Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7656
Peer-review started: August 20, 2023
First decision: October 10, 2023
Revised: October 13, 2023
Accepted: October 26, 2023
Article in press: October 26, 2023
Published online: November 6, 2023
Processing time: 78 Days and 1 Hours
This report delves into the diagnostic and therapeutic journey undertaken by a patient with Sneddon's syndrome (SS) and cerebral venous sinus thrombosis (CVST). Particular emphasis is placed on the comprehensive elucidation of SS's clinical manifestations, the intricate path to diagnosis, and the exploration of potential underlying mechanisms.
A 26-year-old woman presented with recurrent episodes of paroxysmal unilateral limb weakness accompanied by skin mottling, seizures, and cognitive im
This article has reported and analyzed the clinical data, diagnosis, treatment, and prognosis of a case of SS with CVST and reviewed the relevant literature to improve the clinical understanding of this rare condition.
Core Tip: A 26-year-old woman presented with recurrent episodes of paroxysmal unilateral limb weakness accompanied by skin mottling, seizures, and cognitive impairment. Digital subtraction angiography revealed cerebral venous sinus thrombosis (CVST). Despite negative antiphospholipid antibody results, skin biopsy indicated chronic inflammatory cell infiltration. The patient was treated using anticoagulation, antiepileptic therapy, and supportive care, which resulted in symptom improvement. The coexistence of Sneddon's syndrome (SS) and CVST is rare and the underlying pathophysiology remains uncertain. This case underscores the challenge in diagnosis and highlights the need for early clinical differentiation to facilitate accurate assessment and prompt intervention.
- Citation: Heng Y, Tang YF, Zhang XW, Duan JF, Shi J, Luo Q. Sneddon's syndrome concurrent with cerebral venous sinus thrombosis: A case report. World J Clin Cases 2023; 11(31): 7656-7662
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7656.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7656
Sneddon's syndrome (SS) is a complicated and uncommon dermatoneurological syndrome that extensively affects various small- to medium-sized blood vessels throughout the body. It is characterized by recurrent stroke-like episodes and the distinctive presence of widespread reticulated purpura on the skin[1]. SS can directly or indirectly involve multiple systems and organs, including the circulatory, urinary, respiratory, and digestive systems, as well as the eyes and ears. Common associated conditions include hypertension, epilepsy, renal insufficiency, venous thrombosis in the limbs, and migraines. Women with SS may present with a history of recurrent miscarriages[2,3]. The syndrome was first described by Sneddon in 1965[1]. Presently, reports regarding SS with concurrent cerebral venous sinus thrombosis (CVST) are limited. This article presents the clinical data, diagnostic and therapeutic processes, and outcome of a patient with SS and CVST. Our aim is to enhance the clinical understanding of this rare condition.
A 26-year-old woman presented to the Neurology Department of Mianyang Central Hospital on March 6, 2020 with sudden-onset left-sided limb weakness that had been worsening over 3 d. She reported similar episodes in the past that had been recurring over the previous 10 years.
The symptoms began approximately 10 years ago without any apparent triggers. The patient reported difficulty in holding objects in her left hand and required assistance with walking. Previous episodes lasted for approximately 30 min before resolving spontaneously. The patient did not attach much significance to these symptoms. Approximately 3 d prior to admission, she experienced another episode of left-sided limb weakness, which progressed to complete immobility. This time, the weakness was accompanied by dizziness, headache, nausea, and vomiting. She initially sought medical attention at a local county hospital. After she exhibited seizures accompanied by loss of consciousness and urinary incontinence, she was referred to our hospital for further treatment.
On February 28, 2020, the patient underwent a Cesarean section. During her hospitalization, she continued to experience lochia discharge. She reported experiencing elevated blood pressure throughout the pregnancy but specific details were not provided. She also reported having scattered red vascular-like lesions on her face and forehead and reticulated purpura on her trunk and limbs, mainly the lower extremities. These markings were more pronounced when standing or in cold weather, and tended to improve with warmer temperature, immersion in warm water, and lying down. She had never sought medical attention for the skin lesions. The patient denied a history of diabetes, hypertension, coronary heart disease, and renal insufficiency. She also denied a history of infectious disease such as hepatitis or tuberculosis, as well as smoking, alcohol consumption, and substance abuse.
The patient denied any family history of genetic disease or tumors. The patient denied any history of mental illness. And she had one daughter without any history of miscarriages.
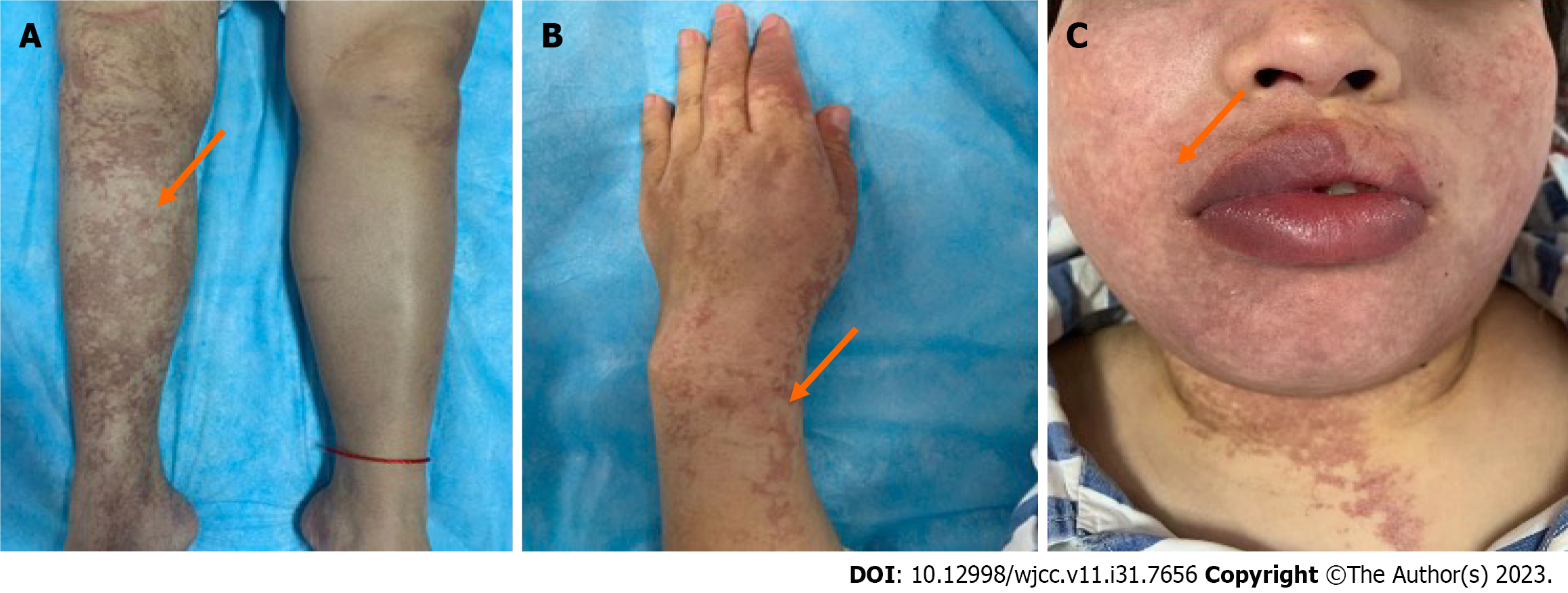
The patient's vital signs were as follows: Body temperature, 36.7°C; pulse rate, 72 beats per minute; respiratory rate, 20 breaths per minute; and blood pressure, 124/91 mmHg. She was well-developed with good nutritional status. Extensive bluish-purple reticulated purpura was observed on the face, trunk, and limbs. The purpura was particularly pronounced on the extremities (especially the hands and right lower limb) and had irregular edges in shades of light red and blue, with some areas appearing darker than others. The lesions did not fade when manual pressure was applied (Figure 1). Neurologically, she was alert with a symmetric face and normal facial muscle strength. Muscle strength of the right upper and lower limbs was normal. Manual muscle testing of the proximal and distal left upper limb was assessed as grade 3, respectively; left lower limb muscle strength was grade 4. Proprioception, sensory perception, and vibratory sense were normal. Deep tendon reflexes of the biceps, triceps, brachioradialis, patellar tendon, and Achilles tendon were normal bilaterally. The Babinski sign was positive on the left. Left finger-to-nose and left heel-to-shin testing were unstable. She was unable to walk in a straight line.
Hematological parameters, liver and kidney function, electrolytes, thyroid function, urinalysis, coagulation function, comprehensive immunological assessment, cardiac phospholipid antibodies testing, tumor markers, and infectious disease screening all showed no significant abnormalities.
On magnetic resonance imaging of the brain, both frontal lobes showed a few punctate dot-like hyperintensities on T2-weighted imaging and fluid-attenuated inversion recovery sequences, with corresponding signal changes on T1-weighted and diffusion-weighted imaging. High signal intensity and swelling were seen in the right parieto-occipital cortex on T2-weighted imaging and fluid-attenuated inversion recovery sequences. Multiple tortuous small vessels were observed superficially on the cortex of both hemispheres. The ventricular system was symmetrically dilated and cerebral sulci and fissures appeared widened; the gyri were mildly diminished. Midline structures were not displaced.
Susceptibility-weighted imaging also showed multiple tortuous vessels on the cortical surface of both hemispheres which were of low signal intensity. An area of decreased signal was observed in the anterior part of the falx cerebri. No abnormal signal was detected elsewhere.
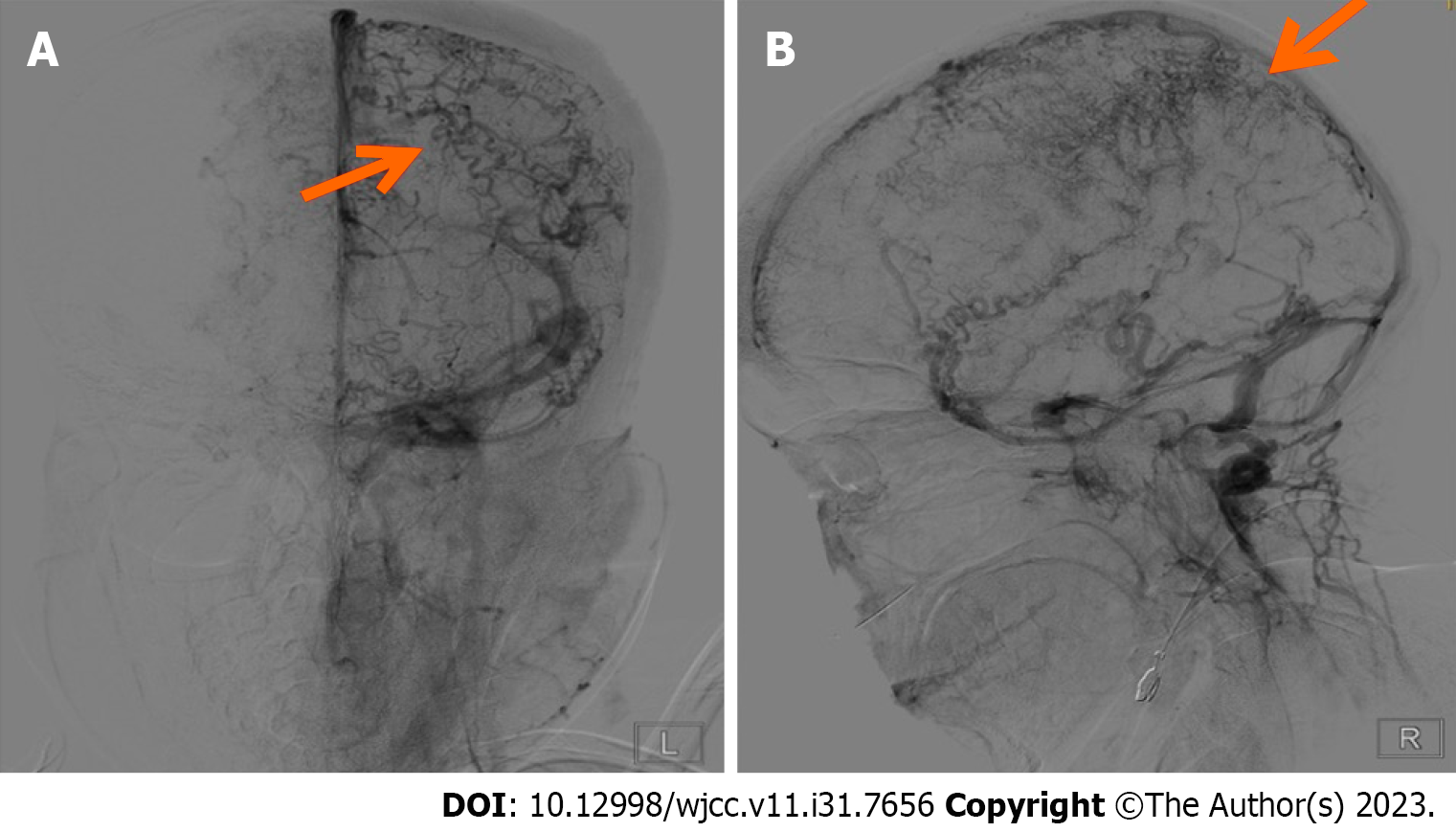
Digital subtraction angiography showed slender middle cerebral and anterior cerebral arteries bilaterally. No significant arterial narrowing or aneurysms were noted along either internal carotid artery. The right vertebral artery appeared thin and the V3 segment was not visualized distally. The left vertebral artery, basilar artery, and both posterior cerebral arteries showed no significant stenosis or aneurysms. The parenchymal phase appeared unremarkable. In the venous phase, extensive dilation of tortuous cortical veins was evident bilaterally. The superior sagittal sinus, inferior sagittal sinus, and straight sinus exhibited faint opacification. Both transverse sinuses were tortuous (Figure 2). Chest computed tomography showed pneumonia.
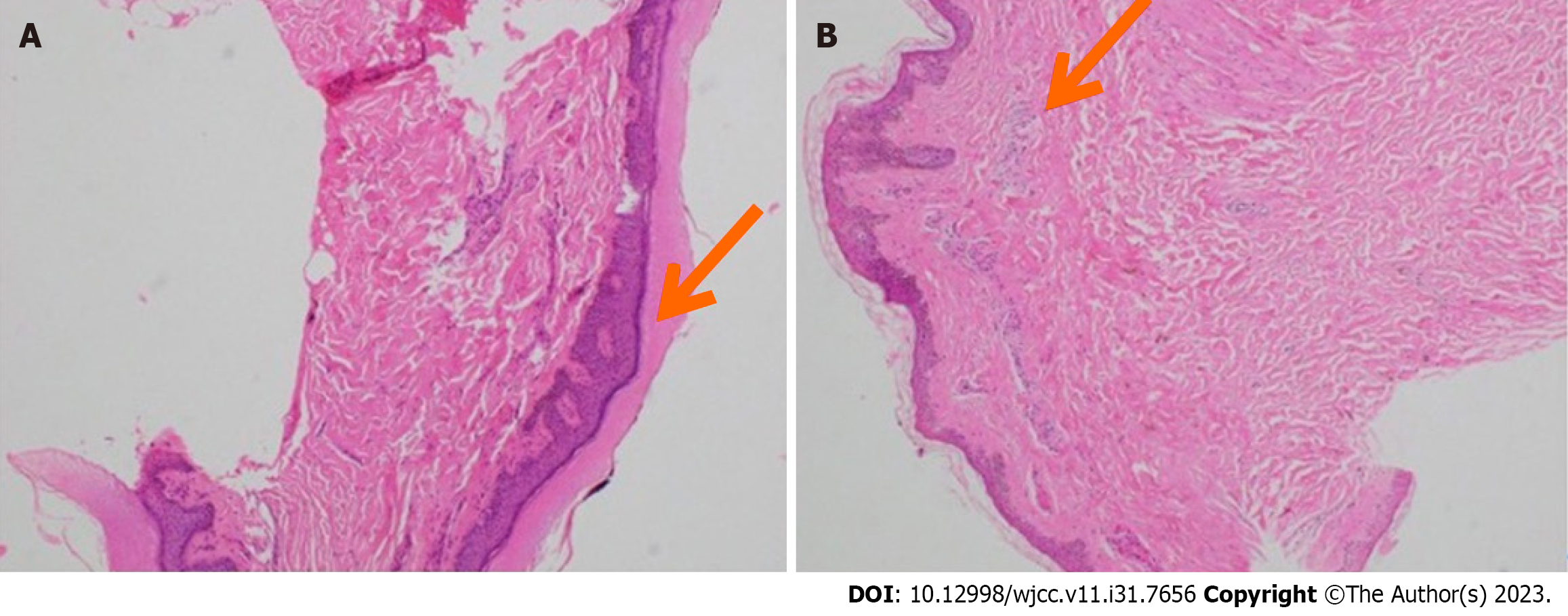
Skin tissue from the right thigh and right hand was submitted for histopathological examination. Both samples showed epidermal hyperkeratosis and subcutaneous chronic inflammatory cell infiltration (Figure 3).
In this case, the young woman has been experiencing recurring episodes of paroxysmal unilateral limb weakness and spontaneous recurrent seizures over the course of several years. These episodes have also been accompanied by reticulated purpura affecting the face, limbs, and trunk. Additionally, she has been diagnosed with CVST. After excluding other potential causes, the clinical diagnosis was SS.
Upon admission, the patient was treated with cefotaxime sodium for pneumonia, oral warfarin for anticoagulation, and sodium valproate for seizure management. These treatments were complemented by supportive care.
The patient's muscle strength on the left side gradually recovered and her mental state cleared. At the time of hospital discharge, her neurological examination was normal. She has continued long-term outpatient treatment with oral warfarin and undergoes regular follow-up assessments of her prothrombin time. In the 10 mo since discharge, there has been no worsening of the reticulated purpura and she has not experienced any further seizures or episodes of impaired consciousness or stroke.
SS is a rare multisystem disorder primarily characterized by reticulated purpura on the skin and recurrent stroke-like events. The prevalence of SS is low, and there are approximately four cases per million individuals. Over two-thirds of cases are diagnosed in adults aged 20 to 40 years[4]; however, symptoms usually begin in childhood and gradually worsen over time. SS is associated with high rates of misdiagnosis and underdiagnosis. The diagnosis is frequently only established once episodes resembling ischemic stroke occur.
SS predominantly affects young women and is marked by recurrent multiple cerebral infarctions, frequent car
The precise etiology and pathogenesis of SS remain unclear. Elevated levels of antiphospholipid antibodies have been observed in SS patients, which contribute to systemic hypercoagulability and thrombus formation. Thrombotic events, spontaneous miscarriages, and thrombocytopenia are associated with these antibodies[8], which appear to play a critical role in SS development.
Common clinical presentations of SS include stroke-like events and reticulated purpura. More than 90% of patients experience acute ischemic stroke or transient ischemic attacks. The lesions are often found in the distal territories of the middle cerebral arteries and posterior cerebral arteries, leading to decreased perfusion. Neurological symptoms encompass limb weakness, sensory disturbances, hemianopia, motor or sensory aphasia, visual impairment, and visual field deficits. Headaches are the most frequent non-specific symptom. Approximately two-thirds of patients with SS experience cognitive impairment, including attention and visual-spatial deficits[9]. Hemorrhagic lesions associated with microvascular disease may cause severe brain atrophy and worsen neurological deficits. SS has also been linked to young-onset dementia, anxiety, depression, hallucinations, and delusions[10].
Reticulated purpura is a prominent clinical feature of SS. They often appear prior to ischemic stroke-like events or along with them. The purpura of SS may be mistaken for systemic vasculitis or idiopathic reticulated purpura. The lesions typically present as irregular, bordered, and ring-shaped bluish-purple patterns that predominantly affect the limbs (100%), trunk (84%–89%), lumbosacral area (68%–74%), face (15%–16%), and extremities (53%–59%)[11]. The lesions may persist for an extended duration. Their color and scope are influenced by various factors. Cold weather, pregnancy, standing, hanging posture, and acute neurological damage exacerbate symptoms, while warmer weather and rest tend to alleviate them. This is likely related to reduced blood flow and oxygenation caused by widespread occlusion of small systemic arteries.
Skin biopsies of reticulated purpura often exhibit non-inflammatory microthrombi within small- to medium-sized arterial vessels. Vessel constriction and fibrosis lead to occlusion, which results in ischemia, hypoxia, and necrosis. The skin biopsy in our patient also reflected these changes, highlighting the importance of skin biopsy in patients with SS.
In addition to neurological and cutaneous manifestations, SS often involves other systems. Hypertension, valvular heart disease, coronary artery disease, chronic renal insufficiency, migraine, Raynaud's phenomenon, epilepsy, venous thrombosis, and repeated miscarriages have all been associated with SS[12].
SS represents a distinctive clinical neurocutaneous syndrome seldom seen in clinical practice. It is primarily characterized by the concurrent presence of livedo reticularis and stroke-like episodes[13]. The diagnosis hinges upon the manifestation of reticular livedo along with ischemic stroke-like episodes after ruling out other diseases that have similar clinical presentation. Of significance, the characteristic histopathological skin biopsy findings hold clinical diagnostic relevance[14]. Optimal therapeutic strategies for SS remain an ongoing subject of exploration. Treatment approaches predominantly emphasize antiplatelet and anticoagulation interventions. In patients with positive antiphospholipid antibodies, long-term administration of warfarin is recommended to maintain an international normalized ratio > 3; however, anticoagulation is associated with a risk of intracranial hemorrhage. For those without antiphospholipid antibodies, long-term antiplatelet therapy with both aspirin and clopidogrel is advised[15]. Alternatively, trials of glucocorticoids and immunosuppressive agents may also be considered, although their effectiveness has not been proven. Irrespective of antiphospholipid antibodies status, SS patients exhibit comparable clinical features pertaining to cerebrovascular disease, livedo reticularis, and cardiovascular risk factors. Differences in associated signs and symptoms are minimal. Therapeutic strategies aimed at averting recurrent stroke and ameliorating residual neurological deficits primarily involve antithrombotic measures, alongside the elimination or management of cardiovascular risk factors.
Currently, there is no consensus regarding SS diagnosis, which is typically derived from symptoms, laboratory testing, imaging, and histopathological findings. Diagnostic criteria often comprise widespread reticular livedo, characteristic histopathological features on skin biopsy, and focal neurological deficits consistent with transient ischemic attack or stroke in conjunction with high-signal intensity lesions on T2-weighted magnetic resonance imaging[14]. Our patient differs from previously reported SS cases in that digital subtraction angiography revealed marked dilation of cortical veins bilaterally, faint opacification of the superior sagittal sinus, inferior sagittal sinus, and straight sinus, and tortuous transverse sinuses, indicative of CVST. CVST accounts for merely 1% of all stroke occurrences, with an annual incidence of five cases per million[15]. Predominantly observed in individuals below 50 years of age, CVST frequently manifests with headaches and visual deterioration. Acquired CVST risk factors include surgical stress, trauma, pregnancy, antiphospholipid antibody syndrome, malignancy, and hormonal fluctuations[16]. Hereditary risk factors encompass hypercoagulable states caused by deficiencies in anticoagulant proteins such as antithrombin III, protein C, and protein S; factor V Leiden gene mutation, oral contraceptive use, and elevated homocysteine level have also been implicated[17]. Some patients may exhibit focal neurological deficit, epilepsy, or altered consciousness. Notably, the superior sagittal sinus and transverse sinus are the primary sites for acute CVST formation[18]. Any factor capable of altering venous sinus structure or blood constituents can potentially precipitate CVST. For instance, in cases of venous stasis, congenital or acquired factors may cause venous sinus stenosis or occlusion, which can obstruct cerebral venous outflow and cerebrospinal fluid absorption. This phenomenon may modulate cortical venous redistribution, impede venous return, activate the immune system, and result in intracranial hypertension and neurological deficits[19]. In our patient, there is no conclusive evidence for causative factors of CVST nor is there a hereditary thrombophilia predisposition. Thus, a more comprehensive exploration of potential etiological factors is warranted. Anticoagulation stands as a recommended CVST treatment in the most recent literature.
This case involved a 26-year-old woman with features of livedo reticularis, stroke-like episodes, and seizures. Skin biopsy suggested chronic inflammatory cell infiltration. Although antiphospholipid antibodies testing was negative, the presence of CVST indicated a hypercoagulable state. Therefore, warfarin anticoagulation therapy was initiated. SS constitutes a multi-system neurocutaneous syndrome characterized by insidious onset and high susceptibility to misdiagnosis and underdiagnosis. Despite the expanding understanding of SS, questions regarding its etiology, diagnosis, and treatment remain. Presently, reliable clinical and pathological standards for diagnosing SS are absent. Prospective multicenter studies are needed to enhance diagnostic and treatment capabilities and improve patient outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tan JK, Malaysia S-Editor: Lin C L-Editor: Wang TQ P-Editor: Zhao S
| 1. | Starmans NLP, van Dijk MR, Kappelle LJ, Frijns CJM. Sneddon syndrome: a comprehensive clinical review of 53 patients. J Neurol. 2021;268:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Lai O, Zillikens D, Shimanovich I. Generalized Net-like Erythema and Stroke in a Young Female. JAMA Dermatol. 2018;154:93-94. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Dutra LA, Braga-Neto P, Pedroso JL, Barsottini OG. Sneddon's syndrome: case report and review of its relationship with antiphospholipid syndrome. Einstein (Sao Paulo). 2012;10:230-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Sayin R, Bilgili SG, Karadag AS, Tombul T. Sneddon syndrome associated with Protein S deficiency. Indian J Dermatol Venereol Leprol. 2012;78:407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Bersano A, Morbin M, Ciceri E, Bedini G, Berlit P, Herold M, Saccucci S, Fugnanesi V, Nordmeyer H, Faragò G, Savoiardo M, Taroni F, Carriero M, Boncoraglio Giorgio B, Perucca L, Caputi L, Parati Eugenio A, Kraemer M. The diagnostic challenge of Divry van Bogaert and Sneddon Syndrome: Report of three cases and literature review. J Neurol Sci. 2016;364:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Samanta D, Cobb S, Arya K. Sneddon Syndrome: A Comprehensive Overview. J Stroke Cerebrovasc Dis. 2019;28:2098-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Assan F, de Zuttere D, Bottin L, Tavolaro S, Courvoisier DS, Barbaud A, Alamowitch S, Francès C, Chasset F. Echocardiographic features in antiphospholipid-negative Sneddon's syndrome and potential association with severity of neurological symptoms or recurrence of strokes: a longitudinal cohort study. Eur Heart J Cardiovasc Imaging. 2021;22:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Collantes-Rodríguez C, Jiménez-Gallo D, Arjona-Aguilera C, Ossorio-García L, Linares-Barrios M. Treatment of Skin Ulcers Secondary to Sneddon Syndrome With Alprostadil (Prostaglandin E1). JAMA Dermatol. 2016;152:726-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Ting S, Kelly RI. Three cases of Sneddon syndrome: A comparison with lymphocytic thrombophilic arteritis. Australas J Dermatol. 2021;62:e272-e275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Francès C, Papo T, Wechsler B, Laporte JL, Biousse V, Piette JC. Sneddon syndrome with or without antiphospholipid antibodies. A comparative study in 46 patients. Medicine (Baltimore). 1999;78:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Yao M, Zhao J, Jiang N, Li L, Ni J. Superficial Siderosis and Microbleed Restricted in Cortex Might Be Correlated to Atrophy and Cognitive Decline in Sneddon's Syndrome. Front Neurol. 2020;11:1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 12. | Yilmaz E, Arsava EM, Gocmen R, Oguz KK, Arat A, Topcuoglu MA. Characteristic imaging features of neurovascular involvement in primary Sneddon's syndrome: an analysis of 12 cases. Neurol Sci. 2021;42:2363-2369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Dominguez F, Pieske B, Kelle S. Cardiac manifestations of Sneddon's syndrome. Int J Cardiol. 2015;190:275-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Einsiedler S, Hödl G, Topakian R. Cerebral venous and sinus thrombosis with complicating thromboangiitis obliterans. CMAJ. 2021;193:E311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Abdalkader M, Shaikh SP, Siegler JE, Cervantes-Arslanian AM, Tiu C, Radu RA, Tiu VE, Jillella DV, Mansour OY, Vera V, Chamorro Á, Blasco J, López A, Farooqui M, Thau L, Smith A, Gutierrez SO, Nguyen TN, Jovin TG. Cerebral Venous Sinus Thrombosis in COVID-19 Patients: A Multicenter Study and Review of Literature. J Stroke Cerebrovasc Dis. 2021;30:105733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Ferro JM, Aguiar de Sousa D. Cerebral Venous Thrombosis: an Update. Curr Neurol Neurosci Rep. 2019;19:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 17. | Kallel N, Saidani A, Kotti A, Moussa N, Maddeh S, Gargouri R, Msaad S, Feki W. Coronavirus disease 19 (COVID-19) and Cerebral venous sinus thrombosis (CVST): A case series and review of the literature. Clin Case Rep. 2022;10:e6143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Aladdin Y, Hamadeh M, Butcher K. The Sneddon syndrome. Arch Neurol. 2008;65:834-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Kong SS, Azarfar A, Bhanusali N. Sneddon syndrome: under diagnosed disease, complex clinical manifestations and challenging diagnosis. A case-based review. Rheumatol Int. 2021;41:987-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |