Published online Oct 26, 2023. doi: 10.12998/wjcc.v11.i30.7469
Peer-review started: August 3, 2023
First decision: September 4, 2023
Revised: September 13, 2023
Accepted: September 28, 2023
Article in press: September 28, 2023
Published online: October 26, 2023
Processing time: 83 Days and 3.5 Hours
Dexmedetomidine (DMED) is frequently used as a sedative in several medical fields. The benefits of DMED include enhanced quality of regional anesthesia, prolonged analgesia, and postoperative opioid-sparing when administered intra
We present two cases of hemodynamic instability that occurred following the initial loading of DMED under supraclavicular BPB. A healthy 29-year-old man without any medical history showed profound bradycardia after receiving a loading dose of DMED 0.9 μg/kg for 9 min. DMED administration was promptly stopped, and after receiving a second dose of atropine, the heart rate recovered. A 62-year-old woman with a history of cardiomyopathy became hypotensive abrup
DMED administration following BPB could trigger hemodynamic instability in patients with decreased cardiac function as well as in healthy individuals.
Core Tip: Although severe, there are few reports of complications with dexmedetomidine (DMED) administration following brachial plexus block. Profound bradycardia can occur even in healthy individuals with DMED administration following brachial plexus block. It can trigger refractory hypotension without apparent bradycardia in patients with decreased cardiac function. Therefore, clinicians must be aware of these potential yet critical consequences while selecting sedatives.
- Citation: Kim YS, Lee C, Oh J, Nam S, Doo AR. Hemodynamic instability following intravenous dexmedetomidine infusion for sedation under brachial plexus block: Two case reports. World J Clin Cases 2023; 11(30): 7469-7474
- URL: https://www.wjgnet.com/2307-8960/full/v11/i30/7469.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v11.i30.7469
Dexmedetomidine (DMED) is one of the most frequently used sedatives in several medical fields, such as balanced general anesthesia, sedation during regional anesthesia, and as part of intensive care unit sedation. Cooperative sedation without respiratory depression, a unique sedative response of DMED, has shown superior safety compared to other sedatives, such as barbiturate, benzodiazepine and propofol[1]. DMED also exhibits sympatholytic, amnesic, and anal
The cardiovascular side effects of DMED, a dose-dependent transient increase in blood pressure (BP) followed by hypotension and bradycardia due to activation of the peripheral α2-adrenergic receptor, are well understood[4]. Severe hemodynamic complications, such as profound bradycardia or asystole, might occur following DMED administration in critically ill patients[5] or overdose of the drug[6]; however, these complications have not been reported in the clinical setting with the conventional regimen of DMED under brachial plexus block (BPB). In this case series, we report two cases of profound bradycardia and refractory hypotension following the administration of the initial loading dose of DMED under BPB for orthopedic upper-extremity surgery.
Case 1: A healthy 29-year-old man (height, 182 cm; weight, 73 kg) was scheduled to undergo BPB for diagnostic arthroscopic triangular fibrocartilage complex repair surgery due to persistent right wrist pain.
Case 2: A 62-year-old woman (height, 159 cm; weight, 56.4 kg) was scheduled to receive supraclavicular BPB for flap coverage of a necrotizing soft tissue infection in the right elbow.
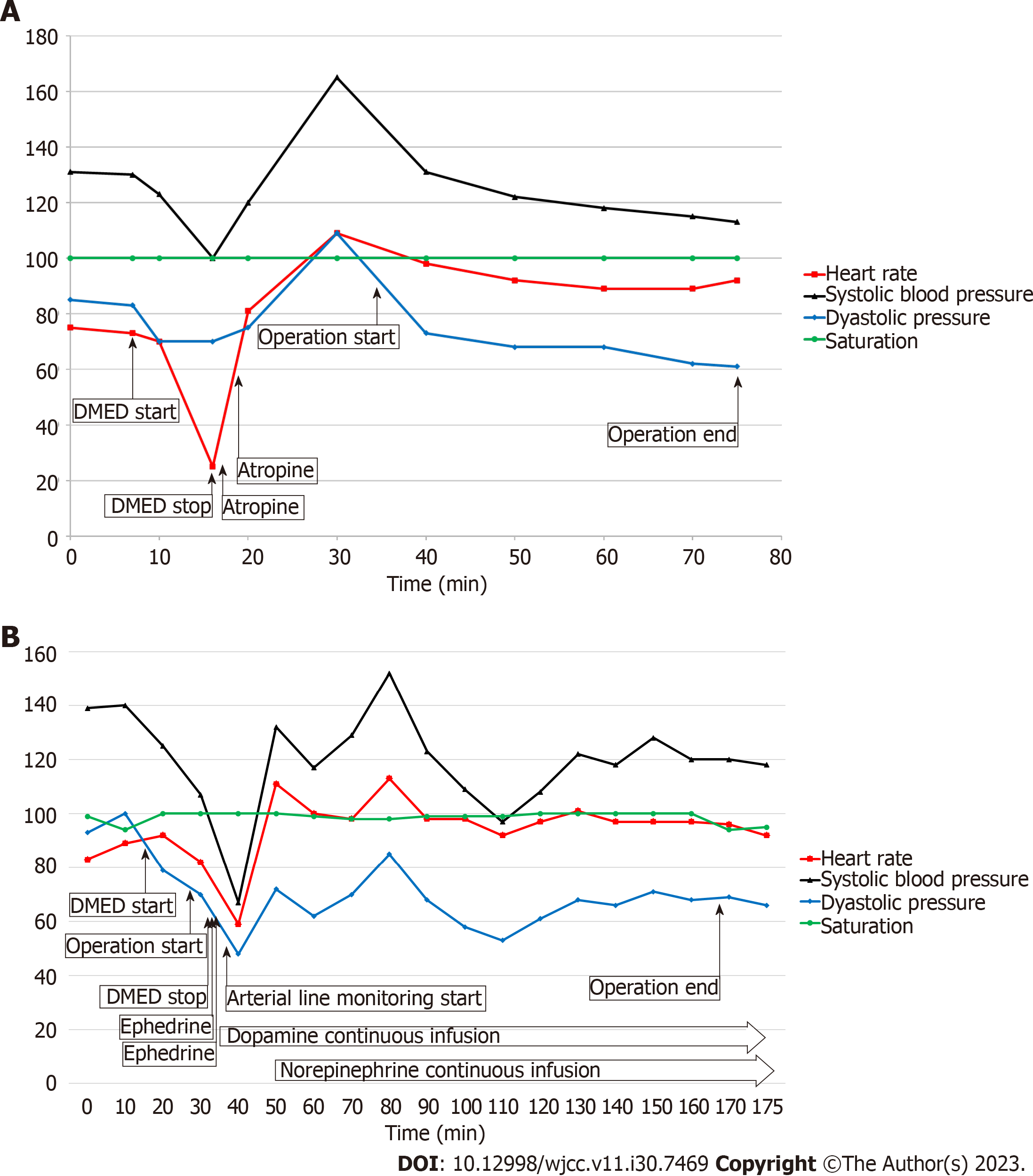
Case 1: Ultrasound-guided supraclavicular BPB was performed on the right supraclavicular fossa without premedication. A 26-gauge 5-cm block needle was advanced toward the brachial plexus lateral to medial direction using real-time ultrasonography after identifying the brachial plexus and adjacent small vessels using color Doppler imaging. We injected 32 mL of 1.5% lidocaine with 5 μg/mL epinephrine. Half the volume (16 mL) entered the main neural cluster, and the remaining half entered the satellite neural clusters via the previously introduced targeted intracluster injection technique. Negative blood aspirations were performed repeatedly with every 5 mL of injection. DMED infusion was initiated to achieve sedation after confirming successful motor and sensory blockade. An initial loading dose of 1 μg/kg (73 μg) was planned at a rate of 6 μg/kg/h. The heart rate (HR) abruptly decreased from 75 to 25 beats per minute (bpm) when approximately 0.9 μg/kg of DMED was administered (9 min after initiation).
Case 2: Supraclavicular BPB was performed using the same technique as in case 1. After confirming successful motor and sensory blockade, half of the recommended loading dose of DMED (0.5 μg/kg) was administered over 10 min at a rate of 3 μg/kg/h due to the underlying heart dysfunction. Immediately after receiving the loading dose, the HR decreased to 59 bpm, and BP abruptly dropped to 67/48 mmHg. The patient experienced nausea and vomiting but showed no signs of local anesthetic systemic toxicity, such as dizziness, tinnitus, or perioral numbness.
Case 1: The patient had no history of allergies or cardiovascular disease. The patient had undergone uncomplicated general anesthesia twice previously, for jaw surgery and then septorhinoplasty.
Case 2: The patient had undergone breast cancer surgery under general anesthesia five years previously, in addition to receiving adjuvant concurrent chemotherapy and radiotherapy. The patient had been diagnosed with stress-induced cardiomyopathy, of which echocardiography revealed severe left ventricular systolic dysfunction (with an ejection fraction of 22%) one month prior. Coronary angiography revealed no significant stenosis in either coronary artery. Proper pharmacological management for cardiomyopathy was ensured before the surgery. The patient was on antihypertensive medications, including beta-blockers (BB), calcium channel blockers (CCB), spironolactone, and angiotensin receptor-neprilysin inhibitor (ARNI). CCB and ARNI were discontinued on the day of surgery.
Case 1: The patient had no specific personal and family history of illnesses.
Case 2: The patient had been diagnosed with stress-induced cardiomyopathy one month prior.
Case 1: The baseline vital signs in the operating room presented noninvasive BP of 131/85 mmHg, HR of 75 bpm, and oxygen saturation by pulse oximetry (SpO2) of 100 % in room air.
Case 2: The patient had intermittent chest discomfort but no symptoms of dyspnea. The patient was on antihypertensive medications, including BB, CCB, spironolactone, and ARNI. CCB and ARNI were discontinued on the day of surgery. On arrival at the operating room, the baseline noninvasive BP, HR, and SpO2 were 139/93 mmHg, 83 bpm, and 99%, respec
Case 1: The preoperative laboratory test results were normal, and electrocardiography (ECG) revealed sinus bradycardia of 58 bpm. However, he exhibited satisfactory functional capacity of 10 estimated metabolic equivalents at the pre-anesthesia visit.
Case 2: A follow-up echocardiography revealed global hypokinesia with moderate left ventricular systolic dysfunction with an ejection fraction of 40%, and ECG showed sinus rhythm with premature atrial complexes and aberrant con
Case 1: The preoperative chest radiography test results were normal.
Case 2: Magnetic resonance imaging of the heart demonstrated non-ischemic cardiomyopathy and an absence of bacterial myocarditis.
The two patients had different clinical presentations and incomparable histories but both patients experienced profound bradycardia after receiving a loading dose of DMED for BPB. The treatment of the bradycardia varied between the two patients in composition and duration of administration. However, both patients stabilized and were discharged with no further cardiovascular events, neurologic deficits, or other sequelae. This suggests that sensitivity and cardiac events in response to DMED sedation is not predictable.
Case 1: DMED administration was immediately halted, and atropine (0.5 mg) was administered twice at a 1-min interval. The patient was drowsy but conscious during the bradycardic event, and the radial artery of the non-operating arm was pulsatile on palpation by an experienced anesthesiologist. The noninvasive BP measured in the non-operating arm was 100/70 mmHg. After receiving a second dose of atropine, the HR recovered to 105 bpm, and BP increased to 165/109 mmHg. Although DMED administration was not resumed, the patient presented as a mild-to-moderate sedative state [Modified Observer’s Assessment of Alertness/Sedation Scale (MOAA/S) score, 3-4] for the duration of the surgery which lasted 45 min (Figure 1A).
Case 2: The depressed BP persisted despite the intravenous administration of 20 mg of ephedrine. After invasive arterial BP monitoring was applied via the radial artery of the non-operating arm, a continuous infusion of dopamine (5 μg/kg/min) and norepinephrine (0.1 μg/kg/min) was started. BP and HR recovered to 132/72 mmHg and 111 bpm, res
Case 1: The surgery was completed without any complications, and no other cardiac events occurred in the post-anes
Case 2: The patient experienced no postoperative cardiovascular or neurological events and was discharged without sequelae.
Hemodynamic complications following DMED administration are well-documented in the literature. As it is a highly selective alpha-2 adrenergic agonist, intraoperative bradycardia and hypotension occur frequently in clinical settings[7]. Bradycardia occurs because of a combination of decreased central sympathetic output with increased parasympathetic output and concomitant decreased release of norepinephrine. Hammer et al[8] reported that DMED significantly depre
There have been several reports of severe bradycardia with or without asystole related to DMED under general anes
In case 2, the patient specifically received half of the recommended loading dose (0.5 μg/kg) and at a reduced infusion rate (3 μg/kg/h) of DMED to accommodate an underlying cardiovascular disease. Nevertheless, BP abruptly decreased by 50%, and the patient showed signs of brain hypoperfusion. The patient was receiving multiple drugs, including BB, CCB, and ARNI, although some were discontinued one day before surgery. The patient may have been dehydrated for the therapeutic purposes of congestive heart failure. Consequently, the sympatholytic action of DMED, in addition to the underlying cardiovascular disease with medications and hypovolemia, could have potentiated myocardial depression and vasodilatation, leading to refractory hypotension. Refractory hypotension was sustained for over 6 h after receiving the initial loading dose (0.5 μg/kg). The elimination half-life of DMED is 2-4 h; however, DMED clearance decreases with increasing age and decreasing cardiac output[15]. A dose of 0.5 μg/kg of DMED could not induce an appropriate level of sedation in case 2. This suggests that the pharmacological effects of DMED may be more intense and longer in the cardiovascular system than that in the central nervous system.
DMED-associated profound bradycardia can occur even in healthy individuals receiving the conventional regimen under BPB. The use of DMED in patients with decreased cardiac function can potentially lead to refractory hypotension without apparent bradycardia.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arslan M, Turkey; Ghannam WM, Egypt S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY; MAC Study Group. Monitored anesthesia care with dexmedetomidine: a prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 2. | Vorobeichik L, Brull R, Abdallah FW. Evidence basis for using perineural dexmedetomidine to enhance the quality of brachial plexus nerve blocks: a systematic review and meta-analysis of randomized controlled trials. Br J Anaesth. 2017;118:167-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 3. | Ahuja V, Thapa D, Chander A, Gombar S, Gupta R, Gupta S. Role of dexmedetomidine as adjuvant in postoperative sciatic popliteal and adductor canal analgesia in trauma patients: a randomized controlled trial. Korean J Pain. 2020;33:166-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Gertler R, Brown HC, Mitchell DH, Silvius EN. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent). 2001;14:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 407] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 5. | Bahraini A, Banerjee O, Ra J. Bradycardia resulting in cardiac arrest in a critically ill patient receiving dexmedetomidine. Trauma Case Rep. 2021;36:100548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 495] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | De Cassai A, Sella N, Geraldini F, Zarantonello F, Pettenuzzo T, Pasin L, Iuzzolino M, Rossini N, Pesenti E, Zecchino G, Munari M, Navalesi P, Boscolo A. Preoperative dexmedetomidine and intraoperative bradycardia in laparoscopic cholecystectomy: a meta-analysis with trial sequential analysis. Korean J Anesthesiol. 2022;75:245-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Hammer GB, Drover DR, Cao H, Jackson E, Williams GD, Ramamoorthy C, Van Hare GF, Niksch A, Dubin AM. The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg. 2008;106:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 9. | Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 879] [Cited by in RCA: 918] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 10. | Aikaterini A, Ioannis D, Dimitrios G, Konstantinos S, Vasilios G, George P. Bradycardia Leading to Asystole Following Dexmedetomidine Infusion during Cataract Surgery: Dexmedetomidine-Induced Asystole for Cataract Surgery. Case Rep Anesthesiol. 2018;2018:2896032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Ingersoll-Weng E, Manecke GR Jr, Thistlethwaite PA. Dexmedetomidine and cardiac arrest. Anesthesiology. 2004;100:738-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 110] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Shah AN, Koneru J, Nicoara A, Goldfeder LB, Thomas K, Ehlert FA. Dexmedetomidine related cardiac arrest in a patient with permanent pacemaker; a cautionary tale. Pacing Clin Electrophysiol. 2007;30:1158-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Kim BJ, Kim BI, Byun SH, Kim E, Sung SY, Jung JY. Cardiac arrest in a patient with anterior fascicular block after administration of dexmedetomidine with spinal anesthesia: A case report. Medicine (Baltimore). 2016;95:e5278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Neal JM, Gerancher JC, Hebl JR, Ilfeld BM, McCartney CJ, Franco CD, Hogan QH. Upper extremity regional anesthesia: essentials of our current understanding, 2008. Reg Anesth Pain Med. 2009;34:134-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Iirola T, Ihmsen H, Laitio R, Kentala E, Aantaa R, Kurvinen JP, Scheinin M, Schwilden H, Schüttler J, Olkkola KT. Population pharmacokinetics of dexmedetomidine during long-term sedation in intensive care patients. Br J Anaesth. 2012;108:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |