Published online Nov 16, 2023. doi: 10.12998/wjcc.v11.i32.7741
Peer-review started: October 1, 2023
First decision: October 24, 2023
Revised: October 27, 2023
Accepted: November 9, 2023
Article in press: November 9, 2023
Published online: November 16, 2023
Processing time: 45 Days and 20.5 Hours
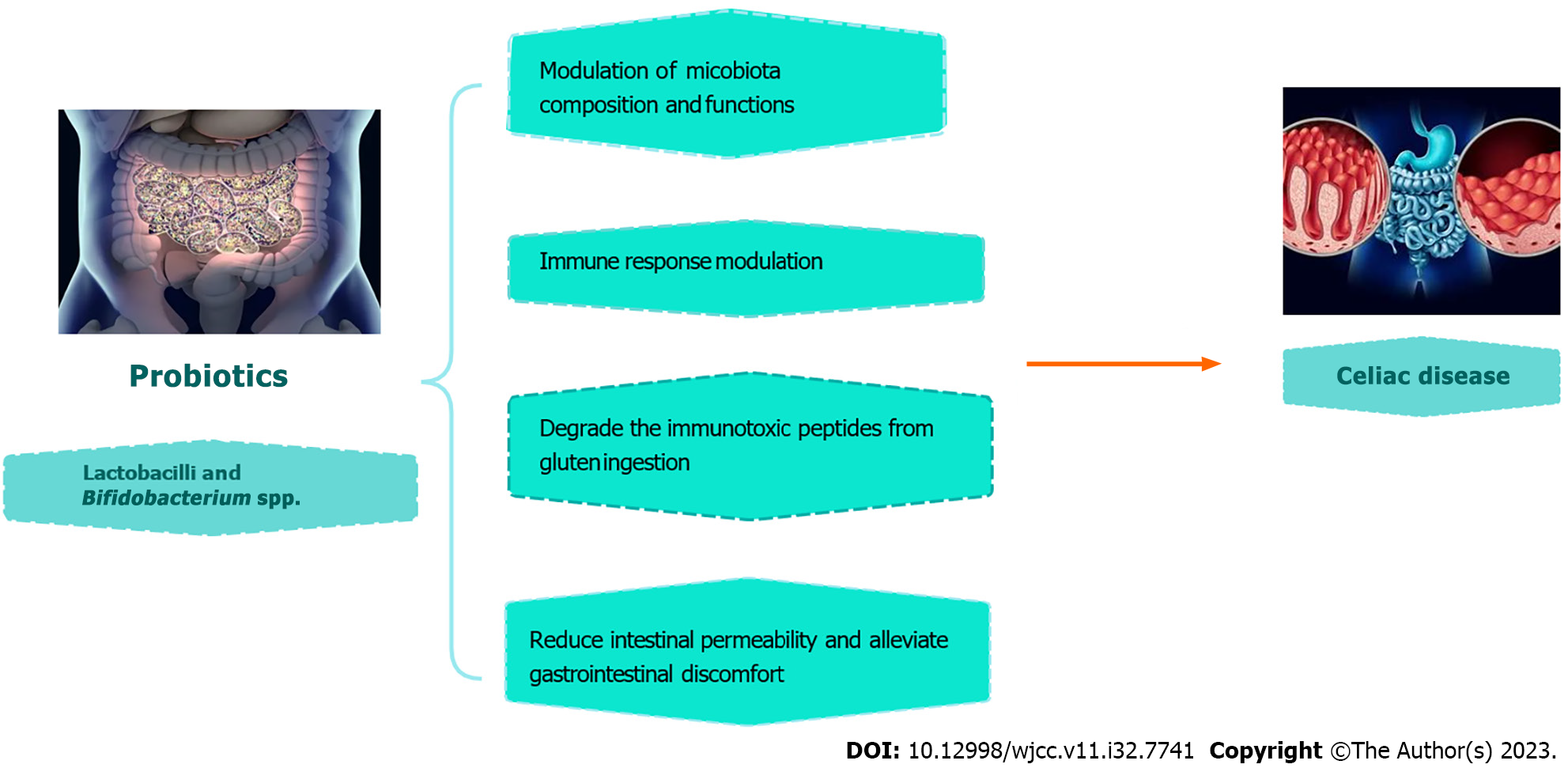
Patients with celiac disease (CD) have a mucosal layer that is unable to regulate the gut microbiota, leaving the host vulnerable to dangerous infections and antigens. When compared to healthy people, this dysbiosis is marked by a decrease in intra- and intergeneric biodiversity, which demonstrates an imbalance between helpful bacteria and possibly harmful or proinflammatory species. The early gut microbiota is influenced by the genotype of newborns with the HLA-DQ2 haplotypes, and this may modify how gluten is handled in the intestinal lumen, polarize innate or adaptive immune responses, and result in glutensensitive enteropathy. The outcome of gluten digestion can vary depending on the composition of the intestinal gut bacteria and the partial conversion of gluten into peptides larger than ten amino acids in the small intestines, which can be immunogenic. In the small intestine, 114 different bacterial strains belonging to 32 different species have 27 of them exhibiting peptidolytic activity. Thus, the individual risk of developing a gluten-related illness is further influenced by microbial composition and gluten degrading capacity. The conclusion that lactobacilli and Bifidobacterium spp. may be used as a probiotic supplement in CD patients is based on their shared possession of the most extensive peptidolytic and proteolytic activity thought to be engaged in the breakdown of gluten among all potential bacterial genera present in the gut microbiota. In children with CD autoimmunity, daily oral dose of Lactobacillus. plantarum HEAL9 and Lactobacillus. paracasei 8700:2 was found to modify the peripheral immune response. Bifidobacterium. breve strains have demonstrated a beneficial effect on reducing pro-inflammatory cytokine TNF- production in CD children on gluten-free diets.
Core Tip: In the context of celiac disease (CD), probiotics emerge as a multifaceted therapeutic approach with promising implications. Clinical trials demonstrate their potential to modulate immune responses, alleviate gastrointestinal symptoms, and reshape the gut microbiota in CD patients. Notably, specific probiotic strains have shown the ability to enzymatically break down immunotoxic gluten peptides, addressing a central challenge in CD pathogenesis. This dual-pronged role positions probiotics as a holistic means of CD management, offering immunomodulation and symptom relief to patients while potentially mitigating the toxicity of gluten peptides. Probiotics thus represent an encouraging avenue for enhancing the quality of life for individuals living with CD, underscoring their significance in the evolving landscape of CD treatment.
- Citation: Moawad MHE, Alkhawaldeh IM, Naswhan AJ. Efficacy of probiotics supplementation in amelioration of celiac disease symptoms and enhancement of immune system. World J Clin Cases 2023; 11(32): 7741-7744
- URL: https://www.wjgnet.com/2307-8960/full/v11/i32/7741.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i32.7741
The small intestine is primarily affected by celiac disease (CD), an autoimmune ailment that develops in those with a hereditary predisposition to gluten and is characterized by symptoms on both the intestinal and extraintestinal levels. The increasing body of research provides support for the hypothesis that changes in the composition and functionality of the gut microbiome are associated with various chronic inflammatory conditions such as inflammatory bowel disease, cancer, and Crohn's disease[1].
The gut microbiota plays a crucial role in promoting tolerance in normal physiological conditions. However, any disruption in the microbiota, known as dysbiosis, can disturb the balance between immune responses that promote tolerance and those that induce inflammation. This imbalance can contribute to the development of diseases such as Crohn's disease, which is characterized by an overactive Th1 immune response. The small intestine of people who have CD makes partial metabolism (due to dysbiosis) to gluten leading to its breakage into large chains of more than 10 amino acids which is immunogenic. The principal probiotic bacteria are Lactobacillus and Bifidobacterium species, which are found in the intestinal environment. Bifidobacteria produce exopolysaccharides that serve as fermentable substrates for additional human intestinal bacteria, whereas lactobacilli can secrete mucins, reinforce the epithelial barrier, improve tight-junction activities, and inhibit the epithelial cells death . It was discovered that microbiota composed of Bifidobacterium and lactobacilli can produce more active peptidases to break down those long chain amino acids, thus protecting the intestine from severe immunological reaction when ingesting gluten[2].
Increased interferon (IFN)-γ synthesis in CD causes TNF-α secretion, which is crucial for causing intestinal mucosal injury and inflammation. Increased production of TNF-α is also associated with lower Firmicutes/Bacteroidetes ratio which is considered a major dysbiosis in CD patients. Klemenak et al[3] and Quagliariello et al[4] demonstrated a decline in TNF-α and the increase of the Firmicutes/Bacteroidetes ratio, respectively, after treatment with B.breve which is in line with Primec et al[5] who showed that after 3 mo of B.breve treatment, TNF-α was reduced with parallel increase in Firmicutes.
Khorzoghi et al[6] studied how probiotic administration affected the composition of the intestinal microbiota and how clinical symptoms in CD patients improved as a result. In this investigation, the ingestion of a probiotic combination containing Bifidobacterium spp., Lactobacillus spp., and S. thermophilus resulted in a reduction in the intensity of clinical symptoms (fatigue, muscle discomfort, bloating, and a gassy feeling) compared to placebo. The relative levels of Lactobacillus, Bacteroidetesspp., Bifidobacterium spp., Clostridium cluster I, Enterobacteriaceae, and Firmicutes were higher in the probiotics group than they were in the control group, with the exception of Staphylococcus spp. In light of this, a 12-week probiotic multi-strain treatment plan could alter the make-up of the intestinal microbiota and lessen gastrointestinal symptoms in CD patients. According to Klemenak et al[3] and Quagliariello et al[4], B. breve strains combined with GFD act on a decrease in TNF- α production, which may have an impact on preventing a pro-inflammatory milieu in children with CD. The effects of this probiotic pill, however, only last while being consumed. Three months following the end of the intervention, Klemenak et al[3] discovered that TNF-α levels have nearly completely returned to baseline levels. Therefore, further trials are required to investigate a long-lasting effect of probiotic pills and control the microbiota environment of the CD patients.
Håkansson et al[7] conducted a randomized, double-blind, placebo-controlled clinical trial for children with CD. The main result was the steady alteration in the peripheral immune response; demonstrated by T cells regulation and decreased their concentration and responses in the probiotic group compared to placebo group indicating that L. paracasei 8700:2 and L. plantarum HEAL9 were capable of modulating the peripheral immune response in CD autoimmunity. The median levels of IgA-tTG also decreased as a result of the probiotic therapy (P = 0.013).
In a major prospective, randomized study, Francavilla et al[8] combined five strains of lactic acid bacteria and bifidobacteria: L. casei LMG 101/37 P-17504, L. plantarum CECT 4528, B. animalis subsp. lactis Bi1 LMG P-17502, B. breve Bbr8 LMG P-17501, and B. breve Bl10 LMG P-17500. They recruited 109 CD patients who strictly adhered to a GFD and had signs and symptoms of irritable bowel syndrome (IBS). They were randomly assigned for six weeks to receive probiotics or a placebo, and then were monitored for another six weeks. The findings showed that this probiotic mixture was effective in reducing the severity of gastrointestinal symptoms, with a decreased feeling of pain on various clinical assessments and a significantly greater percentage of treatment success (defined as at least a 50% decrease in the symptom scores). With a rise in lactic acid bacteria, Bifidobacterium, and Staphylococcus that was remained observable six weeks after the withdrawal of probiotics, they were able to demonstrate a favorable modification of the gut microbiota (Figure 1).
In a different trial[9], 20 CD patients received hydrolyzed wheat gluten bread with L. alimentaris, L. brevis, L. sanfranciscenis, and L. hilgardi for six days. In comparison to healthy controls, the findings revealed no substantial rise in IFN-γ. Thus, clinical trials have recently produced growing and positive outcomes. Serological, histological, and immunohistochemical characteristics were unaffected when CD patients were fed a diet of baked goods with less than 10 ppm of gluten prepared from fermented wheat flour. Similar outcomes were attained when CD patients in remission were exposed to lactobacilli that had already digested gluten for 60 d[10]. Patients' symptoms, intestinal permeability, or serological markers did not worsen, indicating that lactobacilli-derived endopeptidases may be capable of completely breaking down gluten and reducing gluten toxicity in CD patients[10].
Due to this findings, present research aims to identify probiotic strains that can totally degrade the immunotoxic peptides from gluten ingestion. Therefore, probiotics are the uprising mainstay of treatment of CD patients whether by their administration to them or by their effect of gluten peptides that cause autoimmune reactions.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li C, China S-Editor: Liu JH L-Editor: A P-Editor: Yu HG
| 1. | Lynch SV, Pedersen O. The Human Intestinal Microbiome in Health and Disease. N Engl J Med. 2016;375:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1826] [Cited by in RCA: 2325] [Article Influence: 258.3] [Reference Citation Analysis (0)] |
| 2. | Francavilla R, Cristofori F, Vacca M, Barone M, De Angelis M. Advances in understanding the potential therapeutic applications of gut microbiota and probiotic mediated therapies in celiac disease. Expert Rev Gastroenterol Hepatol. 2020;14:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 3. | Klemenak M, Dolinšek J, Langerholc T, Di Gioia D, Mičetić-Turk D. Administration of Bifidobacterium breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig Dis Sci. 2015;60:3386-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Quagliariello A, Aloisio I, Bozzi Cionci N, Luiselli D, D'Auria G, Martinez-Priego L, Pérez-Villarroya D, Langerholc T, Primec M, Mičetić-Turk D, Di Gioia D. Effect of Bifidobacterium breve on the Intestinal Microbiota of Coeliac Children on a Gluten Free Diet: A Pilot Study. Nutrients. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 5. | Primec M, Klemenak M, Di Gioia D, Aloisio I, Bozzi Cionci N, Quagliariello A, Gorenjak M, Mičetić-Turk D, Langerholc T. Clinical intervention using Bifidobacterium strains in celiac disease children reveals novel microbial modulators of TNF-α and short-chain fatty acids. Clin Nutr. 2019;38:1373-1381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 86] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 6. | Soheilian Khorzoghi M, Rostami-Nejad M, Yadegar A, Dabiri H, Hadadi A, Rodrigo L. Impact of probiotics on gut microbiota composition and clinical symptoms of coeliac disease patients following gluten-free diet. Contemp Clin Trials Commun. 2023;35:101201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 7. | Håkansson Å, Andrén Aronsson C, Brundin C, Oscarsson E, Molin G, Agardh D. Effects of Lactobacillus plantarum and Lactobacillus paracasei on the Peripheral Immune Response in Children with Celiac Disease Autoimmunity: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Francavilla R, Piccolo M, Francavilla A, Polimeno L, Semeraro F, Cristofori F, Castellaneta S, Barone M, Indrio F, Gobbetti M, De Angelis M. Clinical and Microbiological Effect of a Multispecies Probiotic Supplementation in Celiac Patients With Persistent IBS-type Symptoms: A Randomized, Double-Blind, Placebo-controlled, Multicenter Trial. J Clin Gastroenterol. 2019;53:e117-e125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (1)] |
| 9. | Mandile R, Picascia S, Parrella C, Camarca A, Gobbetti M, Greco L, Troncone R, Gianfrani C, Auricchio R. Lack of immunogenicity of hydrolysed wheat flour in patients with coeliac disease after a short-term oral challenge. Aliment Pharmacol Ther. 2017;46:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 10. | Francavilla R, De Angelis M, Rizzello CG, Cavallo N, Dal Bello F, Gobbetti M. Selected Probiotic Lactobacilli Have the Capacity To Hydrolyze Gluten Peptides during Simulated Gastrointestinal Digestion. Appl Environ Microbiol. 2017;83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |