Published online Nov 6, 2023. doi: 10.12998/wjcc.v11.i31.7647
Peer-review started: August 11, 2023
First decision: September 26, 2023
Revised: October 15, 2023
Accepted: October 25, 2023
Article in press: October 25, 2023
Published online: November 6, 2023
Processing time: 87 Days and 3.4 Hours
Premature ovarian failure (POF) is the end-stage of a decline in ovarian function prior to the age of 40 years that involves symptoms associated with low estradiol (E2) levels and a minimal probability of pregnancy. This increases the physical and psychological burden experienced by young women of reproductive age, particularly with regards to over-diagnosis.
Here, we report three cases (29, 22, and 33 years-of-age) diagnosed with POF after experiencing secondary amenorrhea for more than one year, serum levels of follicle-stimulating hormone (FSH) > 40 IU/L on two occasions with an interval of more than 4 wk, and negative progesterone withdrawal tests. All three patients were intermittently administered with drugs to create an artificial cycle. During the subsequent discontinuation period, the patients experienced intermittent follicular growth and spontaneous ovulation. One patient experienced two natural pregnancies (both with embryo arrest).
Our findings suggest that young patients with POF can experience unpredictable and intermittent spontaneous follicular development, ovulation, and even natural pregnancy. Clinicians should provide appropriate medical guidance and indi
Core Tip: Three cases (29, 22, and 33 years of age) diagnosed with premature ovarian failure after experiencing secondary amenorrhea for more than one year, serum follicle-stimulating hormone levels > 40 IU/L. During the discontinuation period of artificial cycle, three patients experienced unpredictable and intermittent spontaneous follicle development, ovulation, and even natural pregnancy.
- Citation: Zhang WY, Wang HB, Deng CY. Intermittent spontaneous ovulation in patients with premature ovarian failure: Three case reports and review of literature. World J Clin Cases 2023; 11(31): 7647-7655
- URL: https://www.wjgnet.com/2307-8960/full/v11/i31/7647.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i31.7647
Premature ovarian failure (POF) refers to ovarian failure prior to the age of 40 years and represents a heterogeneous gynecological and endocrine disease with multiple causes. The clinical manifestations of POF are amenorrhea, high levels of gonadotropin (Gn), and symptoms associated with low estradiol (E2) levels, including hot flushes, sweating, vaginal dryness, and infertility[1]. The global incidence of POF is 1%–5% and affects approximately 2 million women of reproductive age[2]. The known causes of POF include genetic, immune, infection, metabolic, environmental, and psy
In this article, we summarize the diagnosis and treatment of three cases of POF experiencing intermittent spontaneous ovulation who were admitted to Peking Union Medical College Hospital (PUMCH) between December 2012 and July 2022. We also review the relevant literature and discuss the characteristics, possible causes, diagnosis, and treatment options for each case.
Secondary amenorrhea for more than one year.
All three patients were experiencing secondary amenorrhea for more than one year and intermittently administered with menopause hormone therapy (MHT) to create artificial cycles.
They had no previous history of mumps, surgery, or chemoradiotherapy, and no special family history.
All three patients were delivered at full term from their mother. The pregnancies was uneventful with no history of special medication. They showed no differences when compared to their peers with regards to growth and intelligence. Menarches occurred at normal age followed by regular/irregular menstruation.
All three patients had negative vulvas, small uterines, negative bilateral adnexal areas and V level breasts.
All three cases experienced serum levels of FSH > 40 IU/L on two occasions with an interval of more than 4 wk, and negative progesterone withdrawal tests. Their thyroid functions were negative and chromosomal statuses were 46, XX with negative fragile X syndrome tests.
Pelvic ultrasound showed that the uterus was small, and no follicles were detected in the ovaries.
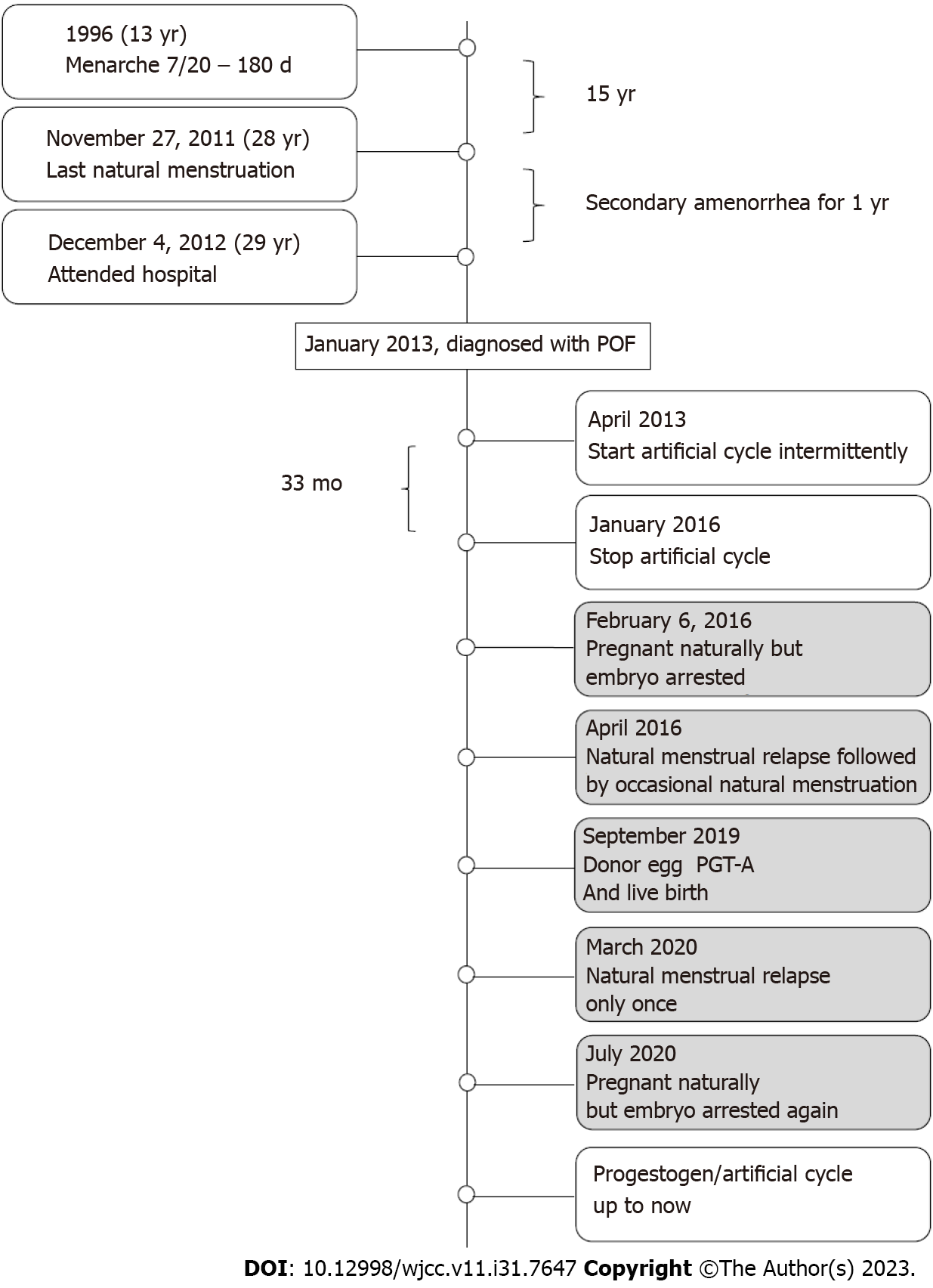
This patient was delivered by cesarean section at full term from her mother’s first pregnancy in 1983. The pregnancy was uneventful with no history of special medication. The patient showed no differences when compared to her peers with regards to growth and intelligence. Menarche occurred at 13 years-of-age followed by irregular menstruation (7/20–180 d). The patient married in 2010 (at 27 years-of-age) and did not use birth control after marriage. The patient experienced normal menstruation on the November 27, 2011 followed by amenorrhea. On the December 4, 2012 (at 29 years-of-age), she attended PUMCH with the chief complaint of secondary amenorrhea for one year. FSH was 110 IU/L, E2 was < 15 pg/mL, and thyroid function was negative. After 41 d, we re-analyzed hormonal activity: FSH was 120 IU/L, and E2 was < 15 pg/mL. Chromosomal status was 46, XX. Pelvic ultrasound showed that the uterus was normal, and no follicles were detected in the ovaries. There was no previous history of mumps, surgery, or chemoradiotherapy, and no special family history. Following a negative progesterone withdrawal test, the patient was diagnosed with POF and administered with medicine (Climen) to generate an intermittent artificial cycle. She stopped taking the drug after menstruation on the January 6, 2016. After having sex only once on the January 21, 2016, examinations revealed that the level of human chorionic gonadotropin was 884 IU/L on the February 6, 2016 due to breast swelling and pain. However, embryo arrest occurred at 9 wk. Uterine evacuation was performed and chorionic villus sampling revealed a chro
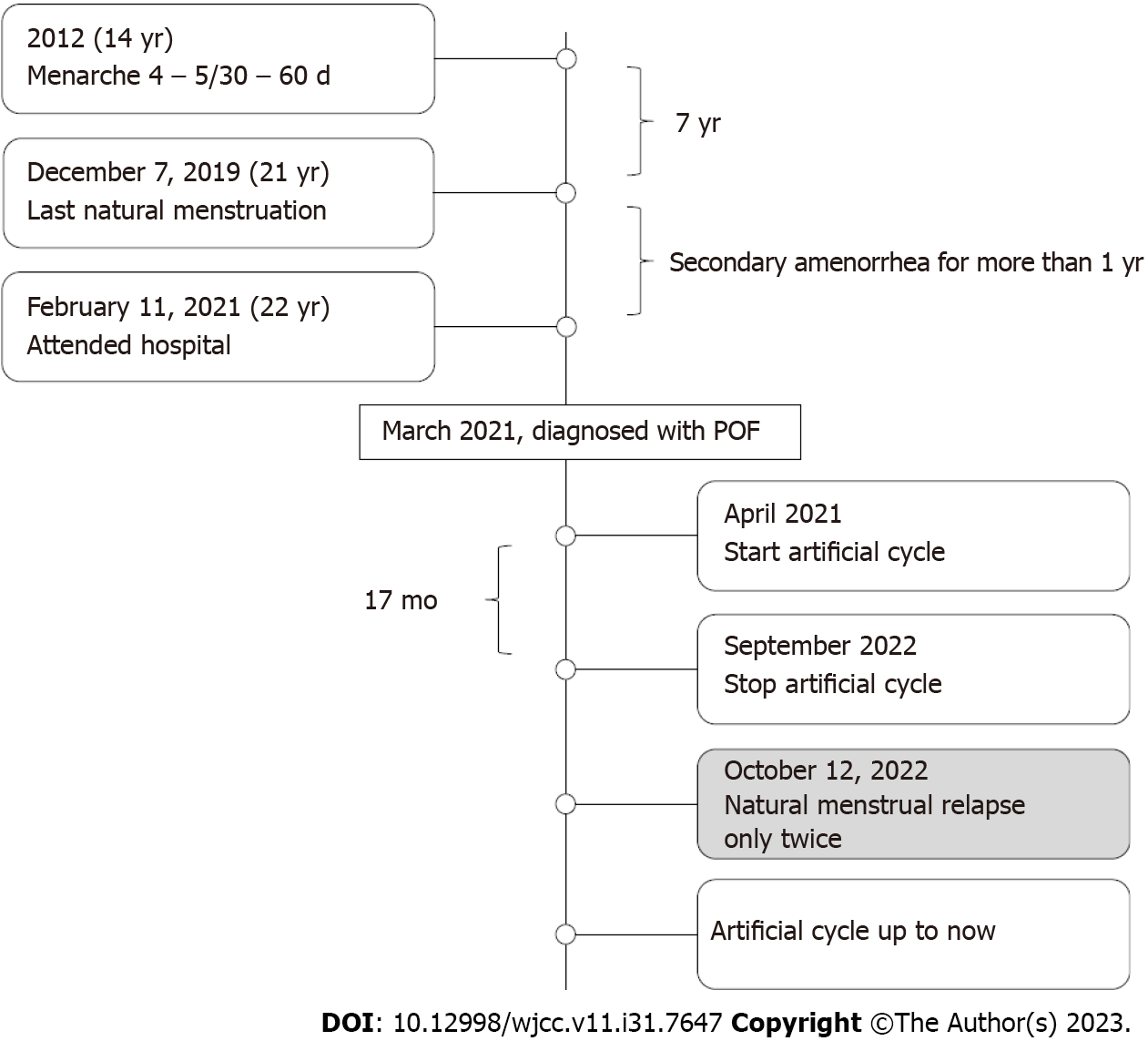
This patient was born from her mother’s first pregnancy. The pregnancy was uneventful with no history of special medication; delivery occurred spontaneously in 1998. Growth and intelligence did not differ significantly from her peers. Menarche occurred at 14 years-of-age (4–5/30–60 d; medium volume). On the December 7, 2019, she underwent normal menstruation but then experienced postmenstrual amenorrhea (at 21 years-of-age). She was unmarried and had no sex. She attended PUMCH with the chief complaint of secondary amenorrhea for more than one year on the February 11, 2021; FSH was 54.75 IU/L, LH was 5.32 IU/L, E2 was 26.01 pg/mL, progesterone (P) was 0.3 ng/mL, testosterone (T) was 0.71 ng/mL, prolactin (PRL) was 10.3 ng/mL, and AMH was 0.03 ng/mL. After 44 d, we re-analyzed hormonal activity: FSH was 72.30 IU/L, LH was 39.58 IU/L, E2 was 26.45 pg/mL, P was 0.42 ng/mL, and T was 0.45 ng/mL; thyroid function was negative. Ultrasound showed that the uterus and both ovaries were small. Chromosomal status was 46, XX and the fragile X syndrome test was normal. The patient denied hot flushes and sweating. She had no previous history of mumps, surgery, radiotherapy, chemotherapy, and no special family history. After a negative progesterone withdrawal test, the patient was diagnosed with POF. Following a MHT safety review, she began an artificial cycle [Femoston (2/10)] in April 2021. During the period of drug administration, she experienced regular menstrual cycles (4–5/28–30 d) without discomfort. On the September 19, 2022, the patient stopped taking the drug by herself. On the October 12, 2022, she experienced natural menstruation, lasting for 4 d, and a hormonal review showed that FSH was 20.70 IU/L, LH was 4.34 IU/L, E2 was < 15 pg/mL, P was 0.81 ng/mL and T was 0.47 ng/mL. Natural menstruation was experienced on the November 21, 2022 followed by repeat episode of amenorrhea. After more than 2 mo, she recommenced the artificial cycle; the patient remains on this course of treatment (Figure 2).
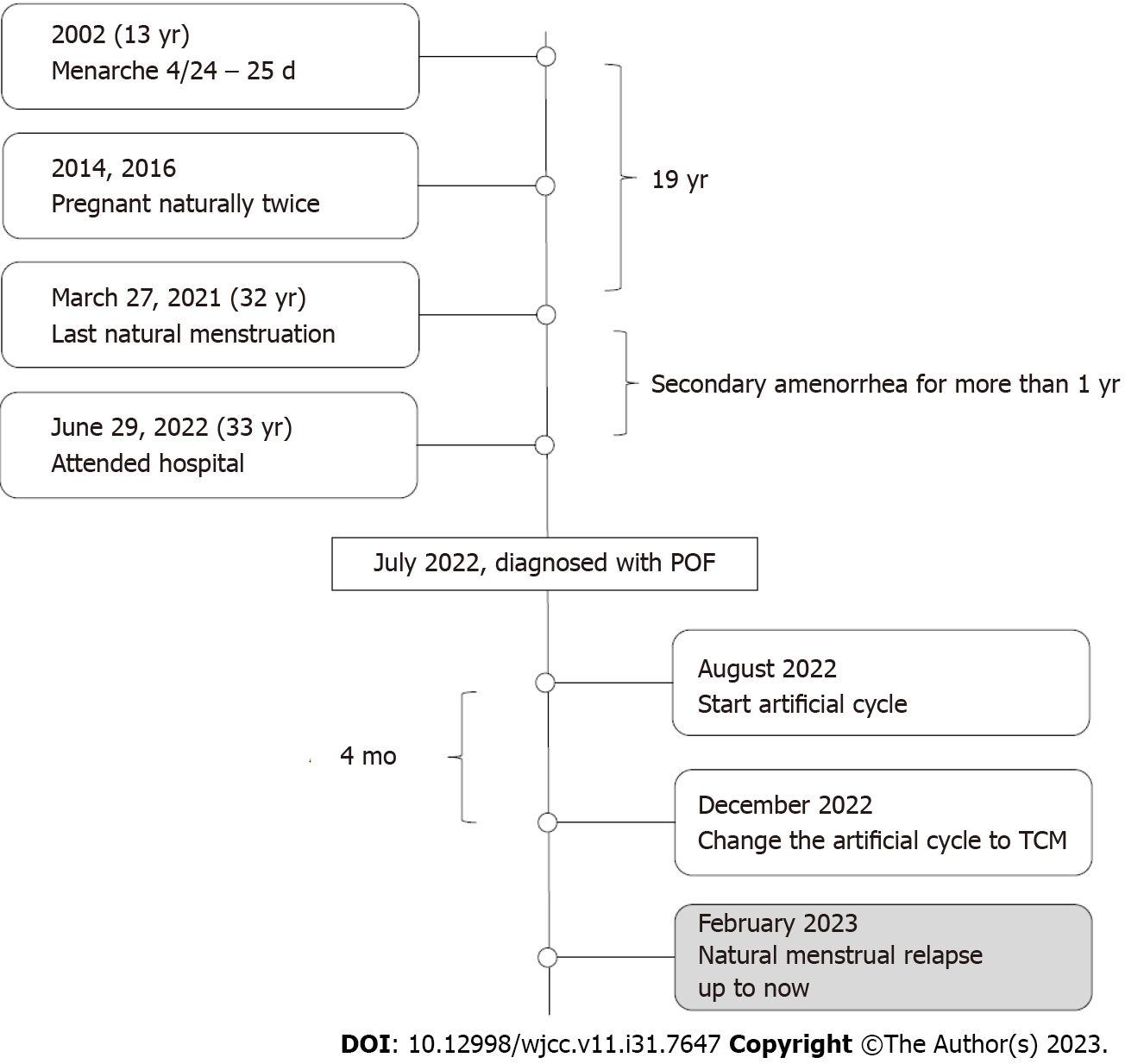
This patient was born from her mother's second pregnancy. Her mother had an uneventful pregnancy with no history of special medication and delivered spontaneously in 1989. Growth and intelligence are no different from peers. Menarche occurred at 13 years-of-age (4/24 – 25 d, medium volume). At 24 years-of-age (2013), she was married and experienced a natural pregnancy in 2014. However, she experienced embryonic arrest after 2 mo of pregnancy and underwent uterine evacuation without chromosomal analysis. In 2016, she became pregnant naturally, and underwent a second-term induction of labor in the fifth month of pregnancy due to social factors (G2P0). Subsequently, she was divorced. On the May 27, 2021, she experienced normal menstruation followed by amenorrhea. On the June 29, 2022 (33 years-of-age), she attended PUMCH with the chief complaint of secondary amenorrhea for more than one year, accompanied by hot flushes and sweating. Hormonal status was as follows: FSH was 44.90 IU/L, LH was 20.65 IU/L, E2 was 8.15 pg/mL, P was 0.51 ng/mL, T was 0.37 ng/mL, PRL was 18.92 ng/mL, and AMH was 0.16 ng/mL. On the July 31, 2022, FSH was 162.47 IU/L, LH was 106.80 IU/L, E2 was < 15 pg/mL, P was 0.62 ng/mL, and T was 0.13 ng/mL; thyroid function was negative. The patient’s chromosomal status was 46, XX. The fragile X syndrome test was normal. Transvaginal ultrasound showed that the uterus and both ovaries were small. The patient had no history of mumps, surgery, radiotherapy, or chemotherapy, no special family history, and there were no abnormalities of menstruation and childbirth with regards to the patient’s mother and sister. The patient was subsequently diagnosed with POF. Following a MHT safety inspection, carried out in August 2022, the patient began an artificial cycle [Femoston (2/10)]. After one month of medication, the symptoms of hot flushes and sweating were significantly relieved. In December 2022, the patient changed the artificial cycle to traditional Chinese medicine (TCM) by herself. From February 2023, the patient experienced natural regular menstruations (3–4/28–30 d) without hot flushes and sweating (Figure 3); the patient’s status remains the same.
Premature ovarian failure.
Following MHT safety reviews, they all began artificial cycles (Climen/Femoston).
During the subsequent discontinuation of MHT, they all experienced intermittent follicular growth and spontaneous ovulation, and one patient experienced two natural pregnancies (both with embryo arrest).
In the present study, we describe three patients who were diagnosed with POF following negative progesterone withdrawal tests at the ages of 29, 22, and 33 years due to secondary amenorrhea for more than 1 year, and serum FSH levels > 40 IU/L on two occasions with an interval of more than 4 wk.
Human follicles begin to develop and reach a maximal number during the fetal period. Subsequently, the number of follicles gradually decreases; there are 6–7 million follicles in the ovaries at 20–28 wk-of-gestation, up to 3 million at birth, and 300000–400000 at puberty[7]. Follicular development is divided into two stages: initial recruitment and cyclic recruitment. Initial recruitment is the process of transition from the primitive follicle pool to a primary follicle until the germ stem cells and primordial follicles are fully depleted; this process is independent of Gn. During the monthly menstrual cycle after puberty, the follicles that have initiated recruitment respond to the periodic changes in Gn and then enter cyclic recruitment[8]. Therefore, the duration of natural menstruation (the period during which the ovaries fulfill their physiological role) is not only related to the original follicle pool reserve, but also depends on the original follicular recruitment rate and the rate of follicular atresia, including accelerated follicular atresia, dominant follicle recruitment abnormalities[9] and follicular maturity disorders[10,11], which may lead to POF.
Globally, the average age at which natural menopause occurs is 48.8 years[12]; approximately 90% of women aged 45–55 years are in menopause. Menopause that occurs between 40 and 45 years-of-age is referred to as early menopause. Ovarian failure before the age of 40 years is referred to as POF and involves a natural state or pathological state that results in the acceleration of follicle utilization. When the number of original follicles is lower than a certain threshold, the natural state no longer activates development or results in extremely slow development; even high levels of FSH are unable to induce the recruitment of follicles.
In 1967, Moraes-Ruehsen and Jones[13] were the first to define POF as an abnormal physiological menopause after puberty and before the age of 40 years. Subsequent research has shown that ovarian failure is a gradual process. In 2006, the American Society for Reproductive Medicine first proposed the term primary ovarian insufficiency (POI)[14]. In 2016, the European Society of Human Reproduction and Embryology (ESHRE)[15] and the International Menopause Society (IMS)[16] changed this definition to POI and clarified the difference between POI and POF by dividing ovarian failure into three stages: The occult phase, the biochemical abnormality phase, and the clinical abnormality phase. Further division included diminished ovarian reserve (DOR), POI, and POF, according to the levels of FSH. The diagnostic criteria for POF are as follows[1]: Amenorrhea for more than 1 year before the age of 40 years[2]; FSH > 40 IU/L on two occasions with an interval of more than 4 wk, and[3] decreasing E2 levels[17]. Significantly, some patients only have a history of amenorrhea that is less than one year when they first attend hospital; some of these patients commence artificial cycles at once. Therefore, it is difficult to distinguish POI from POF.
In addition, POF needs to be differentiated from resistant ovary syndrome (ROS). Patients with ROS present with amenorrhea, high levels of gonadotropin, and low levels of E2; these conditions are often accompanied by infertility. These symptoms are similar to those of POF. However, ROS ovaries contain a large number of primitive follicles, and multiple small follicles can be detected in the ovaries under ultrasound. The pathogenesis of ROS may be related to Gn receptor deficiency, the presence of antibodies that affect receptor activity, structural abnormalities of Gn molecules, or thymic lesions[18].
Although POF is the terminal stage of POI, ovarian function is not necessarily completely lost. The pathophysiology of POF differs from that of menopause in terms of average age. The follicles in the ovaries of menopausal women at a normal menopause age are almost completely exhausted; this condition is permanent. Women with POF are young, and some patients may have a certain number of follicles in a resting state in their ovaries that may be activated under certain conditions[19].
There are two pathological types of POF[1,20]. Type I (with no follicles) involves the complete depletion of follicles due to the absence or inability of germinal cells to develop. This condition is often secondary to disorders of sex development[2]. Type II (with follicles) involves the presence of follicles in the ovaries in a resting state. The younger the patient is, the greater the possibility of being induced or spontaneously restoring follicle development. Type II POF follicles may gradually deplete and develop into type I follicles. The three patients described in this study were all young (29, 22, and 33 years of age) and used artificial cycles for several months. Natural menstruations were experienced after stopping the drug, thus indicating intermittent follicle recovery and development. We hypothesize that all three cases were type II POF, and that follicles in the resting state intermittently resumed growth when driven by high levels of FSH.
In addition to this, natural follicle development in women with POF may also be associated with MHT. Previous research has confirmed that the proliferation and differentiation of granulosa cells (GCs) depend on the levels of FSH, LH, PRL, and their membrane receptors. However, when GCs are exposed to a high Gn state for an extended period of time, the number and sensitivity of their receptors decreases[13,14], thus causing low Gn sensitivity in the residual follicles in the ovarian tissue of patients with POF. In MHT, the levels of E2 is independent of the number, distribution, and affinity of FSH receptors on GCs, but can promote the formation of FSH receptors and improve the sensitivity of FSH receptors on GCs by reducing the levels of FSH, thereby promoting follicle development and maturation. Moreover, levels of LH in the serum of patients with POF continues to rise, thus hindering spontaneous ovulation and the premature luteinization of follicles[21]. MHT can improve the ovulation rate by reducing the levels of LH and by reducing the inappropriate luteinization of follicles. Collectively, these factors make it possible for a very small number of patients with POF to undergo natural menstrual relapse.
The existing literature only features a small body of research relating to spontaneous ovulation and natural pregnancy in POF patients. There are several factors that might be related to this lack of research. For example, a non-standardized diagnosis of POF, such as a duration of amenorrhea for less than 1 year, an inappropriate number of FSH tests, or variable diagnostic thresholds[22-24]. Another factor could be the small number of POI and POF cases in which ovulation and pregnancy are under medical intervention, such as the MHT process[25,26], ovulation induction[27], controlled ovarian stimulation[28], donor egg in vitro fertilization and embryo transfer[29], in vitro activation[30], or ovarian tissue cryopreservation and auto-transplantation[31].
In this study, all three POF patients intermittently resumed spontaneous ovulation when they stopped MHT treatment. One of the patients experienced two spontaneous pregnancies. These data suggest that clinicians should not ignore the intermittent and unpredictable restoration of follicle development in POF patients, and remember that there is still a possibility of unintended pregnancy during intermittent MHT treatment. If there is no reproductive plan, contraception should be recommended. Furthermore, if there are clear genetic factors (e.g., Turner’s syndrome, fragile X syndrome, and pseudohypoparathyroidism), patients should be fully informed of the risks of pregnancy, ovarian dysfunction in the offspring, and other disorders.
At present, there is no effective means of restoring POF ovarian function. Therefore, women with POF should be detected as early as possible so that they can be diagnosed and treated early to alleviate low E2 symptoms, reduce long-term risks and protect residual fertility.
In terms of hormone supplementation, primary POF manifests as undeveloped or delayed female secondary sex characteristics. Secondary POF can cause hot flushes, sweating, osteoporosis, mood swings, cognitive decline, cardiovascular and cerebrovascular symptoms, dryness of the genital tract and dyspareunia[27] which cause adverse effects on the physical and mental health of patients. MHT can effectively alleviate the low levels of E2 and long-term risk of POF patients and improve a patient’s quality-of-life. However, MHT has a small risk of breast cancer and thrombosis. Thus, annual safety checks are required before and during medication. In the present study, one of our three patients experienced hot flushes and sweating symptoms (3 times/d). After safety examination, MHT was given, and the symptoms were significantly relieved after 1 mo of medication.
In terms of fertility, methods for POF patients include the transplantation of previously frozen ovarian tissue, oocyte activation, stem cell therapy, dehydroepiandrosterone, growth hormone, antioxidants, immunosuppressants and egg donation. However, there is no effective treatment to reverse ovarian function.
In addition, TCM has a certain effect on the treatment of POF in younger patients. TCM considers that female reproduction is regulated by the brain-kidney-chong ren-uterine axis which is similar to the hypothalamic-pituitary-ovarian axis in modern medicine. TCM could be used to supplement the kidney, replenish vital energy, and activate the blood[32]. In the present study, one of our POF patients experienced natural menstruation after changing artificial cycle to TCM. This patient has maintained regular natural menstruation thus far, therefore indicating that TCM may be beneficial to younger patients with POF, although multicenter, large-sample, and randomized controlled studies are still needed in future.
POF patients experience several symptoms, including amenorrhea, high levels of Gn, low E2 levels and infertility. Data from the three patients described herein suggest that clinicians should adhere strictly to the diagnostic criteria for POF patients (< 40 years-of-age, amenorrhea for more than 1 year and serum FSH > 40 IU/L on two occasions with an interval > 4 wk), reduce the psychological pressure caused by over-diagnosis among young women, and inform women with POF that there is an intermittent and unpredictable possibility of resuming ovulation. Clinicians should also provide appropriate medical guidance according to whether there are fertility requirements and genetic risks. Furthermore, clinicians should commence individualized treatments as soon as possible, including hormone supplementation and fertility preservation.
The authors would like to thank the three cases for supporting our research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Reproductive biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Menopause subgroup; Chinese society of obstetrics and gynecology; Chinese Medical Association. Menopause subgroup, Chinese society of obstetrics and gynecology, Chinese Medical Association. Menopause subgroup, Chinese society of obstetrics and gynecology, Chinese Medical Association. Chinese guideline on menopause management and menopause hormone therapy. Zhonghua Fu Chan Ke Za Zhi. 2018;53:729. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 2. | Zhang T, He M, Zhang J, Tong Y, Chen T, Wang C, Pan W, Xiao Z. Mechanisms of primordial follicle activation and new pregnancy opportunity for premature ovarian failure patients. Front Physiol. 2023;14:1113684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 3. | Yu L, Qing X. Diagnosis of Idiopathic Premature Ovarian Failure by Color Doppler Ultrasound under the Intelligent Segmentation Algorithm. Comput Math Methods Med. 2022;2022:2645607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Bai X, Wang S. Signaling pathway intervention in premature ovarian failure. Front Med (Lausanne). 2022;9:999440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 5. | Prior JC, Naess M, Langhammer A, Forsmo S. Ovulation Prevalence in Women with Spontaneous Normal-Length Menstrual Cycles - A Population-Based Cohort from HUNT3, Norway. PLoS One. 2015;10:e0134473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Calik-Ksepka A, Grymowicz M, Bronkiewicz W, Urban A, Mierzejewski K, Rudnicka E, Smolarczyk R. Spontaneous pregnancy in a patient with premature ovarian insufficiency - case report. Prz Menopauzalny. 2018;17:139-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Long C, Benny P, Yap J, Lee J, Huang Z. A systematic review of genetics and reproductive health outcomes: Asian perspective. Reprod Sci. 2023;s43032. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 8. | Gong X, Zhang Y, Ai J, Li K. Application of Single-Cell RNA Sequencing in Ovarian Development. Biomolecules. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Wu X, Cai H, Kallianpur A, Li H, Yang G, Gao J, Xiang YB, Ji BT, Yu-Tang, Zheng W, Shu XO. Impact of premature ovarian failure on mortality and morbidity among Chinese women. PLoS One. 2014;9:e89597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Mazziotta C, Pellielo G, Tognon M, Martini F, Rotondo JC. Significantly Low Levels of IgG Antibodies Against Oncogenic Merkel Cell Polyomavirus in Sera From Females Affected by Spontaneous Abortion. Front Microbiol. 2021;12:789991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Usman SF, Shuaibu IR, Durojaiye K, Medugu N, Iregbu KC. The presence of microorganisms in follicular fluid and its effect on the outcome of in vitro fertilization-embryo transfer (IVF-ET) treatment cycles. PLoS One. 2021;16:e0246644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Menopause subgroup; Chinese society of obstetrics and gynecology; Chinese Medical Association. Chinese guideline on menopause management and menopause hormone therapy. Zhonghua Fu Chan Ke Za Zhi. 2023;1:4. [DOI] [Full Text] |
| 13. | de Moraes-Ruehsen M, Jones GS. Premature ovarian failure. Fertil Steril. 1967;18:440-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 104] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2006;86:S148-S155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | European Society for Human Reproduction and Embryology (ESHRE) Guideline Group on POI; Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, Cifkova R, de Muinck Keizer-Schrama S, Hogervorst E, Janse F, Liao L, Vlaisavljevic V, Zillikens C, Vermeulen N. ESHRE Guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31:926-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 576] [Cited by in RCA: 612] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 16. | Baber RJ, Panay N, Fenton A; IMS Writing Group. 2016 IMS Recommendations on women's midlife health and menopause hormone therapy. Climacteric. 2016;19:109-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 526] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 17. | Pastore LM, Christianson MS, Stelling J, Kearns WG, Segars JH. Reproductive ovarian testing and the alphabet soup of diagnoses: DOR, POI, POF, POR, and FOR. J Assist Reprod Genet. 2018;35:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Tsirigotis M, Craft IL. Benign thymoma and resistant ovary syndrome. Br J Obstet Gynaecol. 1994;101:350-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 19. | Gu Y, Xu Y. Successful Spontaneous Pregnancy and Live Birth in a Woman With Premature Ovarian Insufficiency and 10 Years of Amenorrhea: A Case Report. Front Med (Lausanne). 2020;7:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Jankowska K. Premature ovarian failure. Prz Menopauzalny. 2017;16:51-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Sullivan SD, Sarrel PM, Nelson LM. Hormone replacement therapy in young women with primary ovarian insufficiency and early menopause. Fertil Steril. 2016;106:1588-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 280] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 22. | Alper MM, Jolly EE, Garner PR. Pregnancies after premature ovarian failure. Obstet Gynecol. 1986;67:59S-62S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Wright CS, Jacobs HS. Spontaneous pregnancy in a patient with hypergonadotrophic ovarian failure. Br J Obstet Gynaecol. 1979;86:389-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Jeppsson S, Ljungberg O, Rannevik G. Hypergonadotrophic hypogonadism with preserved fertility--a new syndrome? Acta Endocrinol (Copenh). 1980;95:388-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Anna Liza R, Alik RZ, Ahmad Murad Z, Ghazali I. Spontaneous twin pregnancy in premature ovarian failure. Med J Malaysia. 2008;63:263-264. [PubMed] |
| 26. | Vandborg M, Lauszus FF. Premature ovarian failure and pregnancy. Arch Gynecol Obstet. 2006;273:387-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Yang Y, Ma XL, Zhang XH. Successful pregnancy with tripterygium glycoside-induced premature ovarian insufficiency: a case report. J Int Med Res. 2019;47:2274-2279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Zouboulis CC, Achenbach A, Makrantonaki E. Acne tarda and male-pattern baldness unmasking primary ovarian insufficiency: a case and review. Dermatology. 2014;229:51-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Egbe TO, Wafo CY, Bollo BB, Pany C, Onomo MJ, Sandjon G. Successful pregnancy with donor eggs in-vitro fertilization after premature ovarian insufficiency in a tertiary hospital in a low-income setting: a case report. Fertil Res Pract. 2016;2:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 325] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 31. | Revelli A, Marchino G, Dolfin E, Molinari E, Delle Piane L, Salvagno F, Benedetto C. Live birth after orthotopic grafting of autologous cryopreserved ovarian tissue and spontaneous conception in Italy. Fertil Steril. 2013;99:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Ma K, Li JN, Fan XD, Zhang H, Ma LN. [Mechanism of tonifying kidney and activating blood therapy for premature ovarian failure:a review]. Zhongguo Zhong Yao Za Zhi. 2023;48:1808-1814. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |