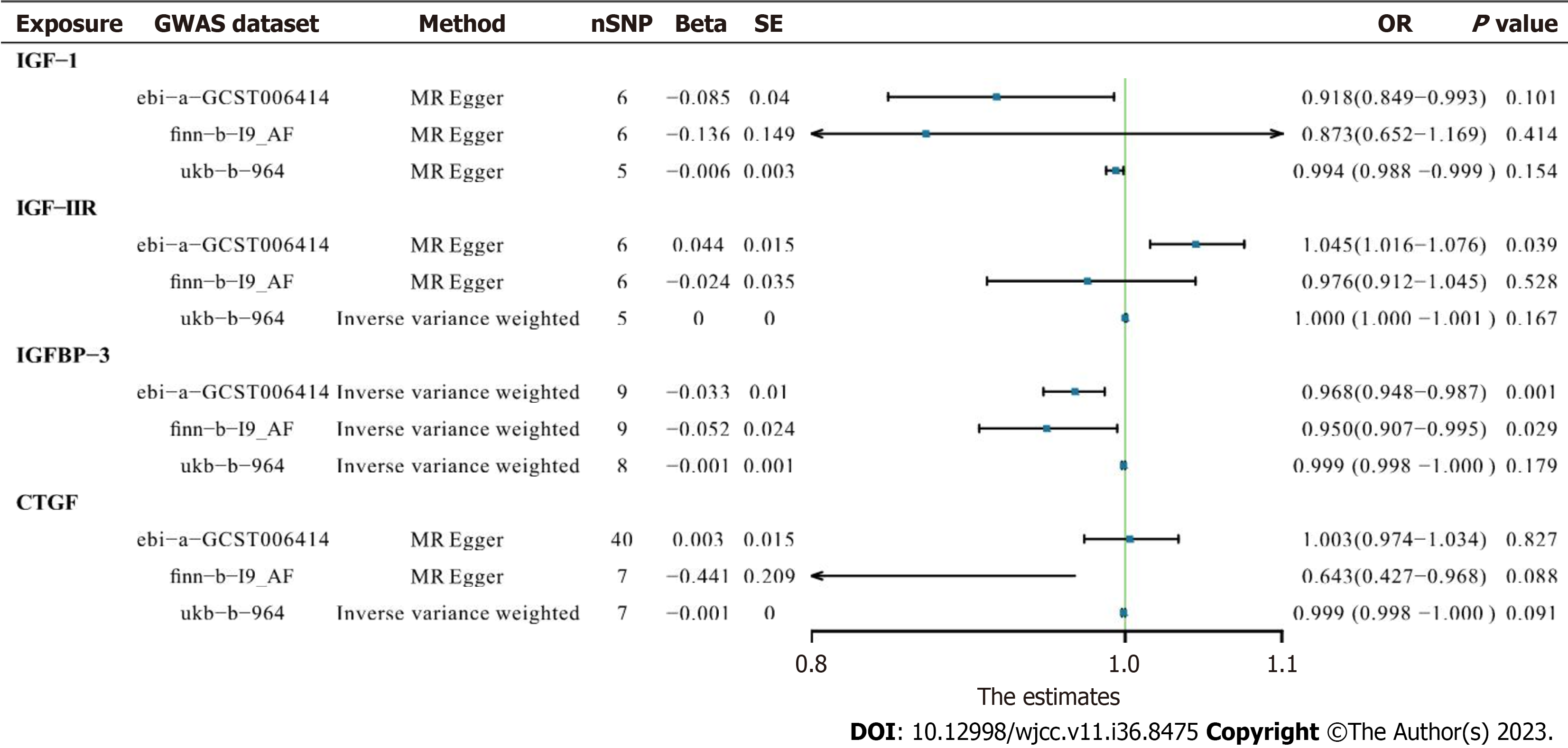
In IGF1 and IGF2R assessments, neither finn-b-19_AF (OR 0.873, 95%CI: 0.652–1.169, P = 0.414 and OR 0.976, 95%CI: 0.912–1.045, P = 0.528, respectively) nor ukb-b-964 (OR 0.994, 95%CI: 0.988–0.999, P = 0.154 and OR 1.000, 95%CI: 1.000–1.001, P = 0.167, respectively) yielded any significant results in MR-Egger or IVW analyses. The significant negative correlation between IGFBP3 and AF was confirmed in finn-b-19_AF trait analysis (OR 0.950, 95%CI: 0.907–0.955, P = 0.029), indicating that lower serum IGFBP3 contributes to AF (Figure 2).
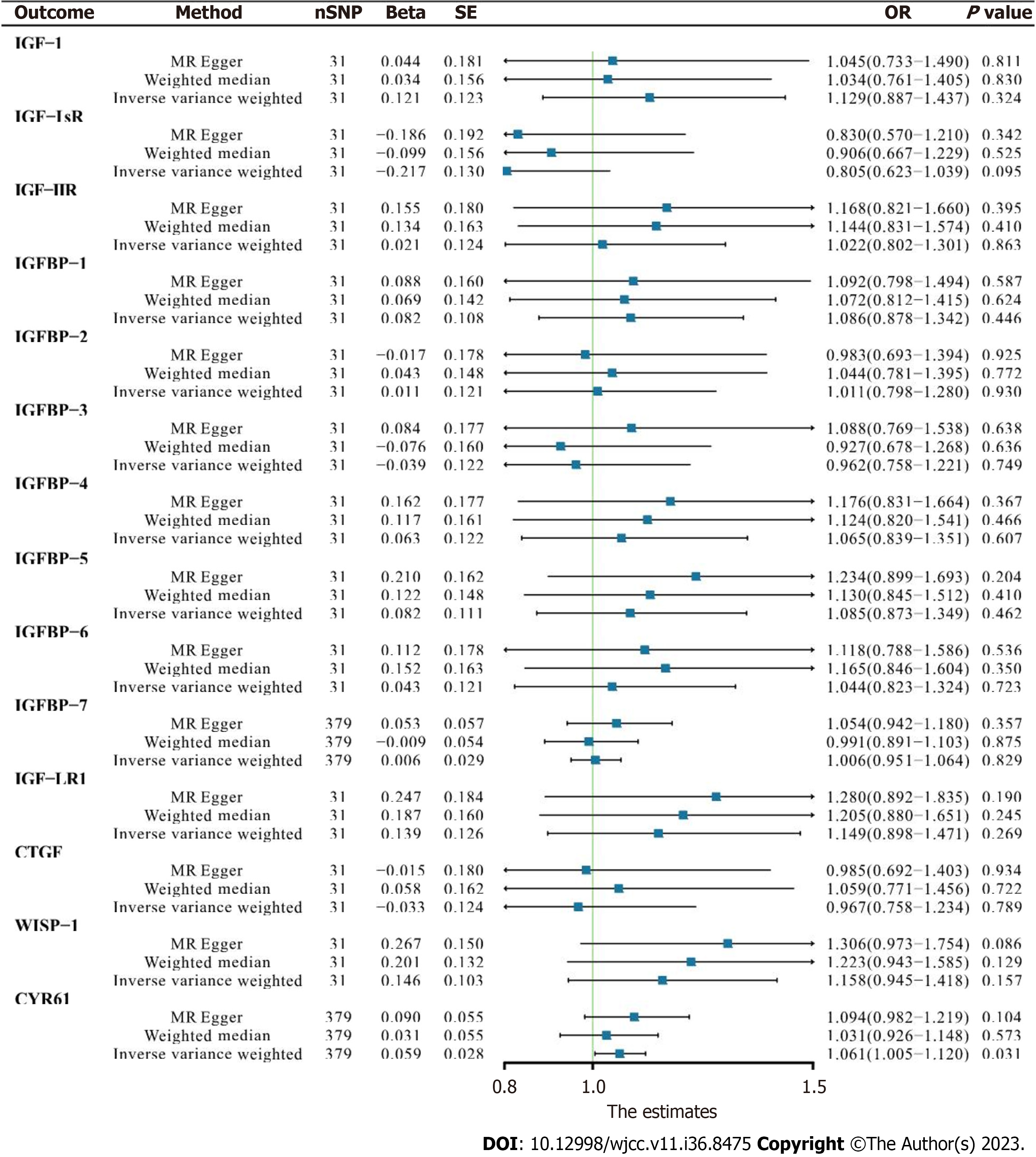
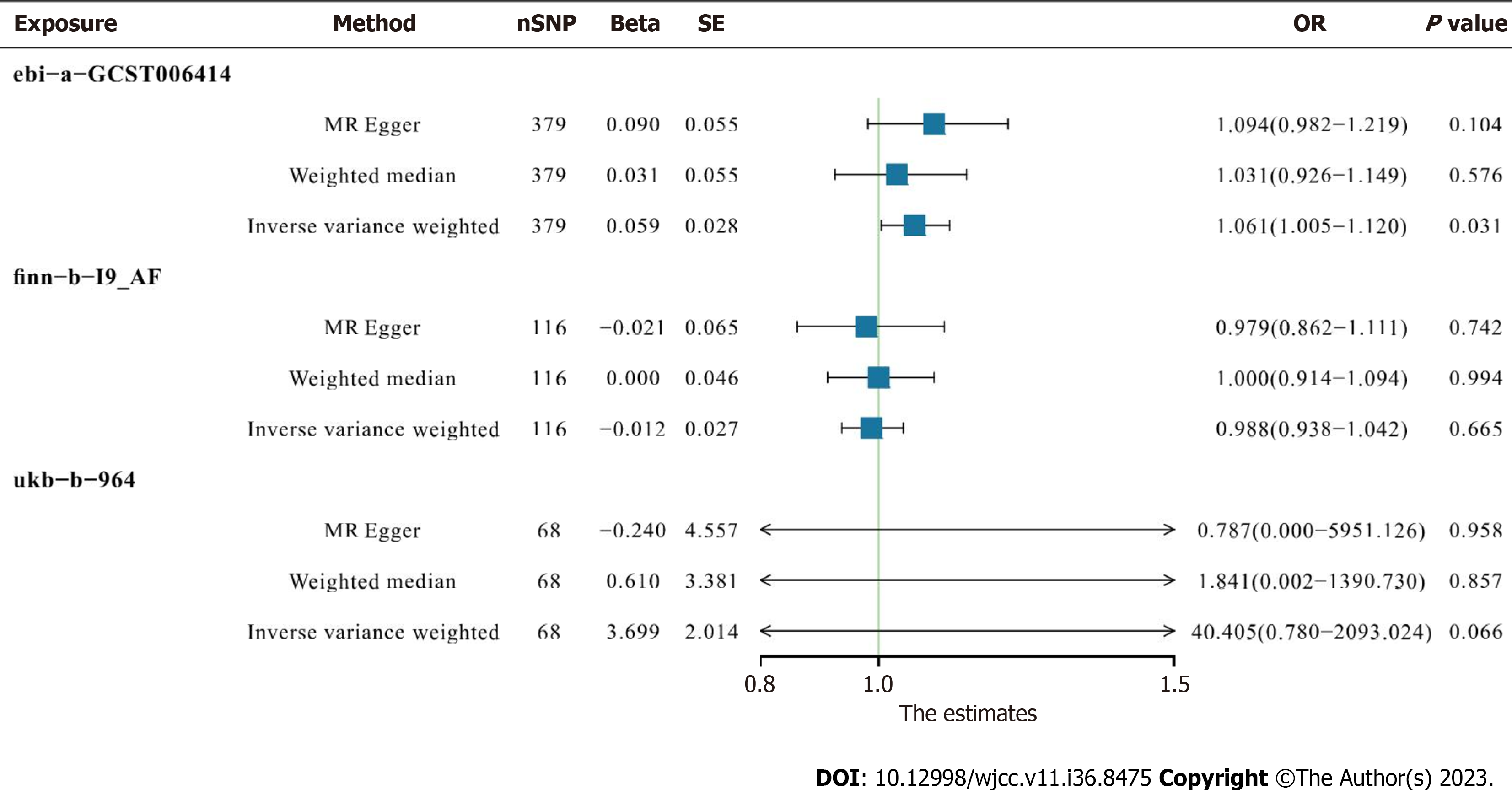
Analyses were conducted to identify causal associations between serum IGF family member levels and AF. There was no convincing evidence of genetic associations between IGF family member expression and AF. In basic IVW analysis based on the ebi-a-GCST006414 trait, CYR61 was significantly positively correlated with AF (OR 1.060, 95%CI: 1.005–1.119, P = 0.031, Figure 3). In a more detailed validation test however, CYR61 was not positively correlated with finnb19_AF or ukb-b-964 traits as determined via any analysis methods (Figure 4).
In this study two-sample MR analyses using multiple GWAS datasets was conducted to assess relationships between individual IGF family members and AF. Results indicated that genetically determined lower levels of IGF1 and IGFBP3, as well as genetically determined higher levels of IGF2R, contribute to increased risk of AF. The presence of AF was genetically associated with elevated CYR61 levels. To the best of our knowledge the current study is the first comprehensive MR analysis systematically investigating associations between multiple IGF family members and AF.
In the present study a genetically determined decrease in circulating IGF1 was associated with an increased incidence of AF, a finding consistent with previously published MR analyses[20] and observational trials[10,21]. IGF1, a 70-amino acid peptide, is primarily synthesized in the liver and regulated by hypothalamic growth hormone-releasing hormone and pituitary growth hormone[22]. Notably IGFBP3—the most abundant binding partner of IGF1—was significantly positively associated with AF. Numerous studies have identified effects of IGF1 on the cardiovascular system, linking abnormalities in IGF1 levels to elevated risks of cardiovascular diseases, including atherosclerosis, hypertension, and coronary artery disease. Furthermore, IGF1 levels are age-dependent, peaking during puberty and declining throughout the remainder of life. IGFBP3, like IGF1, exhibits growth hormone-dependent regulation. Recent in vivo studies indicate that fetal growth restriction in mice leads to IGF1 deficiency and an increased risk of adult cardiovascular diseases. Moreover, intrauterine administration of additional IGF1 can mitigate the risk of adult cardiovascular diseases in a mouse fetal growth restriction model[23]. These effects are reportedly mediated by a deficiency in the mTORC1 pathway[24], a downstream component of the IGF1 pathway[25]. Studies in elderly populations have revealed significantly lower mean serum levels of IGF1 (P = 0.02) and IGFBP3 (P = 0.03) in AF patients than in non-AF participants[21]. A population-based study yielded similar results, further suggesting that low IGF1/IGFBP3 ratios are associated with a higher prevalence of AF[10]. Therefore our findings align with previous research suggesting that insufficient levels of IGF1 and IGFBP3 throughout life, particularly during periods of higher circulating IGF1 and growth hormone, significantly contribute to the onset of AF. These biomarkers hold potential for the prevention of AF.
In addition to IGF1 and IGFBP3, in the current study elevated levels of IGF2R were associated with AF. IGF2R, also known as the cation-independent mannose-6-phosphate receptor, comprises a substantial N-terminal extracellular region, a single membrane-spanning region, and a small cytoplasmic tail. Its primary role is to regulate circulating and tissue levels of IGF2 by targeting it for lysosomal degradation, thereby modulating IGF2 activity[26]. Both IGF2 and IGF2R have been implicated in placental and fetal growth and development. SNPs within IGF2R have been linked to increased risks of growth abnormalities, reduced growth rates during the first 3 years of life, and certain cancers[27-29]. Recent research suggests that an unfavorable intrauterine environment can induce epigenetic changes in the IGF2/H19 and IGF2R genes, subsequently altering the expression of IGF2 and IGF2R[30,31]. In animal studies fetal myocardial levels of IGF2 and IGF2R increased in response to reduced placental substrate supply in lambs[32]. In mice inactivation of the maternal IGF2R allele resulted in excessive growth and perinatal lethality, a phenotype that could be rescued with an IGF2 null allele[33]. Notably, protein abundance was inversely associated with relative left ventricle weight in models with reduced placental function, whereas it exhibited a positive correlation in the control group. This suggests that the IGF2R signaling pathway may be pathologically activated, leading to ventricular hypertrophy[32].
Previous reports have discussed the potential benefits of suppressing the IGF2R signaling pathway, such as protecting against myocardial cell apoptosis and preventing the progression of heart failure[34]. The present study provides the first indication of a potential correlation between IGF2R and AF, underscoring the potential for fetal pathological effects on the occurrence of adult cardiovascular diseases, including AF. Notably however, our literature review did not identify any observational studies investigating relationships between IGF2R and AF. Further research is therefore warranted, to investigate IGF2R as a potential biomarker of AF, and to deepen our understanding of its role in AF pathogenesis.
In the present study there was a correlation between AF and alterations in CYR61 Levels in circulation. CYR61, also known as cellular communication network factor 1 (CCN1), belongs to the CCN family of matricellular proteins and plays pivotal roles in angiogenesis, inflammation, and the repair of fibrotic tissue[35-37]. Observational studies have consistently indicated significant increases in CYR61 within atherosclerotic lesions rich in vascular smooth muscle cells. Such studies have also identified CYR61 increases in the cardiomyocytes of individuals with ischemic cardiomyopathy, and STelevation in myocardial infarction patients[38-40]. Furthermore, the addition of CYR61 to the reference GRACE risk score led to improved risk stratification for all-cause mortality, surpassing the predictive capacity of high-sensitivity troponin T in subsequent analyses[40]. In the current study a preliminary CYR61-related result suggested that AF may induce increased CYR61 expression, but more detailed investigations did not confirm this. Thus a causal association between AF and CYR61 was not convincingly demonstrated. Notably however, levels of circulating CYR61 may assist the functional assessment of cardiovascular diseases.
The present study investigated associations between fourteen IGF family members and AF, and identified potential relationships with respect to three of them. Bidirectional analysis indicated that AF may influence CYR61. The use of MR analysis and large European GWAS datasets in the study conferred a substantial advantage with respect to reduced susceptibility to inverse causality, confounding, and biases inherent in the use of small sample sizes. The study also had some limitations. Primarily, the use of a higher significance threshold (P < 1 × 10-5) for SNP selection from GWAS datasets on IGF family members was necessitated by the limited number of IGF family members that yielded at least one genome-wide significant SNP when using the conventional threshold of P < 5 × 10-8. Thirteen IGF family members exhibited multiple genome-wide significant SNPs under the P < 1 × 10-5 threshold. A combined GWAS dataset was used for AF and atrial flutter, precluding distinction between these two arrhythmia subtypes. Due to unavailability of relevant datasets for each specific AF subtype, the study focused solely on associations between IGF family members and AF onset. We were unable to stratify our results based on different AF phenotypes. Given the multifaceted nature of AF pathogenesis, ascertaining the precise roles played by the identified IGF family members in AF pathogenesis remains elusive. The results of the current study emphasize the urgent need for further observational and experimental studies.
Based on GWAS summary datasets derived from AF and circulating IGF family members, we identified causal relationships between three IGF family members and AF via MR analysis. IGFBP3 was negatively correlated with AF prevalence in both WM analysis and IVW analysis. The study results provide novel insights into AF pathogenesis and the implications of serum IGF family member concentrations with respect to AF risk. Further observational and experimental studies are critically required.
The etiology of atrial fibrillation is still unknown, and insulin-like growth factor had been suspected to be involved in atrial fibrillation.
The relationship between insulin-like growth factor and atrial fibrillation had not be well addressed.
This study was carried out to evaluate the causal effect of serum insulin-like growth factor family concentration on risk of atrial fibrillation.
Mendelian Randomization analysis was performed based on genome-wide association study datasets of insulin-like growth factor family concentration and atrial fibrillation.
Lower levels of circulating insulin-like growth factor binding protein 3 was associated with atrial fibrillation.
The study generated evidence on the potential roles of developmental pathological effects in the pathogenesis of atrial fibrillation.
Further observational and experimental studies are critically needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ong H, Malaysia S-Editor: Liu JH L-Editor: A P-Editor: Xu ZH
| 1. | Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56-e528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4294] [Cited by in RCA: 5897] [Article Influence: 982.8] [Reference Citation Analysis (5)] |
| 2. | Anné W, Willems R, Roskams T, Sergeant P, Herijgers P, Holemans P, Ector H, Heidbüchel H. Matrix metalloproteinases and atrial remodeling in patients with mitral valve disease and atrial fibrillation. Cardiovasc Res. 2005;67:655-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 3. | Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1075] [Cited by in RCA: 1121] [Article Influence: 53.4] [Reference Citation Analysis (0)] |
| 4. | Patton KK, Ellinor PT, Heckbert SR, Christenson RH, DeFilippi C, Gottdiener JS, Kronmal RA. N-terminal pro-B-type natriuretic peptide is a major predictor of the development of atrial fibrillation: the Cardiovascular Health Study. Circulation. 2009;120:1768-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 254] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 5. | Sinner MF, Stepas KA, Moser CB, Krijthe BP, Aspelund T, Sotoodehnia N, Fontes JD, Janssens AC, Kronmal RA, Magnani JW, Witteman JC, Chamberlain AM, Lubitz SA, Schnabel RB, Vasan RS, Wang TJ, Agarwal SK, McManus DD, Franco OH, Yin X, Larson MG, Burke GL, Launer LJ, Hofman A, Levy D, Gottdiener JS, Kääb S, Couper D, Harris TB, Astor BC, Ballantyne CM, Hoogeveen RC, Arai AE, Soliman EZ, Ellinor PT, Stricker BH, Gudnason V, Heckbert SR, Pencina MJ, Benjamin EJ, Alonso A. B-type natriuretic peptide and C-reactive protein in the prediction of atrial fibrillation risk: the CHARGE-AF Consortium of community-based cohort studies. Europace. 2014;16:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 149] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Aviles RJ, Martin DO, Apperson-Hansen C, Houghtaling PL, Rautaharju P, Kronmal RA, Tracy RP, Van Wagoner DR, Psaty BM, Lauer MS, Chung MK. Inflammation as a risk factor for atrial fibrillation. Circulation. 2003;108:3006-3010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1097] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 7. | Ho JE, Yin X, Levy D, Vasan RS, Magnani JW, Ellinor PT, McManus DD, Lubitz SA, Larson MG, Benjamin EJ. Galectin 3 and incident atrial fibrillation in the community. Am Heart J. 2014;167:729-34.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 8. | Nortamo S, Ukkola O, Lepojärvi S, Kenttä T, Kiviniemi A, Junttila J, Huikuri H, Perkiömäki J. Association of sST2 and hs-CRP levels with new-onset atrial fibrillation in coronary artery disease. Int J Cardiol. 2017;248:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 9. | Ravassa S, Ballesteros G, López B, Ramos P, Bragard J, González A, Moreno MU, Querejeta R, Vives E, García-Bolao I, Díez J. Combination of Circulating Type I Collagen-Related Biomarkers Is Associated With Atrial Fibrillation. J Am Coll Cardiol. 2019;73:1398-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 10. | Busch M, Krüger A, Gross S, Ittermann T, Friedrich N, Nauck M, Dörr M, Felix SB. Relation of IGF-1 and IGFBP-3 with prevalent and incident atrial fibrillation in a population-based study. Heart Rhythm. 2019;16:1314-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Chihara K, Kato Y, Kohno H, Takano K, Tanaka T, Teramoto A, Shimatsu A. Safety and efficacy of growth hormone (GH) during extended treatment of adult Japanese patients with GH deficiency (GHD). Growth Horm IGF Res. 2008;18:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Derar DR, Taya K, Watanabe G, Miyake YI. Characterization of immunoreactive IGF-I pattern during the peri-ovulatory period of the oestrous cycle of thoroughbred mares and its relation to other hormones. Reprod Domest Anim. 2012;47:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Tuleta I, Frangogiannis NG. Fibrosis of the diabetic heart: Clinical significance, molecular mechanisms, and therapeutic opportunities. Adv Drug Deliv Rev. 2021;176:113904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 14. | van der Laan SW, Fall T, Soumaré A, Teumer A, Sedaghat S, Baumert J, Zabaneh D, van Setten J, Isgum I, Galesloot TE, Arpegård J, Amouyel P, Trompet S, Waldenberger M, Dörr M, Magnusson PK, Giedraitis V, Larsson A, Morris AP, Felix JF, Morrison AC, Franceschini N, Bis JC, Kavousi M, O'Donnell C, Drenos F, Tragante V, Munroe PB, Malik R, Dichgans M, Worrall BB, Erdmann J, Nelson CP, Samani NJ, Schunkert H, Marchini J, Patel RS, Hingorani AD, Lind L, Pedersen NL, de Graaf J, Kiemeney LA, Baumeister SE, Franco OH, Hofman A, Uitterlinden AG, Koenig W, Meisinger C, Peters A, Thorand B, Jukema JW, Eriksen BO, Toft I, Wilsgaard T, Onland-Moret NC, van der Schouw YT, Debette S, Kumari M, Svensson P, van der Harst P, Kivimaki M, Keating BJ, Sattar N, Dehghan A, Reiner AP, Ingelsson E, den Ruijter HM, de Bakker PI, Pasterkamp G, Ärnlöv J, Holmes MV, Asselbergs FW. Cystatin C and Cardiovascular Disease: A Mendelian Randomization Study. J Am Coll Cardiol. 2016;68:934-945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 15. | Nielsen JB, Thorolfsdottir RB, Fritsche LG, Zhou W, Skov MW, Graham SE, Herron TJ, McCarthy S, Schmidt EM, Sveinbjornsson G, Surakka I, Mathis MR, Yamazaki M, Crawford RD, Gabrielsen ME, Skogholt AH, Holmen OL, Lin M, Wolford BN, Dey R, Dalen H, Sulem P, Chung JH, Backman JD, Arnar DO, Thorsteinsdottir U, Baras A, O'Dushlaine C, Holst AG, Wen X, Hornsby W, Dewey FE, Boehnke M, Kheterpal S, Mukherjee B, Lee S, Kang HM, Holm H, Kitzman J, Shavit JA, Jalife J, Brummett CM, Teslovich TM, Carey DJ, Gudbjartsson DF, Stefansson K, Abecasis GR, Hveem K, Willer CJ. Biobank-driven genomic discovery yields new insight into atrial fibrillation biology. Nat Genet. 2018;50:1234-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 592] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 16. | Suhre K, Arnold M, Bhagwat AM, Cotton RJ, Engelke R, Raffler J, Sarwath H, Thareja G, Wahl A, DeLisle RK, Gold L, Pezer M, Lauc G, El-Din Selim MA, Mook-Kanamori DO, Al-Dous EK, Mohamoud YA, Malek J, Strauch K, Grallert H, Peters A, Kastenmüller G, Gieger C, Graumann J. Connecting genetic risk to disease end points through the human blood plasma proteome. Nat Commun. 2017;8:14357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 280] [Cited by in RCA: 482] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 17. | Sun BB, Maranville JC, Peters JE, Stacey D, Staley JR, Blackshaw J, Burgess S, Jiang T, Paige E, Surendran P, Oliver-Williams C, Kamat MA, Prins BP, Wilcox SK, Zimmerman ES, Chi A, Bansal N, Spain SL, Wood AM, Morrell NW, Bradley JR, Janjic N, Roberts DJ, Ouwehand WH, Todd JA, Soranzo N, Suhre K, Paul DS, Fox CS, Plenge RM, Danesh J, Runz H, Butterworth AS. Genomic atlas of the human plasma proteome. Nature. 2018;558:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1269] [Cited by in RCA: 1369] [Article Influence: 195.6] [Reference Citation Analysis (0)] |
| 18. | Bulik-Sullivan BK, Loh PR, Finucane HK, Ripke S, Yang J; Schizophrenia Working Group of the Psychiatric Genomics Consortium, Patterson N, Daly MJ, Price AL, Neale BM. LD Score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat Genet. 2015;47:291-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2705] [Cited by in RCA: 3544] [Article Influence: 354.4] [Reference Citation Analysis (0)] |
| 19. | Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, Laurin C, Burgess S, Bowden J, Langdon R, Tan VY, Yarmolinsky J, Shihab HA, Timpson NJ, Evans DM, Relton C, Martin RM, Davey Smith G, Gaunt TR, Haycock PC. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4747] [Cited by in RCA: 4859] [Article Influence: 694.1] [Reference Citation Analysis (0)] |
| 20. | Zanetti D, Gustafsson S, Assimes TL, Ingelsson E. Comprehensive Investigation of Circulating Biomarkers and Their Causal Role in Atherosclerosis-Related Risk Factors and Clinical Events. Circ Genom Precis Med. 2020;13:e002996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Duron E, Vidal JS, Funalot B, Brunel N, Viollet C, Seux ML, Treluyer JM, Epelbaum J, Bouc YL, Hanon O. Insulin-like growth factor I, insulin-like growth factor binding protein 3, and atrial fibrillation in the elderly. J Gerontol A Biol Sci Med Sci. 2014;69:1025-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253:2769-2776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 822] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Alsaied T, Omar K, James JF, Hinton RB, Crombleholme TM, Habli M. Fetal origins of adult cardiac disease: a novel approach to prevent fetal growth restriction induced cardiac dysfunction using insulin like growth factor. Pediatr Res. 2017;81:919-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Hennig M, Fiedler S, Jux C, Thierfelder L, Drenckhahn JD. Prenatal Mechanistic Target of Rapamycin Complex 1 (m TORC1) Inhibition by Rapamycin Treatment of Pregnant Mice Causes Intrauterine Growth Restriction and Alters Postnatal Cardiac Growth, Morphology, and Function. J Am Heart Assoc. 2017;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3052] [Cited by in RCA: 4441] [Article Influence: 555.1] [Reference Citation Analysis (0)] |
| 26. | Brown J, Jones EY, Forbes BE. Keeping IGF-II under control: lessons from the IGF-II-IGF2R crystal structure. Trends Biochem Sci. 2009;34:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Kaku K, Osada H, Seki K, Sekiya S. Insulin-like growth factor 2 (IGF2) and IGF2 receptor gene variants are associated with fetal growth. Acta Paediatr. 2007;96:363-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 28. | Petry CJ, Ong KK, Wingate DL, Brown J, Scott CD, Jones EY, Pembrey ME, Dunger DB; Alspac Study Team. Genetic variation in the type 2 insulin-like growth factor receptor gene and disparity in childhood height. Growth Horm IGF Res. 2005;15:363-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Hébert E. Mannose-6-phosphate/insulin-like growth factor II receptor expression and tumor development. Biosci Rep. 2006;26:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Li CC, Maloney CA, Cropley JE, Suter CM. Epigenetic programming by maternal nutrition: shaping future generations. Epigenomics. 2010;2:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 31. | Ollikainen M, Smith KR, Joo EJ, Ng HK, Andronikos R, Novakovic B, Abdul Aziz NK, Carlin JB, Morley R, Saffery R, Craig JM. DNA methylation analysis of multiple tissues from newborn twins reveals both genetic and intrauterine components to variation in the human neonatal epigenome. Hum Mol Genet. 2010;19:4176-4188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 32. | Wang KC, Zhang L, McMillen IC, Botting KJ, Duffield JA, Zhang S, Suter CM, Brooks DA, Morrison JL. Fetal growth restriction and the programming of heart growth and cardiac insulin-like growth factor 2 expression in the lamb. J Physiol. 2011;589:4709-4722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Sandovici I, Georgopoulou A, Pérez-García V, Hufnagel A, López-Tello J, Lam BYH, Schiefer SN, Gaudreau C, Santos F, Hoelle K, Yeo GSH, Burling K, Reiterer M, Fowden AL, Burton GJ, Branco CM, Sferruzzi-Perri AN, Constância M. The imprinted Igf2-Igf2r axis is critical for matching placental microvasculature expansion to fetal growth. Dev Cell. 2022;57:63-79.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 34. | Chu CH, Tzang BS, Chen LM, Liu CJ, Tsai FJ, Tsai CH, Lin JA, Kuo WW, Bau DT, Yao CH, Huang CY. Activation of insulin-like growth factor II receptor induces mitochondrial-dependent apoptosis through G(alpha)q and downstream calcineurin signaling in myocardial cells. Endocrinology. 2009;150:2723-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Hinkel R, Trenkwalder T, Petersen B, Husada W, Gesenhues F, Lee S, Hannappel E, Bock-Marquette I, Theisen D, Leitner L, Boekstegers P, Cierniewski C, Müller OJ, le Noble F, Adams RH, Weinl C, Nordheim A, Reichart B, Weber C, Olson E, Posern G, Deindl E, Niemann H, Kupatt C. MRTF-A controls vessel growth and maturation by increasing the expression of CCN1 and CCN2. Nat Commun. 2014;5:3970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 36. | Jun JI, Kim KH, Lau LF. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat Commun. 2015;6:7386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 37. | Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 826] [Cited by in RCA: 757] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 38. | Hilfiker A, Hilfiker-Kleiner D, Fuchs M, Kaminski K, Lichtenberg A, Rothkötter HJ, Schieffer B, Drexler H. Expression of CYR61, an angiogenic immediate early gene, in arteriosclerosis and its regulation by angiotensin II. Circulation. 2002;106:254-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, Drexler H. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 91] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Klingenberg R, Aghlmandi S, Liebetrau C, Räber L, Gencer B, Nanchen D, Carballo D, Akhmedov A, Montecucco F, Zoller S, Brokopp C, Heg D, Jüni P, Marti Soler H, Marques-Vidal PM, Vollenweider P, Dörr O, Rodondi N, Mach F, Windecker S, Landmesser U, von Eckardstein A, Hamm CW, Matter CM, Lüscher TF. Cysteine-rich angiogenic inducer 61 (Cyr61): a novel soluble biomarker of acute myocardial injury improves risk stratification after acute coronary syndromes. Eur Heart J. 2017;38:3493-3502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |