Published online Nov 16, 2023. doi: 10.12998/wjcc.v11.i32.7881
Peer-review started: September 4, 2023
First decision: September 28, 2023
Revised: October 11, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 16, 2023
Processing time: 73 Days and 2.5 Hours
Median arcuate ligament syndrome (MALS) is a rare disease caused by compre
A 55-year-old woman was admitted due to abdominal pain, nausea and vomiting. On admission, the patient presented with epigastric pain that worsened after eating, without signs of peritoneal irritation. Computed tomography angiography of the upper abdomen showed compression of the proximal segment of the abdominal trunk, local luminal stenosis with angular “fishhook” changes, which changed significantly during forceful inspiration and expiration; gallbladder stones; and multiple cysts in the liver. Abdominal duplex ultrasonography showed that peak systolic velocity was 352 cm/s. After diagnosis of MALS was confirmed, an arch ligament release procedure was performed. MALS has no specific symptoms and can be misdiagnosed as other abdominal diseases. Awareness of MALS should be improved to avoid misdiagnosis. The commonly used treatment option is MAL release and resection of the peripheral ganglion of the celiac trunk artery.
The diagnosis and treatment of MALS must be individualized, and MAL release is effective and provides immediate symptomatic relief.
Core Tip: Median arcuate ligament syndrome (MALS) is a rare disorder caused by compression of the median arcuate ligament against the celiac trunk. Patients with MALS often present with chronic postprandial abdominal pain, nausea, vomiting, diarrhea, and unexplained weight loss. Imaging examination is the preferred screening method. MALS is confused with many common diseases, and definitive diagnosis requires exclusion of other causes of abdominal pain. In this case, combination of gallbladder stones and chronic cholecystitis made it easy to miss diagnosis of MALS. Clinicians should be more aware of MALS. Surgery can provide immediate symptomatic relief and can be an effective treatment for MALS.
- Citation: Dang JQ, Wang QQ, Yang YL, Shang L, Bian QT, Xiang HJ. Median arcuate ligament syndrome complicated with gallbladder stones: A case report. World J Clin Cases 2023; 11(32): 7881-7887
- URL: https://www.wjgnet.com/2307-8960/full/v11/i32/7881.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i32.7881
Median arcuate ligament syndrome (MALS), also known as celiac artery compression syndrome or Dunbar syndrome, is due to the celiac trunk artery or celiac ganglion being compromised by the fibrous ligaments that connect the fibrous crus of the diaphragm on both sides (forming the anterior edge of the aortic hiatus). Caused by external compression of the median arcuate ligament (MAL). The MAL is usually located above the starting point of the celiac trunk, and 10%–24% of people have MAL located in the front and upper part of the celiac trunk, so it is easy to compress arteries or adjacent nerves and cause chronic, recurrent abdominal pain and other clinical symptoms. The typical symptoms of MALS are postprandial abdominal pain, weight loss, nausea, and vomiting[1]. The aim of this report is to document a case of MALS treated with surgical decompression. This case report was elaborated in accordance with the SCARE criteria.
CASE PRESENTATION
A 55-year-old female patient presented with intermittent epigastric pain for 20 d, and the postprandial abdominal pain was aggravated without other specific manifestations.
Symptoms started 20 d before presentation with postprandial abdominal pain. Abdominal pain was intermittent and did not radiate from the back or perineum. She visited the local hospital and underwent computed tomography (CT) examination, which suggested: (1) Gallbladder stones (Figure 1A); (2) MALS; and (3) multiple hepatic cysts (Figure 1B). Treatment with anti-inflammatory and choleretic drugs and nonsteroidal analgesic drugs, but the abdominal pain was not significantly relieved. For further treatment, she came to our hospital.
The patient had a past history of right upper abdominal pain, did not go to the hospital for examination, and the abdominal pain was relieved by self-administration of anti-inflammatory and choleretic drugs.
The patient denied any family history of abdominal pain.
On physical examination, the vital signs were: Body temperature, 36.5 ℃; blood pressure, 120/78 mmHg; heart rate, 76 beats/min; respiratory rate, and 18 breaths/min. There was no yellow sclera, flat and soft abdomen, upper abdominal tenderness, and no rebound tenderness. No vascular murmur was heard in the upper abdomen. The visual pain score was 6.
Before the surgical procedure, routine blood analysis, liver and kidney function tests, amylase, blood coagulation function, inflammatory indexes (C-reactive protein, high-sensitivity C-reactive protein and procalcitonin) and tumor markers (carbohydrate antigens 19-9 and 125, carcinoembryonic antigen) were normal.
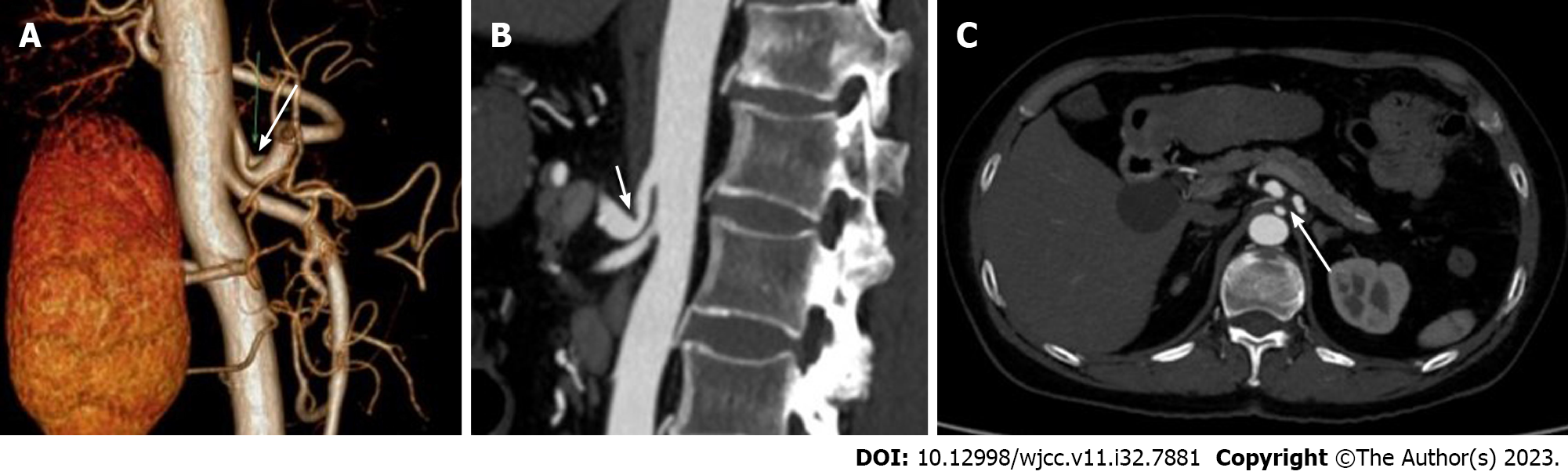
Upper abdominal B-ultrasound and abdominal contrast-enhanced magnetic resonance imaging showed multiple gallbladder stones and multiple liver cysts. No obvious abnormality was found in esophagogastroduodenoscopy. CT angiography (CTA) showed that the origin of the celiac trunk was high, and the adjacent MAL compressed the initial segment of the celiac trunk with severe V-shaped stenosis (Figure 2A-C). When exhaling hard, the degree of stenosis was aggravated.
Giving antispasmodic, analgesic, cholagogic and other symptomatic treatment, the symptoms of abdominal pain were not significantly improved. Abdominal duplex ultrasound (DUS) was performed, which showed hemodynamically significant extrinsic compression of the abdominal trunk (> 70% reduction of the lumen) with peak systolic velocity of 352 cm/s.
Combined with the patient’s medical history, the final diagnosis was: (1) MALS, (2) gallbladder stones with chronic cholecystitis; and (3) multiple liver cysts.
After diagnosis of MALS was confirmed and since the patient’s symptoms remained unimproved (epigastric pain and worsening after meals), MAL release was recommended. Under general anesthesia, the patient was placed in a supine position, and a median epigastric incision was made to enter the abdomen layer by layer. Exploration showed multiple cysts in the liver, full gallbladder, slightly thickened cyst wall, and no other abnormalities. The lesser sac was opened to reveal the superior margin of the pancreas, the No.8a lymph node was removed to expose the common hepatic artery, and the proximal dissection revealed the left gastric artery and splenic artery. The anterior wall of the celiac trunk was dissected toward the root to reveal the dense and thickened fibrous tissue compressing the celiac trunk, and the ligament was freed and severed (Figure 3A and B), and the celiac trunk to the anterior wall of the abdominal aorta was fully exposed. The lateral and posterior aspects of the celiac trunk were exposed, the surrounding fibrous tissue and celiac ganglion were removed, and the junction of the abdominal trunk and abdominal aorta was skeletonized (Figure 3C). The gallbladder was resected in the conventional way, and a large hepatic cyst (3.1 cm × 3 cm) on the surface of the caudate lobe of the liver (Figure 1B) was drained by opening the cyst. The patient had an uneventful operation with intraoperative bleeding of 50 mL. The postoperative recovery was smooth, with significant relief of self-perceived abdominal pain symptoms, and she was discharged 10 d later.
Postoperative pathological results showed chronic cholecystitis and liver cyst. The VAS score was 2 again before discharge, and the patient was free of epigastric pain symptoms at the 3 mo follow-up.
MALS, also known as mid-foot of the diaphragm compression syndrome, celiac artery compression syndrome, celiac artery cord syndrome, and Dunbar syndrome, is a rare disorder caused by compression of the celiac trunk by the MAL[2]. The MAL is a tough fibrous arch that connects the right and left aortic fissures (T12–L1) at the level of the diaphragmatic feet on both sides and crosses anterior to the aorta above the root of the celiac trunk. The origin of the celiac trunk is in approximately 85% of the anterior wall of the abdominal aorta between the upper third of the T11 vertebral body and the upper third of the T12 vertebral body[3]. Compression of the celiac trunk can result from a high starting point of the celiac trunk or a low attachment point of the MAL diaphragm foot. In 1971, Lindner et al[4] carried out 75 autopsy studies and noticed the anatomical variation of MAL. Harjola reported for the first time that MALS, Dunbar et al. passed angiography, summarized and reported 15 cases of MALS[5]. However, the pathophysiological mechanism of MALS is still controversial, and the related theories are as follows. (1) Compression of the celiac trunk by MAL leads to a reduction in its blood flow. Long-term compression of MAL can lead to narrowing of the arterial lumen, and histological studies have shown that arterial smooth muscle, elastic fibers and inner and outer membrane proliferation in patients with MALS may lead to complete occlusion of the artery, resulting in organ ischemia and epigastric pain[2]. (2) When the blood flow within the larger collateral circulation is inhibited by the celiac trunk stenosis, the collateral vessels proliferate to compensate for part of the blood supply. For example, blood is supplied to the celiac trunk via the collateral vessels such as the pancreaticoduodenal artery arch. Clinical symptoms are caused by a decrease in blood flow shunted to the celiac trunk supply area after feeding due to dilatation of the superior mesenteric artery[6]. (3) Neuropathic factors: long-term chronic compression and overstimulation of the nerve tissues around the celiac stem can lead to abdominal pain symptoms. This neurological compression can directly stimulate sympathetic pain fibers, and combined with sympathetic excitation causes visceral vasoconstriction, resulting in local ischemia. Pathological studies have shown that the compressed nerve fibers and perineural fibrosis with small nerve fiber proliferation, and that blockade targeting the celiac ganglion relieves abdominal pain symptoms[7]. Therefore, it has been hypothesized that neuropathic pain is one of the causes of MALS.
The true prevalence of MALS is not known due to the diverse clinical presentation. However, it is more common in women aged 30–50 years (female: male: 4:1) and in patients with a lean body mass[6], and has been reported in pediatric patients. The symptoms of MALS are: chronic abdominal pain after meals; nausea, vomiting, diarrhea and unexplained weight loss. Clinical signs include epigastric tenderness, abdominal vascular murmur and an increased end-expiratory murmur[2]. In a study of 43 patients with surgically treated MALS, 91% of patients presented with abdominal pain[8]. Of these, 62% presented with postprandial pain, 32% with postexercise pain, and 6% had abdominal pain of unknown origin. In addition, 40% of the patients had significant weight loss, 30% had nausea and vomiting, and 47% had an audible epigastric vascular murmur. Since the symptoms of MALS are similar to those of other abdominal diseases, the diagnosis of MALS requires the exclusion of other causes of abdominal pain. As in the present case, gallbladder stones were found on physical examination for > 1 year, and there was a history of epigastric pain that was relieved by oral anti-inflammatory and choleretic drugs, during which she did not seek medical attention in the hospital. The patient had no specific abdominal symptoms, no epigastric paroxysmal severe pain and radiating pain in the right back or shoulder, and abdominal symptoms did not improve after administration of antispasmodic, analgesic and choleretic drugs. Therefore, this patient’s abdominal pain may have had another cause. A thorough examination of the digestive system is required to rule out other conditions that more commonly cause abdominal pain. This includes various imaging tests: upper gastrointestinal endoscopy, colonoscopy, abdominal ultrasound (US), abdominal CT, CTA or angiography, and related laboratory tests. In this case, imaging (CT and magnetic resonance imaging) confirmed gallbladder stones and cholestasis (Figure 1). CT of the upper abdomen suggested (Figure 2C) compression and narrowing of the abdominal trunk, and further CTA and DUS examination confirmed diagnosis of MALS.
When MALS is clinically suspected, abdominal DUS can be used as the screening tool of choice. DUS during maximal inspiration and expiration can dynamically demonstrate the site and extent of celiac arterial stenosis[9]. DUS is cheaper and radiation-free compared to CTA, which has the advantage of 3D reconstruction to obtain images of the celiac arteries, facilitating the observation of the compressed arteries from different angles. The CTA features of MALS include a sharp V-shaped depression or a characteristic hook-like appearance of the proximal wall of the celiac trunk[10]. This hooked appearance is helpful in differentiating the stenosis from atherosclerotic and aortitis stenosis. CTA also allows dynamic observation of arterial compression, and the degree of arterial compression stenosis in MALS patients varies with respiration, with heavier luminal stenosis in the expiratory phase than in the inspiratory phase. CTA can also show the establishment of collateral circulation after celiac trunk stenosis, and studies have shown that collateral circulation can be established when the degree of celiac trunk stenosis exceeds 65%, including pancreaticoduodenal artery arch type, dorsal pancreatic artery type, and intrahepatic type (phrenic artery). It is important to note that the presence of typical stenosis on imaging does not necessarily have clinical symptoms[11]. In a retrospective observational study, only one of eight patients with > 50% stenosis of the celiac artery was symptomatic[12].
Due to the rich collateral circulation between the superior mesenteric artery and the celiac trunk, abdominal trunk stenosis does not necessarily cause ischemic manifestations in the organs. In the diagnosis of gastrointestinal ischemia, gastric exercise tonometry (GET) has a high sensitivity (76%) and specificity (92%)[13]. Mensink et al[14] found that 29 (67.4%) of 43 patients with celiac trunk compression (> 70%) with significant abdominal pain had positive GET, 22 with MAL release alone, and seven with MAL release combined with celiac artery reconstruction. Follow-up at 39 mo revealed normal GET results in asymptomatic patients (83%) and abnormal GET results in patients with persistent symptoms (25%). This suggests that MALS is an ischemic syndrome with a good surgical outcome. Despite its high diagnostic accuracy, GET is a complex and time-consuming procedure that is not easily used as a routine test.
The treatment of MALS focuses on its possible pathophysiological mechanisms, which involves MAL release to relieve compression of the celiac trunk, combined with or without abdominal lymph node dissection. The combination of MAL release with peripheral ganglionectomy of the celiac trunk may be an option for treating the neurological cause of pain. In contrast, MAL release combined with periceliac trunk ganglionectomy is effective and is currently a widely accepted treatment[15]. Traditionally, MALS is treated by open surgery. After dissecting and separating the MAL and celiac trunk, the MAL is cut and the proximal part of the celiac trunk is completely exposed to release the compression; at the same time, the peripheral ganglion of the celiac trunk is removed. In recent years, there has been a trend to release the pressure on the celiac trunk through laparoscopy, and laparoscopic and surgical robots have been reported abroad for the treatment of MALS[16]. Jimenez et al[17] retrospectively analyzed data from 400 patients who underwent surgical treatment for MALS. Of these, 279 underwent open surgery and 121 underwent laparoscopic surgery. Overall, 85% of the patients experienced postoperative symptom relief. Postoperative symptom recurrence rates were 6.8% and 5.7% for patients in the open and laparoscopic groups, respectively; 9.1% of laparoscopic procedures were intermediate to open due to bleeding. There were no surgery-related deaths in either group. The laparoscopic group had the advantages of shorter hospital stay, shorter fasting time, less risk of postoperative complications, less intraoperative bleeding, better postoperative pain relief, and smaller incisions. Intraoperative US multispectral or angiography can visualize the effect of postoperative abdominal stem decompression, which can be addressed by arterial reconstruction if stenosis persists. These include: Abdominal aortic bypass or peritoneal artery patch angioplasty. Percutaneous transluminal angioplasty with or without stenting provides an adjunctive treatment for persistent stenosis after MAL release. However, endovascular intervention alone does not address extrinsic compression of the celiac artery and, therefore, intervention alone is ineffective in the treatment of MALS. One report found that patients who had a percutaneous peritoneal plexus block preoperatively and whose symptoms were relieved had a better postoperative outcome. However, high-quality evidence to support this claim is lacking[18].
MALS is a rare clinical syndrome for which there is no general consensus on diagnostic and therapeutic criteria. MALS can be easily confused with many common diseases. In this case, the patient also had gallbladder stones with chronic cholecystitis (which was confirmed by postoperative pathology). Gallbladder stones can also cause epigastric pain and intolerance to fried or high-fat foods (characterized by nausea and bloating), making it likely that the diagnosis of MALS will be missed. Clinicians should raise awareness of MALS, especially in patients with chronic abdominal pain, and consider it earlier. Surgery can provide immediate relief and can be an effective treatment for MALS. However, there is no consensus on the best surgical treatment. As for whether there is an intrinsic pathological link between gallstones and MALS, it remains to be confirmed by more similar cases.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Handra-Luca A, France S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Rubinkiewicz M, Ramakrishnan PK, Henry BM, Roy J, Budzynski A. Laparoscopic decompression as treatment for median arcuate ligament syndrome. Ann R Coll Surg Engl. 2015;97:e96-e99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Goodall R, Langridge B, Onida S, Ellis M, Lane T, Davies AH. Median arcuate ligament syndrome. J Vasc Surg. 2020;71:2170-2176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 3. | Koç M, Artaş H, Serhatlıoğlu S. The investigation of incidence and multidetector computed tomography findings of median arcuate ligament syndrome. Turk J Med Sci. 2018;48:1214-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lindner HH, Kemprud E. A clinicoanatomical study of the arcuate ligament of the diaphragm. Arch Surg. 1971;103:600-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 118] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Dunbar JD, Molnar W, Beman FF, Marable SA. Compression of the celiac trunk and abdominal angina. Am J Roentgenol Radium Ther Nucl Med. 1965;95:731-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 263] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Arazińska A, Polguj M, Wojciechowski A, Trębiński Ł, Stefańczyk L. Median arcuate ligament syndrome: Predictor of ischemic complications? Clin Anat. 2016;29:1025-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Chaum M, Shouhed D, Kim S, Walts AE, Marchevsky AM. Clinico-pathologic findings in patients with median arcuate ligament syndrome (celiac artery compression syndrome). Ann Diagn Pathol. 2021;52:151732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Ho KKF, Walker P, Smithers BM, Foster W, Nathanson L, O'Rourke N, Shaw I, McGahan T. Outcome predictors in median arcuate ligament syndrome. J Vasc Surg. 2017;65:1745-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Björck M, Koelemay M, Acosta S, Bastos Goncalves F, Kölbel T, Kolkman JJ, Lees T, Lefevre JH, Menyhei G, Oderich G; Esvs Guidelines Committee; Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Kakkos S, Koncar I, Sanddal Lindholt J, Vega de Ceniga M, Vermassen F, Verzini F, Document Reviewers, Geelkerken B, Gloviczki P, Huber T, Naylor R. Editor's Choice - Management of the Diseases of Mesenteric Arteries and Veins: Clinical Practice Guidelines of the European Society of Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. 2017;53:460-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 430] [Article Influence: 61.4] [Reference Citation Analysis (1)] |
| 10. | Kazmi SSH, Safi N, Berge ST, Kazmi M, Sundhagen JO, Hisdal J. Laparoscopic Surgery for Median Arcuate Ligament Syndrome (MALS): A Prospective Cohort of 52 Patients. Vasc Health Risk Manag. 2022;18:139-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Squizzato F, Oderich GS, Tenorio ER, Mendes BC, DeMartino RR. Effect of celiac axis compression on target vessel-related outcomes during fenestrated-branched endovascular aortic repair. J Vasc Surg. 2021;73:1167-1177.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Baskan O, Kaya E, Gungoren FZ, Erol C. Compression of the Celiac Artery by the Median Arcuate Ligament: Multidetector Computed Tomography Findings and Characteristics. Can Assoc Radiol J. 2015;66:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | van Noord D, Sana A, Moons LM, Pattynama PM, Verhagen HJ, Kuipers EJ, Mensink PB. Combining radiological imaging and gastrointestinal tonometry: a minimal invasive and useful approach for the workup of chronic gastrointestinal ischemia. Eur J Gastroenterol Hepatol. 2013;25:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Mensink PB, van Petersen AS, Kolkman JJ, Otte JA, Huisman AB, Geelkerken RH. Gastric exercise tonometry: the key investigation in patients with suspected celiac artery compression syndrome. J Vasc Surg. 2006;44:277-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Iqbal S, Chaudhary M. Median arcuate ligament syndrome (Dunbar syndrome). Cardiovasc Diagn Ther. 2021;11:1172-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 16. | Fernstrum C, Pryor M, Wright GP, Wolf AM. Robotic Surgery for Median Arcuate Ligament Syndrome. JSLS. 2020;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Jimenez JC, Harlander-Locke M, Dutson EP. Open and laparoscopic treatment of median arcuate ligament syndrome. J Vasc Surg. 2012;56:869-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Weber JM, Boules M, Fong K, Abraham B, Bena J, El-Hayek K, Kroh M, Park WM. Median Arcuate Ligament Syndrome Is Not a Vascular Disease. Ann Vasc Surg. 2016;30:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |